Abstract
Background & Aims:
Clearance of hepatitis B surface antigen (HBsAg) from serum is the most desirable endpoint and a proposed definition of functional cure for hepatitis B virus (HBV) infection. However, little is known about the long-term durability of HBsAg loss, and there is controversy over whether the development of antibodies against HBsAg (anti-HBs) is required for maintenance. We aimed to assess the durability of spontaneous or treatment-related (interferon or nucleos(t)ide analogue [NA]) loss of HBsAg.
Methods:
We performed a retrospective study of patients with chronic HBV infection followed at the National Institutes of Health from February 1980 through November 2017. We identified those with HBsAg loss, confirmed on 2 visits at least 24 weeks apart. Patients with HCV, HDV, HIV, or HTLV-1 co-infection or HBsAg loss after liver transplantation were excluded. Patients were assigned to the following groups: spontaneous clearance (cleared HBsAg without ever receiving treatment or those who received treatment with a NA or interferon and discontinued therapy more than 5 years before HBsAg loss), interferon-treated (cleared HBsAg either during treatment or within 5 years after stopping interferon), and NA-treated (cleared HBsAg either during treatment or within 5 years after stopping NA).
Results:
Among the 787 HBsAg-positive patients, 89 achieved HBsAg loss; 65/89 had confirmed HBsAg loss, which was spontaneous in 19 of the patients (29%), after interferon in 22 (34%), and after NA in 24 (37%). Of the 65 patients with confirmed loss of HBsAg, 62 patients (95%) remained HBsAg negative after a mean time of 9.6 years from first negative HBsAg test result. HBsAg sero-reversion occurred in 3 of the 46 treated patients (7%) (1 interferon and 2 NA), 1 of whom was positive for anti-HBs. At time of HBsAg loss, 33/65 (51%) were anti-HBs positive. At the last follow-up evaluation, anti-HBs was detectable in 50 of the 62 patients (81%) assessed. The rate of development of anti-HBs was proportionally higher among interferon-treated patients (19/21, 90%) than NA-treated patients (17/22, 77%) or patients with spontaneous loss of HBsAg (14/19, 74%).
Conclusions:
In a retrospective study of 787 HBsAg-positive patients, loss of HBsAg (either spontaneous or after treatment) was confirmed in 8% and was durable. Seroconversion to anti-HBs increased over time and appeared to be more frequent after interferon treatment. HBsAg loss is therefore a robust endpoint for functional cure.
Keywords: Vaccination, Seroconversion, CHB, Outcome
Introduction:
Chronic hepatitis B (CHB) is the leading cause of liver cirrhosis and hepatocellular carcinoma (HCC) globally.1, 2 Prevention of these complications is the major goal of treatment. However, these hard-clinical endpoints may take years or decades to develop and therefore are not suitable to use as endpoints to assess effectiveness of antiviral treatment. Consequently, biochemical (normalization of serum alanine aminotransferase [ALT] level), virological (undetectable HBV DNA level), serological (hepatitis B e antigen [HBeAg] seroconversion and hepatitis B surface antigen [HBsAg] seroconversion) and histological (improvement in necroinflammation and/or fibrosis) surrogate markers have been used to evaluate effectiveness of therapy. Among these, loss of HBsAg is considered the ideal treatment endpoint because it is strongly associated with sustained HBV DNA suppression and a low rate of complications from CHB including HCC and liver-related death.3-5 Unfortunately, HBsAg loss is rarely achieved with current antiviral therapy.6 With the development of novel antiviral agents to treat CHB, there has been re-newed interest in using HBsAg loss as a therapeutic endpoint. However, there has been some debate as to whether HBsAg loss alone or HBsAg loss and development of antibody to HBsAg (anti-HBs) (HBsAg seroconversion) should be the endpoint of therapy.7 Detection of anti-HBs indicates development of immune control and is believed to be necessary for any definition of “cure”. The aims of the current study were to evaluate the durability of HBsAg loss either spontaneously or after stopping treatment with interferon or nucleos(t)ide analogues (NA), and to assess whether the development of anti-HBs was necessary for sustained, off-treatment HBsAg loss.
Patients and Methods:
Patient selection
We retrospectively searched an electronic database for all patients with CHB who had been followed at the National Institutes of Health between February 1980 to November 2017 for evidence of HBsAg loss. Demographic (age, gender, race), clinical (date of initial diagnosis, date of HBsAg loss, length of follow up, mode of HBsAg loss, presence of cirrhosis, prior treatment history) and laboratory (ALT and aspartate aminotransferase [AST] levels, total bilirubin, serum albumin, platelet count, HBsAg, anti-HBs, HBeAg status, and HBV DNA) data were extracted from the electronic medical record and when necessary from the paper record. To be included in the analysis, patients had to have CHB defined as 2 positive HBsAg tests a minimum of 6 months apart and then to have confirmed HBsAg loss defined as testing HBsAg negative on two separate occasions at least 6 months apart. Patients co-infected with hepatitis C virus (HCV), hepatitis D virus (HDV), human immunodeficiency virus-1 (HIV-1), human T lymphocyte virus (HTLV-1), and those who underwent liver transplantation were excluded.
Prior to HBsAg loss, untreated patients were typically followed every 3-6 months or more frequently if clinically indicated. After HBsAg loss, patients were usually monitored every 6-12 months. Treated patients were monitored during and after treatment per protocol which ranged from monthly for studies utilizing interferon to every 3 months for studies using NAs. Treatment, if administered, was generally through a clinical protocol although a minority of patients received standard of care treatment under a Natural History protocol if they did not qualify for a clinical trial. The treatment regimens with interferon were standard interferon 5 million international units (IU) subcutaneously daily, 10 million IU three times weekly for 16-24 weeks, or pegylated interferon alfa-2a (180 mg subcutaneously weekly for 48 weeks). If HBsAg loss occurred during interferon therapy, patients remained on treatment per protocol until the course of treatment was completed. Treatment regimens for NAs included adenine arabinoside monophosphate (ARA/AMP) 10 mg/kg/day given initially intravenously three times daily for 5 days followed by intramuscular injections every 12 hours for 23 days, fialuridine (FIAU) 0.1 mg/kg/day for 28 days, lamivudine 100 mg orally daily, adefovir 10 mg orally daily, lamivudine 100 mg / adefovir 10 mg orally daily, entecavir 0.5 mg orally daily, tenofovir 300 mg orally daily and tenofovir 300 mg / emtricitabline 100 mg orally daily. Patients treated with NAs received treatment indefinitely with the exception of pilot studies using ARA/AMP and FIAU or if the NA was withdrawn. If HBsAg loss occurred during treatment with NAs, patients received a minimum of 6 months consolidation treatment before the NA was stopped. Following HBsAg loss, hepatitis B vaccination was administered to induce anti-HBs at the discretion of the treating physician. If administered, the standard dose of 20 ug intramuscularly was used at a frequency of 1-5 doses. Some of the patients were previously reported in other publications.8-11
Study Design
For analysis, patients were categorized into 3 groups depending on the mode of HBsAg loss. The spontaneous clearance group was defined as those who cleared HBsAg without ever receiving treatment or who received treatment with a NA or interferon and discontinued therapy more than 5 years before HBsAg loss. The interferon-treated group was defined as patients who cleared HBsAg either during treatment or within 5 years after stopping interferon. Finally, the NA-treated group was defined as those who cleared HBsAg either during treatment or within 5 years after stopping NA. HBsAg seroconversion was defined as loss of HBsAg with the development of anti-HBs.
HBV DNA Quantification, HBV Genotype and HBV Serological Testing
HBV DNA levels were quantified using the Roche COBAS TaqMan HBV Assay (Roche Molecular Systems, Branchburg, New Jersey); lower limit of quantification 20 IU/ml. In cases where HBV DNA results were not measured by a PCR based assay, serum samples stored at −20°C or −80°C were retested based on availability. Viral genotyping was performed using the Inno-Lipa HBV genotyping assay (Innogenetics N.V., Ghent, Belgium). Pre-1998 HBV serology (HBsAg, anti-HBs, HBeAg and anti-HBe) was tested with RIA and then EIA (Abbott Laboratories, North Chicago, IL). Post-1998 HBV serology were tested using the VITROS® ECi/ECiQ Immunodiagnostic System (Ortho Clinical Diagnostics, Raritan, NJ).
Statistical Analysis
Continuous outcomes were summarized using means and standard deviation (SD) and categorical outcomes were summarized using frequencies. Either the Chi-squared test or Fisher’s Exact test was used to compare whether two or more proportions were equal. The Kaplan-Meier curve or cumulative incidence rate plot were used to present cumulative proportion of events of interest. All analyses were two-tailed tests based on a significance level of 0.05 and conducted using SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Patient Cohort and Clinical Characteristics at time of HBsAg loss
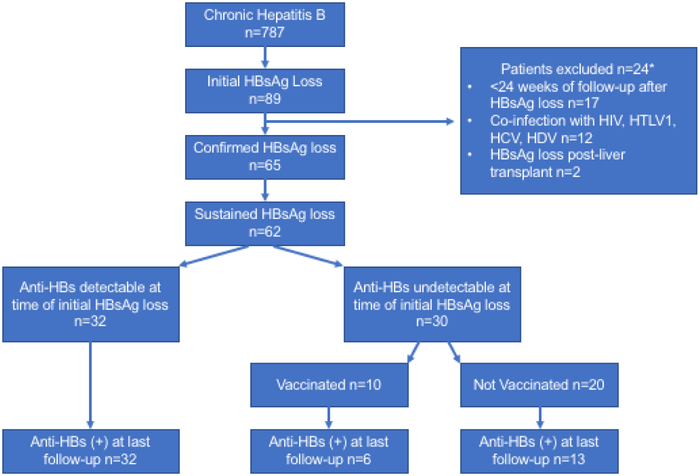
We identified 787 patients with CHB, 89 (11.3%) of whom achieved initial HBsAg loss. Twenty-four of eighty-nine patients were excluded because HBsAg loss was not confirmed primarily because patients were lost to follow-up (n=17), due to co-infection with HCV (n=5), HDV (n=2), HIV (n=4), HTLV-1 (n=1) and because HBsAg loss occurred after liver transplantation (n=2), Figure 1. Patients may have met more than one criterion for exclusion. Overall, 65/89 immunocompetent patients had confirmed HBsAg loss. The mean age was 49 (SD:14, range: 5-78) years, 52 (80%) were male, 48 (72%) were Caucasian, 5 (10%) African American, and 12 (18%) Asian and 44/65 (71%) were initially HBeAg positive. The mean ALT and AST at time of HBsAg loss were 40 U/L and 35 U/L, respectively. Other labs are shown in Table 1. The mode of HBsAg loss was spontaneous in 19 (29%), interferon-related in 22 (34%) and NA-related in 24 (37%). Among patients with treatment-related HBsAg loss (n=46), HBsAg loss occurred on treatment in 23 (50%) patients, (NA, n=20; interferon, n=3) and off treatment in 23 (50%) patients (NA, n=4; interferon, n=19). The mean duration of follow-up after HBsAg loss was 115 months and was longer for interferon treated patients compared to those with spontaneous or NA-related HBsAg loss. The mean number of HBsAg and anti-HBs tests performed during follow-up was 9 and was similar among the three groups (range 7-11).
Figure 1: Study Consort Diagram.
Participants included in the analysis. HIV-human immunodeficiency virus, HTLV1-Human T-cell leukemia virus type 1, HCV-hepatitis C virus, HDV-hepatitis D virus
Table-1:
Baseline Demographics and Clinical Characteristics of the Cohort
| Characteristic | Mode of HBsAg loss | |||
|---|---|---|---|---|
| All n=65 | Spontaneous | Interferon- related |
Nucleos(t)ide analogue- related |
|
| n=19 | n=22 | n=24 | ||
| Mean Age (Years) | 49 (SD:14) | 50 (SD:14) | 42 (SD:16) | 54 (SD:11) |
| Male gender | 52 (80%) | 16 (84%) | 14 (64%) | 22 (92%) |
| Race: | ||||
| Caucasian | 48 (74%) | 14 (74%) | 19 (86%) | 15 (63%) |
| Asian | 12 (18%) | 5 (26%) | 1 (5%) | 6 (25%) |
| African | 5 (8%) | 0 (0%) | 2 (9%) | 3 (12%) |
| Genotype (GT): | ||||
| GT A | 14 (22%) | 1 (5%) | 4 (18%) | 9 (38%) |
| GT B | 1 (2%) | 1 (5%) | 0 (0%) | 0 (0%) |
| GT C | 4 (6%) | 1 (5%) | 0 (0%) | 3 (12%) |
| GT D | 2 (3%) | 0 (0%) | 0 (0%) | 2 (8%) |
| GT Indeterminate | 2 (3%) | 0 (0%) | 1 (5%) | 1 (4%) |
| GT Missing | 42 (64%) | 16 (84%) | 17 (77%) | 9 (38%) |
| HBeAg positive | 46 (71%) | 10 (52%) | 22 (100%) | 14 (58%) |
| ALT U/L | 40 (SD:64) | 30 (SD:28) | 59 (SD:99) | 30 (SD:15) |
| AST U/L | 35 (SD:63) | 24 (SD:10) | 58 (SD:102) | 23 (SD: 5.9) |
| Albumin g/dL | 4.22 (SD:0.36) | 4.4 (SD: 0.32) | 4.2 (SD:0.34) | 4.1 (SD:0.34) |
| Total Bilirubin | 0.75 (SD:0.51) | 0.7 (SD:0.27) | 0.94 (SD:0.72) | 0.7 (SD:0.36) |
| Platelet count K/mm3 | 195 (SD:51.4) | 199 (SD:55) | 186 (SD:63) | 200 (SD:33) |
| Cirrhosis based on Biopsy n=51 | 5 (10%) | 1 (9%) | 3 (15%) | 1 (5%) |
| Cirrhosis based on APRI n=65≥2.0 | 3 (5%) | 0 (0%) | 3 (14%) | 0 (0%) |
| Cirrhosis based on FIB-4 n=65≥3.5 | 2 (3%) | 0 (0%) | 1 (5%) | 1 (4%) |
| Total with cirrhosis | 7(11%) | 1 (9%) | 4 (18%) | 2 (8%) |
| Mean duration of follow up since initial presentation (months) | 208 (SD:107) | 204 (SD:138) | 227 (SD:100) | 186 (SD:85) |
| Mean duration of follow up after first negative HBsAg test (months) | 115 (SD:96) | 84 (SD:94) | 192 (SD:91) | 69 (SD:53) |
| Mean number of f/u HBsAg (−) tests done after initial HBsAg (−) | 9 (SD:7) | 7 (SD:7) | 9 (SD:7) | 11 (SD:8) |
| Mean number of f/u Anti-HBs tests done after initial HBsAg (−) | 9 (SD:7) | 7 (SD:7) | 8 (SD:6) | 11 (SD:8) |
Prior Treatment History of Patients with Spontaneous- and Treated-related HBsAg Loss
Among the spontaneous HBsAg loss group (n=19), 11 (58%) received prior treatment (6 patients received interferon, 4 patients received NA, and 1 patient received both NA and interferon), Figure 2a. Among the interferon-related HBsAg loss group (n=22), three patients received short courses of NA before receiving interferon (2 patients received ARA/AMP for 28 days, and 1 patient received FIAU for 28 days); 1 patient received 3 separate courses of interferon, Figure 2b. HBsAg loss occurred while on interferon in 3 (14%) patients and off interferon in 19 (86%) patients, a mean of 26.9 months (SD: 21.9, range 1-59 months) after stopping treatment. In the NA-related HBsAg loss group (n=24), three patients received a prior 6-month course of interferon and one patient received combination therapy with interferon for 6 months, Figure 2c. Twenty patients lost HBsAg while on treatment and all received consolidation treatment for a minimum of 6 months after HBsAg loss. Four patients lost HBsAg a mean of 32 months after stopping treatment.
Figure 2a:
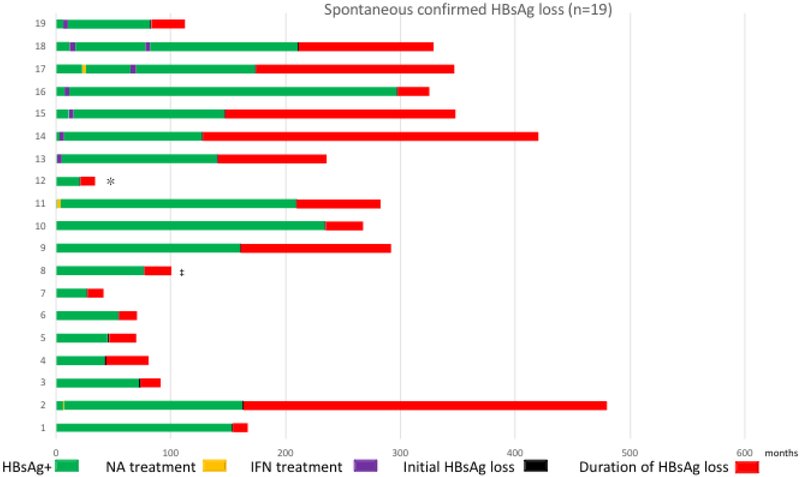
Prior Treatment History and Course of Patients with Spontaneous HBsAg Loss. *Patient received adefovir for 5 years and then discontinued treatment 7 years prior to initiating care at NIH. ‡Patient received entecavir for 3 years and then discontinued therapy before initiating care at NIH.
Figure 2b:
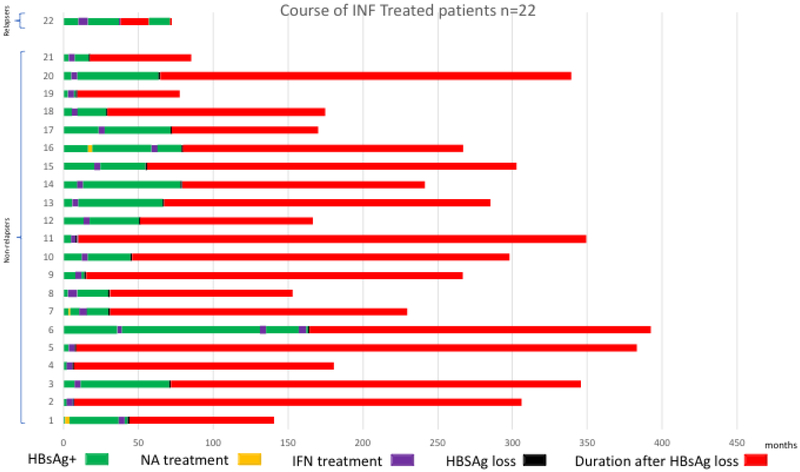
Prior Treatment History and Course of Patients with Interferon-related HBsAg loss.
Figure 2c:
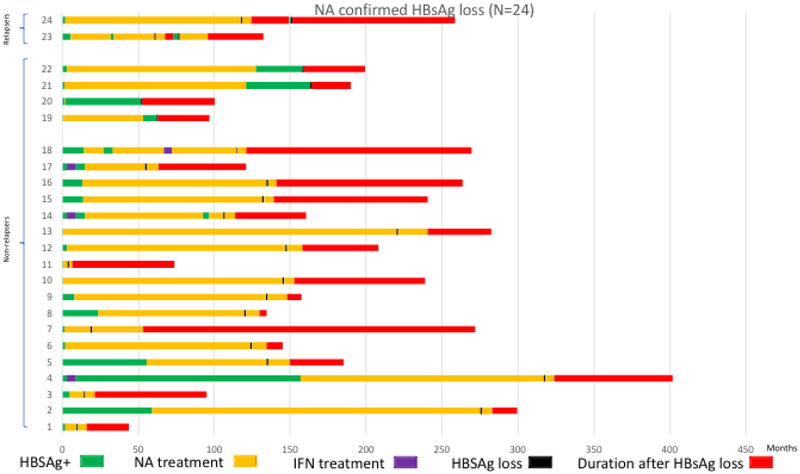
Prior Treatment History and Course of Patients with Nucleos(t)ide analogue-related HBsAg loss. Prior NA treatment indicated by yellow bar, an asterisk and a double cross; prior interferon treatment indicated by purple bar. Green bar indicates period of HBsAg positivity since initial visit. Black bar indicates time of HBsAg loss. Red bar indicates duration of follow-up after HBsAg loss.
Incidence and Durability of HBsAg loss
The annual incidence of HBsAg loss was estimated to be 20 per 100-person years. It was 50 per 100-person years among interferon-treated patients, 20 per 100-person years among NA-treated patients and 10 per 100-person years among those with spontaneous HBsAg loss. No patient developed HCC in follow-up.
Sixty-two of sixty-five (95%) patients with confirmed HBsAg loss remained HBsAg negative after a mean follow-up 115 months from the first negative HBsAg test, Figure 3a. The one- and five-year cumulative risk of relapse was 1.6% and 5.4% respectively. Only 3 of 65 (5%) patients experienced HBsAg seroreversion, one patient (patient-1) received standard interferon for 6 months and 2 patients (patients 2 and 3) received NAs for 60 and 122 months respectively, Supplemental Figure 1. The mean time to HBsAg seroreversion was 20 months (range: 11-30 months). In the interferon-treated patient, HBsAg was positive on a single occasion 19 months after initial loss and was negative when re-tested 14 months later. During HBsAg reappearance, no increase in ALT or HBV DNA levels were noted. Of the 2 NA-treated patients, both lost HBsAg on-treatment, and both received consolidation treatment for 6 months. Patient 3 had a single positive HBsAg test 31 months after the initial loss of HBsAg and was negative when re-tested 2 weeks later. The HBV DNA level remained undetectable and ALT level normal. Patient 2 became HBsAg positive 11 months after stopping NA and HBsAg was repeatedly positive 3 times over the course of 6 weeks. HBV DNA level increased to 20,600 IU/ml but serum ALT level remained normal. The patient was restarted on NA treatment and after a re-treatment period of 19 months cleared HBsAg and was able to stop NA. The patient remained HBsAg negative for a period of 37 months and then was lost to follow-up. Thus, only a single patient had confirmed detection of HBsAg.
Figure 3a:
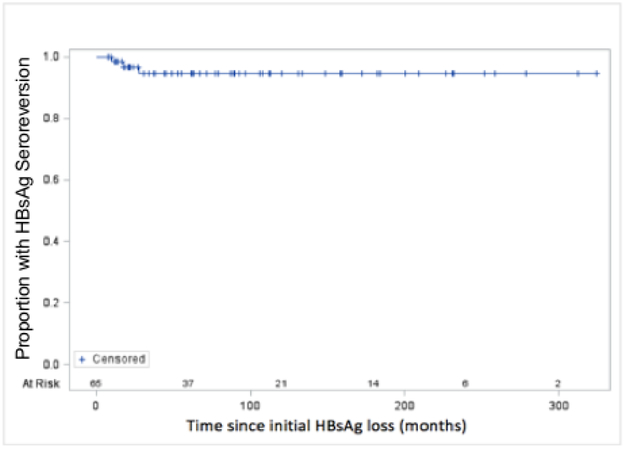
Kaplan-Meier Analysis of Durability of HBsAg loss
Development of anti-HBs and its role in maintenance of HBsAg loss
At the time of HBsAg loss, 33/65 (51%) had developed concomitant anti-HBs. Anti-HBs was detected in 9/19 (47%) of those with spontaneous, 15/22 (68%) of those with interferon-related and 9/24 (37%) of those with NA-related HBsAg loss, p=0.1, Figure 4a. At last follow up, a mean of 115 months after HBsAg loss, anti-HBs had increased to 50/62 (81%). The proportion anti-HBs positive, was higher among interferon-treated patients (interferon 19/21 (90%) versus spontaneous 14/19 (74%) and NA 17/22 (77%), but was not significant, p=0.35, Figure 4b. The mean time from initial HBsAg loss to development of anti-HBs was 21 (range 0-209) months. The cumulative incidence of anti-HBs is shown in figure 3b. The rate of HBsAg relapse among anti-HBs positive vs anti-HBs negative patients was 1/33 (3%) and 2/32 (6%), p=0.53, Supplementary Figure 2. The influence of the duration of consolidation therapy on development of anti-HBs in NA-treated persons who were anti-HBs negative at time of HBsAg loss and who experienced HBsAg loss on treatment (n=12) was analyzed. The mean period of consolidation was similar among those who developed anti-HBs (n=7) compared to those who did not (n=5), 13 (range 6-33) months compared to 9 (range 6-15) months respectively, p=0.43.
Figure 4a:
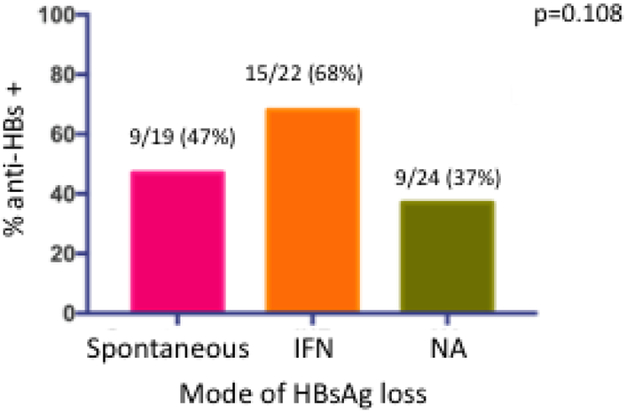
Proportion with anti-HBs at time of initial HBsAg loss by mode of HBsAg loss.
Figure 4b:
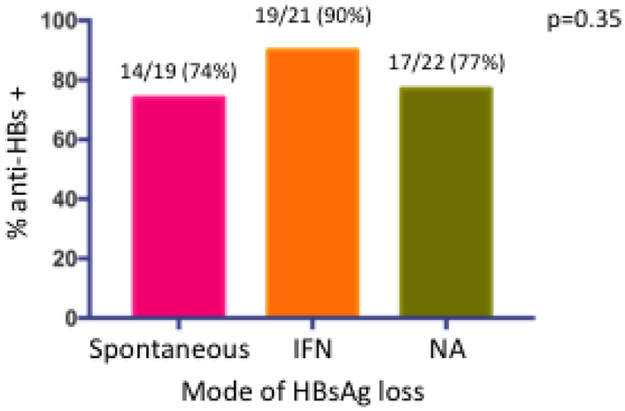
Proportion with anti-HBs at time of last follow up visit by mode of HBsAg loss. Magenta bar spontaneous HBsAg loss; orange bar interferon-related HBsAg loss; green bar nucleos(t)ide-related HBsAg loss.
Figure 3b:
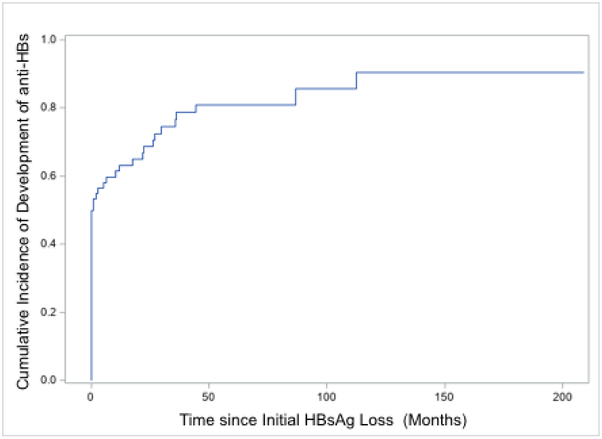
Cumulative Incidence of Development of anti-HBs
Role of HBV vaccine in inducing anti-HBs seroconversion
We evaluated the effect of administration of hepatitis B vaccination to induce anti-HBs seroconversion after clearance of HBsAg in patients without anti-HBs. Among the 30 patients who were anti-HBs negative at time of HBsAg loss and had not relapsed, 10 received a mean of 3 doses (range 1-5) of hepatitis B vaccine. The mean time to administration of the first dose of hepatitis B vaccine was 14 months after initial loss of HBsAg (range: 1.3-32.8 months). The proportion who developed anti-HBs at last follow-up was similar among vaccinated versus non-vaccinated patients, 6/10 (60%) versus 13/20 (65%), respectively, p=0.78, Figure 1. Time to development of anti-HBs was shorter in the unvaccinated group 25.3 months (SD: 25.6) compared to the vaccinated group, 44.3 months (SD: 46.1) but this was not significant.
Discussion
This retrospective analysis was conducted to investigate the durability of HBsAg loss and whether anti-HBs was necessary for maintenance of HBsAg loss. We demonstrate that confirmed spontaneous or treatment-related HBsAg loss is a durable endpoint in 95% of patients over a mean follow-up of 115 months. Three, (5%), treated patients experienced HBsAg seroreversion a mean of 20 months after stopping therapy compared to no patient with spontaneous HBsAg loss. In two of these patients HBsAg seroreversion was transient and likely represented a false positive as it was never confirmed on follow-up testing, HBV DNA was not detected, and ALT remained normal. A single patient had recurrent viremia and required retreatment. Thus, the durability of HBsAg loss may be as high as 98%. Anti-HBs seroconversion was detectable in 51% at time of initial HBsAg loss and increased to 81% at time of last follow-up and was unrelated to HBsAg seroreversion. Administration of HBV vaccine neither increased the frequency nor rate of development of anti-HBs.
The overall incidence of HBsAg loss in this retrospective study was 20 per 100 person years which was comparable to the annual seroclearance rate range of 0.15% to 3.02% reported in a recent systematic review.12 A major finding of this study is that HBsAg loss is durable over time. This finding is in keeping with the limited studies that have reported on durability of HBsAg loss with follow-up that ranged from an average of 2 to 6 years11, 13-15 and one study with a follow-up of 9.8 years.16 Collectively, these studies reported that among patients with spontaneous or treatment-related HBsAg loss, the rate of HBsAg seroreversion ranged from 4 to 12%. A particular strength of our study was the long-duration of follow-up after HBsAg loss, 9.6 years, and the frequent monitoring of HBsAg and anti-HBs, thus, providing robust data regarding the durability of HBsAg loss. In addition, this study included patients of all races whereas other studies have included predominantly Asian patients. No patient developed HCC in follow-up probably due to the low rate of cirrhosis in the cohort (11%) and the relatively young mean age at HBsAg loss (51 years).
An important issue is whether anti-HBs is necessary for maintenance of HBsAg loss. The level of anti-HBs has been reported to be associated with reactivation of HBV infection in patients receiving immunosuppressive therapy. This is controversial as some but not all studies suggested that negative or low anti-HBs levels (<100 mIU/mL) prior to receipt of immunosuppressive therapy in patients who have recovered from CHB were associated with a greater likelihood of HBV reactivation.17, 18 In this study of immunocompetent patients, the HBsAg seroreversion rate in those who were anti-HBs negative was numerically twice that compared to those who were anti-HBs positive at time of HBsAg loss, 2/32 (6%) versus 1/33 (3%), respectively. However, the numbers are too small to draw a definite conclusion as to whether presence of anti-HBs is associated with a lower HBsAg seroreversion rate. Age, gender, race and presence of cirrhosis had no influence on the rate of HBsAg seroreversion.
In this study, detection of anti-HBs increased from 51% at time of HBsAg loss to 81% after a mean of 9.6 years. A similar observation was noted in another study after NA-related HBsAg clearance where the rate of detection anti-HBs doubled from 34.3% at year one to 67.4% at year five.16 What determines the detection of anti-HBs after HBsAg loss is unknown. During recovery from acute infection detection of anti-HBs is usually delayed after HBsAg loss, taking several weeks or months to appear.19 In a study of Alaskan Natives following recovery of acute hepatitis B, anti-HBs was detected in only 42% 1-year after onset of illness, but increased to 80% at 5 years.20 A possible explanation is that HBsAg continues to be produced at low levels beyond detection of current commercial assays coupled with low anti-HBs production that is complexed to HBsAg. Indeed, in a small study evaluating a more sensitive Lumipulse HBsAg-HQ assay in patients with spontaneous and entecavir-related HBsAg loss, HBsAg could be detected up to 10 months following spontaneous HBsAg loss and was intermittently positive for almost 12 months in a patient with entecavir-related HBsAg loss when measured by the Lumipulse HBsAg-HQ assay despite testing HBsAg negative by the Abbott Architect assay.21 Alternatively, anti-HBs may not be produced due to B-cell or T-helper cell defect/s that initially persist after HBsAg loss but recover over time. The observation that the rate of anti-HBs positivity increased over time suggests that HBsAg loss may lead to restoration of a B- or T-helper response that is required for production of anti-HBs. A third explanation is that anti-HBs intermittently fall below the level of detection and may have been missed because of the annual follow-up. Importantly, we could not identify any host or virological characteristics that were associated with anti-HBs positivity at time of HBsAg loss.
We were able to examine the effect of HBV vaccination on the subsequent development of anti-HBs. Administration of single or multiple doses of standard HBV vaccination did not hasten the development of anti-HBs or increase the proportion of patients who developed anti-HBs compared to those who did not receive vaccination. Therefore, based on this small cohort, we do not recommended administration of HBV vaccine to induce anti-HBs after HBsAg loss.
Current management guidelines recommend that discontinuation of NAs may be considered in persons who have demonstrated loss of HBsAg but cite lack of evidence to support this recommendation. Among NA-treated patients in this analysis 22/24 (92%) had durable HBsAg loss. Among the two relapsers only one experienced virological relapse. Despite the small cohort, these data suggest that the majority of NA-treated patients with documented confirmation of HBsAg loss could be safely withdrawn after a consolidation period of 6 months.
Our study has a several limitations. This was a retrospective analysis and therefore not all subjects were necessarily monitored at the same schedule and data was not available annually on all patients although the mean number of tests was 9 over 9 years of follow-up. A sensitivity analysis (supplementary material) highlighted that in some instances the interval between visits may have exceeded 21 months. Thus, we cannot exclude the possibility of HBsAg seroreversion during these periods. However, if this had occurred it would have been transient, as all available HBsAg tests were negative except in the three patients. We did not have complete HBV genotype data for a majority of patients and therefore could not analyze the contribution of HBV genotype on durability of HBsAg loss or development of anti-HBs. Given the low HBsAg seroreversion rate it is unlikely that HBV genotype would have influenced the results. A proportion of patients with spontaneous HBsAg loss had received short courses of interferon, NA or both prior to HBsAg loss. We reasoned that HBsAg loss that occurred 5 years or longer after the course of treatment was unlikely to be related to prior treatment. Reallocating these patients to the interferon or NA-related HBsAg clearance groups would not alter the results in any meaningful way since none of these patients experienced HBsAg reappearance.
In summary, spontaneous and treatment-related HBsAg loss is durable in the majority of patients and development of anti-HBs does not appear to be required for maintenance of HBsAg loss. After HBsAg loss, anti-HBs develops in a majority of patients over time and appears to be higher in interferon-treated patients compared to NA-treated or spontaneous HBsAg loss suggesting that novel therapies that can restore the immune response may be associated with higher rates of development of anti-HBs. These data support the definition of functional cure as HBsAg loss either with or without the development of anti-HBs and use of HBsAg loss as a treatment endpoint for novel antiviral/immunomodulatory agents.
Supplementary Material
Need to Know.
Background: Little is known about the long-term durability of hepatitis B surface antigen (HBsAg) loss, and there is controversy over whether the development of antibodies against HBsAg (anti-HBs) is required for maintenance. We aimed to assess the durability of spontaneous or treatment-related (interferon or nucleos(t)ide analogue [NA]) loss of HBsAg
Findings: In a retrospective study of 787 HBsAg-positive patients, we found loss of HBsAg (either spontaneous or after treatment) to be durable. Seroconversion to anti-HBs increased over time and appeared to be more frequent after interferon treatment.
Implications for Patient Care: Loss of HBsAg (either spontaneous or after treatment) is a robust endpoint for functional cure.
Acknowledgments
Funding: This work was supported by the Intramural Research Program of NIDDK, NIH
Abbreviations:
- IFN
interferon
- NA
nucleos(t)ide analogues
- HBsAg
hepatitis B surface antigen
- Anti-HBs
hepatitis B surface antibody
- HBeAg
hepatitis B e antigen
- Anti-HBe
hepatitis B e antibody
- HCC
hepatocellular carcinoma
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AFP
alpha fetoprotein
- HCV
hepatitis C virus
- HDV
hepatitis D virus
- HIV-1
human immunodeficiency virus-1
- HTLV-1
human T lymphocyte virus
- ARA/AMP
adenine arabinoside monophosphate
- FIAU
fialuridine
- ETV
entecavir
Footnotes
Financial Disclosure: The authors are employees of the U.S. Government and have no financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009;49:S45–55. [DOI] [PubMed] [Google Scholar]
- 2.Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuen MF, Wong DK, Fung J, et al. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology 2008;135:1192–9. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Yang HI, Lee MH, et al. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma. Gut 2014;63:1648–57. [DOI] [PubMed] [Google Scholar]
- 5.Yip TC, Wong GL, Chan HL, et al. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J Hepatol 2019;70:361–370. [DOI] [PubMed] [Google Scholar]
- 6.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lok AS, Zoulim F, Dusheiko G, et al. Hepatitis B cure: From discovery to regulatory approval. Hepatology 2017;66:1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong TL, Di Bisceglie AM, Gerber MA, et al. Persistence of hepatitis B virus DNA in the liver after loss of HBsAg in chronic hepatitis B. Hepatology 1993;18:1313–8. [PubMed] [Google Scholar]
- 9.Lau DT, Everhart J, Kleiner DE, et al. Long-term follow-up of patients with chronic hepatitis B treated with interferon alfa. Gastroenterology 1997;113:1660–7. [DOI] [PubMed] [Google Scholar]
- 10.Ghany MG, Feld JJ, Zhao X, et al. Randomised clinical trial: the benefit of combination therapy with adefovir and lamivudine for chronic hepatitis B. Aliment Pharmacol Ther 2012;35:1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingala S, Lau DT, Koh C, et al. Long-term lamivudine therapy in chronic hepatitis B. Aliment Pharmacol Ther 2016;44:380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeo YH, Ho HJ, Yang HI, et al. Factors Associated With Rates of HBsAg Seroclearance in Adults With Chronic HBV Infection: A Systematic Review and Meta-analysis. Gastroenterology 2019;156:635–646 e9. [DOI] [PubMed] [Google Scholar]
- 13.Stelma F, van der Ree MH, Jansen L, et al. HBsAg loss after peginterferon-nucleotide combination treatment in chronic hepatitis B patients: 5 years of follow-up. J Viral Hepat 2017;24:1107–1113. [DOI] [PubMed] [Google Scholar]
- 14.Yip TC, Wong GL, Wong VW, et al. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J Hepatol 2018;68:63–72. [DOI] [PubMed] [Google Scholar]
- 15.Suarez E, Buti M, Rodriguez M, et al. Hepatitis B surface antigen loss after discontinuing nucleos(t)ide analogue for treatment of chronic hepatitis B patients is persistent in White patients. Eur J Gastroenterol Hepatol 2019;31:267–271. [DOI] [PubMed] [Google Scholar]
- 16.Kim GA, Lim YS, An J, et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut 2014;63:1325–32. [DOI] [PubMed] [Google Scholar]
- 17.Paul S, Dickstein A, Saxena A, et al. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: A meta-analysis. Hepatology 2017;66:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy KR, Beavers KL, Hammond SP, et al. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:215–9; quiz e16-7. [DOI] [PubMed] [Google Scholar]
- 19.Hoofnagle JH. Type B hepatitis: virology, serology and clinical course. Semin Liver Dis 1981;1:7–14. [DOI] [PubMed] [Google Scholar]
- 20.McMahon BJ, Bender TR, Berquist KR, et al. Delayed development of antibody to hepatitis B surface antigen after symptomatic infection with hepatitis B virus. J Clin Microbiol 1981;14:130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinkai N, Matsuura K, Sugauchi F, et al. Application of a newly developed high-sensitivity HBsAg chemiluminescent enzyme immunoassay for hepatitis B patients with HBsAg seroclearance. J Clin Microbiol 2013;51:3484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.