Abstract
Objective
To provide a standardized protocol for the measurement of cervical strain elastography, present its reproducibility, and analyze baseline clinical factors affecting the measurement of elastographic parameters.
Methods
This study was performed by the Korean Research Group of Cervical Elastography. We enrolled pregnant women according to our study protocol. After measuring the cervical length, elastography was performed using the E-Cervix™ quantification tool to measure the strain of the cervix using intrinsic compression. We evaluated 5 elastographic parameters, namely, the strain of the internal os of the cervix (IOS), strain of the external os of the cervix (EOS), ratio of the strain of IOS and EOS, elasticity contrast index, and hardness ratio. For baseline clinical factors, we examined the maternal body mass index, blood pressure, heart rate, uterine artery Doppler indices, and fetal presentation.
Results
We established a specific protocol for the measurement of cervical elastography using the E cervix program. For all elastographic parameters, the intra-observer intraclass correlation coefficient (ICC) ranged from 0.633 to 0.723 for single measures and from 0.838 to 0.887 for average measures, and the inter-observer ICC ranged from 0.814 to 0.977 for single measures and from 0.901 to 0.988 for average measures. Regression analysis showed that the measurement of the elastographic parameter was not affected by baseline clinical factors.
Conclusion
We present a standardized protocol for the measurement of cervical elastography using intrinsic compression. According to this protocol, reproducibility was acceptable and the measurement of elastographic parameters was not affected by the baseline clinical factors studied.
Keywords: Cervix uteri, Elastography, Standardization, Reproducibility of results, Premature birth
Introduction
Although short cervical length is an important risk factor for preterm delivery, the actual positive predictive value for predicting preterm delivery by cervical length ≤2.5 cm measured between 20 to 25 weeks is <20% in low-risk populations [1,2]. The current American College of Obstetrics and Gynecology guidelines do not mandate universal cervical length measurement for low-risk populations but rather state that screening may be considered [3]. Despite the lack of strong evidence, cervical length measurement has become routine, even in low-risk populations in Korea.
Recently, elastography has emerged as an adjunctive tool to conventional ultrasound in organs such as the breast, liver, and thyroid [4,5,6]. For example, current guidelines recognize that elastography is a complementary technique to B-mode imaging in breast ultrasounds or diffuse liver disease [4,6]. However, unlike breast or liver tissues, the cervix is a relatively small organ and has no control tissues, precluding the practical application of cervical elastography in pregnancy.
There are 2 methods of elastography, namely, shear-wave and strain elastography; the latter can be further divided according to the method of compression—extrinsic or intrinsic. Because standardization of cervical elastography measurement is crucial irrespective of the methods used, it is important to implement useful standards to retain reproducibility. The E-cervix program is designed to use intrinsic compression by internally generated fine vibrations by organ motion, such as adjacent arterial pulsation; therefore, in theory, multiple baseline clinical factors may affect the measurement of elastographic parameters. We presumed that several baseline clinical factors such as maternal body mass index (BMI), blood pressure, heart rate, uterine artery Doppler indices, and fetal presentation can affect the elastographic parameter values.
Considering the above, the aim of our study was 3-fold: 1) to provide a standardized protocol for the measurement of cervical strain elastography using intrinsic compression, 2) to present its intra- and inter- observer reproducibility, and 3) to analyze baseline clinical factors that affect the measurement of elastographic parameters.
Materials and methods
1. Study population
This study was performed by the Korean Research Group of Cervical Elastography, which included 7 institutions (Kyung Hee University Hospital at Gangdong, Kangbuk Samsung Hospital, Kyungpook National University Hospital, Dongguk University Ilsan Hospital, Konkuk University Medical Center, Yonsei University Severance Hospital, and Samsung Medical Center). The ultimate aim of this on-going prospective multicenter study is to verify cervical elastography as a predictor of spontaneous preterm birth in high or low risk populations. The study period is planned to run from June 2018 to December 2020. According to the study protocol, we will recruit 6 groups of pregnant women according to the indications as follows: 1) women with short cervical length (≤2.5 cm) between 16 and 32 weeks of gestation (the short cervix group); 2) women with threatened preterm labor (PTL), defined as those showing 6 or more contractions in 1 hour on cardiotocography with intact membranes and who were between 20 and 34 weeks of gestation (the PTL group); 3) women with twin pregnancies between 16 and 30 weeks of gestation (the twin group); 4) women with a history of cervical surgery, such as loop electrosurgical excision procedure (LEEP), between 14 and 24 weeks of gestation (the LEEP group); 5) women who were treated with progesterone between 16 and 28 weeks of gestation due to short cervix or history of prior spontaneous preterm birth (the progesterone group); and 6) asymptomatic women who underwent routine mid-trimester ultrasound between 20 and 24 weeks of gestation (the normal group). The study population was also categorized into 2 groups as follows: the high-risk population comprising the short cervix, PTL, twin, LEEP, and progesterone groups, and the low-risk population of the normal group. The exclusion criteria of all study groups were triplet pregnancy, placental abruption, and placement of cervical cerclage at elastography. Participants were allowed to be enrolled into one or more groups according to the clinical indications following elastography.
For the assessment of potential baseline clinical factors that affect the measurement of cervical elastography, participants (n=256) who were enrolled during the first 3 months after outset of the study period (from June 2018 to October 2018) were included. In this analysis, if a participant was enrolled into more than one group, she was assigned to one representative group. For reproducibility and correlation analysis of this study, an extended number of participants enrolling up to June 2019 (n=895) were included.
2. Baseline clinical factors that potentially affect the measurement of cervical elastography
As for baseline factors that could affect the measurement of cervical elastographic parameters, we examined following variables at the time of cervical elastographic measurement: maternal blood pressure, heart rate, body weight, and height. The mean arterial pressure was calculated using the following equation: MAP = (systolic blood pressure + 2×diastolic pressure)/3. Uterine artery Doppler velocimetry was performed only in the low-risk population, and the pulsatility index and resistance index were used for analysis. Because of concerns that active fetal limb movements near the cervix could induce fine internal vibration and may, thus, affect cervical elastography, we also checked fetal presentation during elastography, which was dichotomized into cephalic or non-cephalic.
3. Obtaining proper plane and measurement of cervical elastography
Cervical elastography was measured via vaginal ultrasound (WS80A Ultrasound System, Samsung Medison, Seoul, Korea), which used a 6-MHz transvaginal probe. After measuring the cervical length, elastography was performed at least 3 times in the same plane with the same transvaginal probe using the E-Cervix™ system, a quantification tool to measure the strain of the cervix.
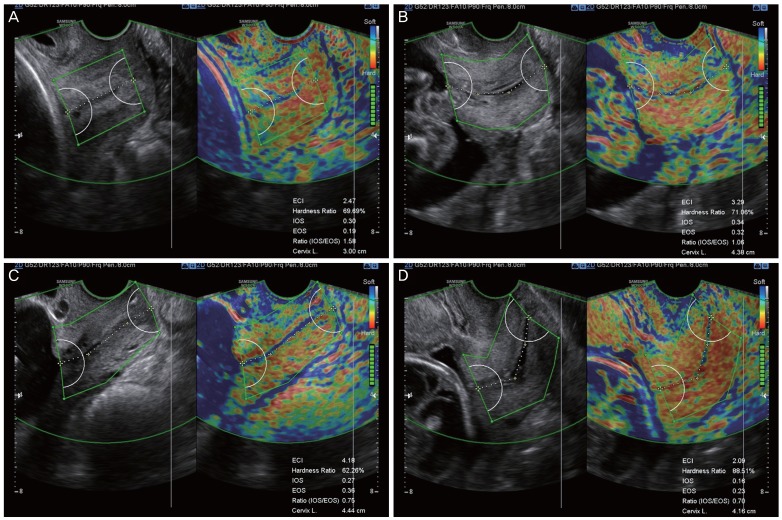
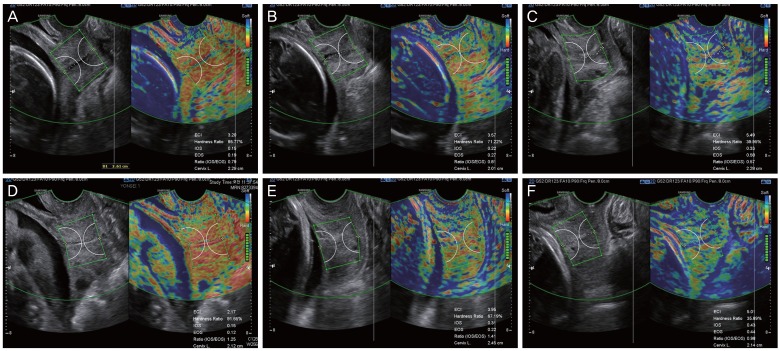
At the beginning of this study, all investigators from the 7 participating institutions had several research meetings to discuss how best to measure and define proper images or images that should be excluded from the final data analysis. After multiple discussions based on practical experiences of performing cervical elastography for more than 2 or 3 years, we summarized the protocol for the performance and measurement of cervical elastography, which are presented in Table 1 and Fig. 1 demonstrates the standard plane of cervical elastography using this suggested protocol. The acquisition of the cervical elastography image is the same as when measuring cervical length. Thus, obtaining the mid-sagittal plane in which the endocervical canal is clearly delineated and the anterior width of the cervix is equal to the posterior width is the most important step. We used the default setting of preset sector (set at 5 cm), as it was enough to cover entire cervix. Fig. 2 shows examples of a short cervix as measured on cervical elastography using the E cervix program, showing relatively low, intermediate, and high strain values. We checked 5 elastographic parameters, namely, the strain of the internal os of the cervix (IOS), external os of the cervix (EOS), ratio of the strain of the internal and external os (ratio [IOS/EOS]), elasticity contrast index (ECI), and hardness ratio, as previously defined [7], as well as cervical length.
Table 1. Suggested protocol for the performance of cervical elastography and measurement of cervical strain using the E cervix program.
| Protocol | ||
|---|---|---|
| 1. The maternal bladder should be empty prior to examination | ||
| 2. Set image orientation | ||
| The apex of the image is displayed at the top of the monitor, and the fetal part is displayed on the left side of the image sector (Fig. 1). | ||
| 3. Activate (click on) the E cervix program and obtain an optimal cervical image | ||
| The radius of the default preset sector is set at 5 cm (Fig. 1). | ||
| The image plane used for cervical elastography is basically the same as the one used in measuring cervical length. | ||
| 1) Obtain the mid-sagittal plane of the cervix in which the endocervical canal is clearly delineated and the anterior width of the cervix is equal to the posterior width. | ||
| 2) Do not apply pressure with the probe onto the anterior cervix.a) | ||
| 4. Acquisition of cervical strain | ||
| When the optimal cervical image is obtained, hold still and wait for all motion bars (reliability indicator) to turn green (use auto-freeze setting for motion bars). | ||
| 1) The patient is allowed to breathe normally during the time of acquisition. | ||
| 2) Discard the image when active fetal movements occur during the acquisition, especially fetal limb movement in breech presentation, as it may affect cervical strain. | ||
| 5. ROI caliper placement for strain measurement | ||
| 1) Calipers are placed on the grayscale image displayed on the left of the screen as the elastographic image displayed on the right may be blurred at the margin. | ||
| 2) By selecting either a 2- or 4-point ROI, draw a line along the endocervical canal between the internal and external os of the cervix. If the endocervical line is straight, use a 2-point ROI tool. With a curved cervix, use a 4-point ROI to trace the endocervical lining as best as possible. | ||
| 3) After the cervical canal is defined, green points will automatically appear (Fig. 1). Place the points on the 4 corner edges of the cervix so that the ROI box includes the entire cervix area. Try to include the entire cervix without including adjacent structures such as the bladder or vaginal wall. | ||
| 6. Recording measurement | ||
| Obtain at least 3 separate images of cervical strain, and the average is usually recorded. | ||
ROI, region of interest.
a)Additional external pressure on the cervix may affect cervical strain.
Fig. 1. Standard plane for cervical elastography using the E cervix™. During cervical length measurement, the midline sagittal plane, in which the endocervical canal is clearly visible and the anterior width of the cervix is equal to the posterior width, is obtained. Care must be taken not to apply unnecessary pressure to the anterior cervix and to keep the vaginal probe still during data acquisition. After all motion bars turn green, the screen will freeze automatically. Define the cervix area using either 2-point ROI or 4-point ROI tools. Aim to cover the entire cervix without including other organs, such as the bladder or vaginal wall. (A) Cervical elastogram using 2-point ROI when the endocervical line is straight. (B-D) Cervical elastogram using 4-point ROI when the endocervical line is curved. IOS, strain of internal os of the cervix; EOS, strain of external os of the cervix; Ratio (IOS/EOS), ratio of the strain of the internal and external os; ECI, elasticity contrast index; ROI, region of interest.
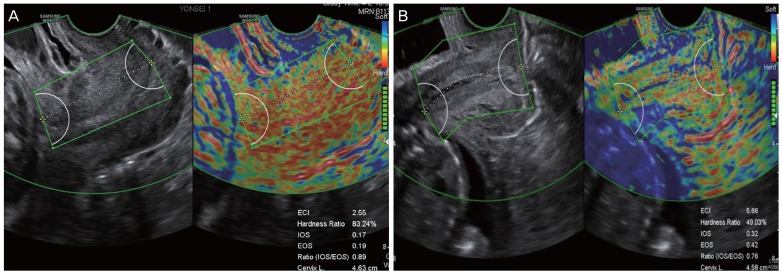
Fig. 2. Cases of cervical elastography performed in short cervix using E cervix™. (A, D) Short cervix showing relatively low strain values, reflecting a hard cervix. In (A), the values of cervical length (CL), elasticity contrast index (ECI), hardness ratio, strain of internal os of cervix (IOS), and strain of external os of cervix (EOS) were 2.29 cm, 3.20, 85.77%, 0.15, and 0.19, respectively, and in (D), these were 2.12 cm, 2.17, 91.65%, 0.15, and 0.12, respectively. (B, E) Short cervix showing relatively intermediate strain values. In (B), the values of CL, ECI, hardness ratio, IOS, and EOS were 2.01 cm, 3.57, 71.22%, 0.22, and 0.27, respectively, and in (E), these were 2.45 cm, 3.95, 67.19%, 0.31, and 0.22, respectively. (C, F) Short cervix showing relatively high strain values, reflecting a soft cervix. In (C), the values of CL, ECI, hardness ratio, IOS, and EOS were 2.28 cm, 5.49, 39.95%, 0.33, and 0.58, respectively, and in (F), these were 2.14 cm, 5.01, 35.89%, 0.43, and 0.44, respectively. Ratio (IOS/EOS), ratio of the strain of the internal and external os.
4. How to measure a funneling cervix
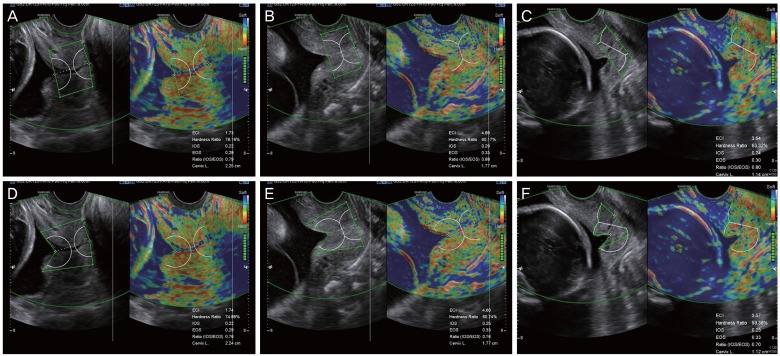
In cases of a funneling cervix, how to delineate the region of interest (ROI) caliper over the area of the cervix can be an issue. The first method involves including the remaining cervical area (functional cervix) only without the funneling area, and the second option is to extend the margin of the internal os to include the funneling area (Fig. 3). We compared these 2 methods in cases of a short cervix with funneling (91 images from 29 patients) and found no significant differences in the elastographic parameters between 2 methods (Supplementary Table 1). However, with regard to clinical settings, all experienced investigators in this research group agreed that the first method (to include the remaining cervical area only) is the preferred way to minimize inter-observer variance and to avoid possible technical difficulties of ROI caliper placement. Thus, it was decided to adhere to the first method in the subsequent studies.
Fig. 3. Performing cervical elastography using the E cervix™ in a short cervix with funneling: 2 methods. Cervical elastography measurements were performed using 2 different methods in the same patients (A vs. D, B vs. E, C vs. F). In the upper panel, measurement of cervical elastography was performed in a way that it only encompassed the functional cervix (A-C). With this method, the lateral margin of the internal os is perpendicular to the endocervical canal, excluding funneling area. In the lower panel, cervical elastography measurements were performed in a way that it encompassed the whole cervix, including the region of funneling by maximal extension of the lateral margin of the internal os (D-F). IOS, strain of internal os of the cervix; EOS, strain of external os of the cervix; Ratio (IOS/EOS), ratio of the strain of the internal and external os; ECI, elasticity contrast index.
5. Inadequate images and images requiring re-measurement
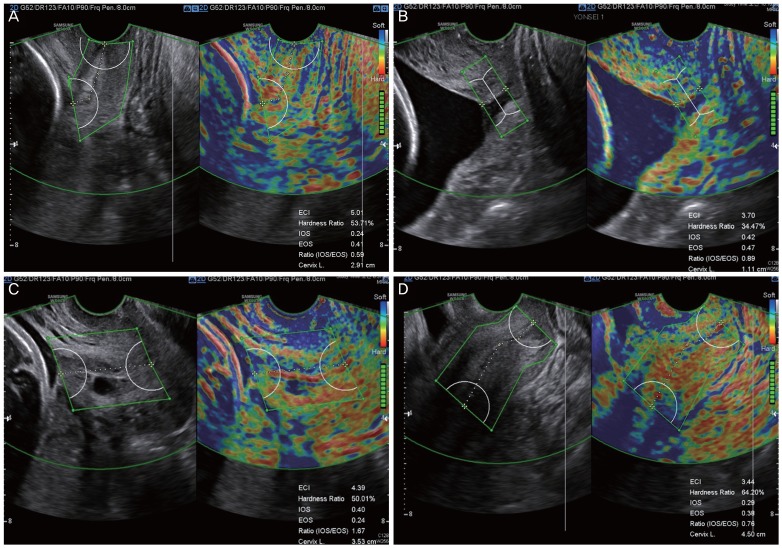
We defined an inadequate image for elastographic analysis of the cervix as one that demonstrated any of the following characteristics: asymmetric cervix (defined when the width of the anterior cervix was less than half of the posterior cervix or severely distorted cervix), dilated cervix precluding the measurement of the cervical length itself, cervix with Nabothian cyst, and no-clear visualization of internal os due to shadowing caused by the lower uterine segment. Fig. 4 shows examples of inadequate elastographic cervix images, and we excluded these images from the final data analysis. We also defined images requiring re-measurement as cervical elastographic images; these themselves were satisfactory, but the ROI caliper placement required redefinition for the optimal coverage of the cervix.
Fig. 4. Inadequate images for cervical elastography. (A) Asymmetric cervix defined as the width of anterior cervix less than half of the posterior cervix. (B) Dilated cervix. Note that the dilated endocervical canal is depicted in blue. (C) Cervix with Nabothian cyst. The Nabothian cyst, depicted in blue due to fluid collection, may increase the ECI. (D) Case in which the internal os of the cervix cannot be clearly visualized, hindering reliable measurement of cervical elastography and cervical length as well. ECI, elasticity contrast index; IOS, strain of internal os of the cervix; EOS, strain of external os of the cervix; Ratio (IOS/EOS), ratio of the strain of the internal and external os.
6. Reproducibility analysis
To determine the intra- and inter- observer reproducibility of cervical elastography measurement, the intraclass correlation coefficient (ICC) was calculated. For intra-observer reproducibility, the ICC was obtained using values from 3 repeated examinations (per measurement) obtained from all (seven) participating institutions. For inter-observer reproducibility, the ICC was obtained from the study population from one institution (Samsung Medical Center) in the following way: in detail, the first examiner captured the image of cervical strain and measured the elastographic parameters, and the second examiner retrieved the pre-captured elastographic images, defined the ROI, and measured the elastographic parameters and cervical length blinded to the result of first examination. The ICC were presented as single measures (an index for the reliability of the ratings for 1, typical, single rater) and as average measures (an index for the reliability of different raters averaged together). The ICC was interpreted as follows: <0.50: poor reproducibility, between 0.50 and 0.75: moderate reproducibility, between 0.75 and 0.90: good reproducibility, >0.90: excellent reproducibility [8].
7. Statistical analysis
Correlations between elastographic parameters and baseline clinical factors (or cervical length and gestational age at examinations) were analyzed using Spearman's correlation. Unadjusted and adjusted regression analysis was also used to determine the association between baseline clinical factors and elastographic parameters. All analyses were performed using SPSS software version 24.0 (SPSS Inc, Chicago, IL, USA) and R software packages version 3.5.1 (R-Project, R Foundation for Statistical Computing, Vienna, Austria; https://r-project.org). A P-value <0.05 was considered statistically significant.
Results
1. Inappropriate case selection and inappropriate measurement
For quality control, we collected all elastographic images from 7 institutions during the first 3 months of the study period. A total of 1,279 images from 256 women were reviewed by 2 examiners (SYO and HJS). Overall, the rate of inadequate images, defined in the materials and methods, was 9.8% (126/1,279), including 66 images of asymmetric cervix, 12 of dilated cervix, 12 of Nabothian cyst, and 36 with no-clear visualization of internal os due to shadowing caused by the lower segment of the uterus. The rate of images requiring re-measurement using pre-captured elastographic images was also 10.0% (129/1,279). Fig. 5 shows examples of images requiring re-measurement. The most common reason for requiring re-measurement was inadvertent application of the 2-point ROI, despite the fact that the endocervical line was not straight.
Fig. 5. The common representative images requiring re-measurement. (A) In a curved cervix, a straight line without measuring along the cervical canal would not reflect a correct cervical canal. (B) Measurements that inadvertently contain the bladder or amniotic fluid in the ROI box. This image did not follow the standardized protocol because the bladder was not empty. Ratio (IOS/EOS), ratio of the strain of the internal and external os; ECI, elasticity contrast index; ROI, region of interest.
2. Reproducibility
For intra-observer reproducibility, 1,247 measurements from 895 women were analyzed (because some women underwent follow-up cervical elastography measurements, the number of measurements outweighed the number of patients; and 1 measurement included at least 3 images), and for inter-observer reproducibility, 166 individual images from 43 women were used. Table 2 demonstrates the intra- and inter- observer ICCs for each elastographic parameter as well as cervical length. Overall, the intra-observer ICC value ranged from 0.633 to 0.723 for single measures and from 0.838 to 0.887 for average measures, indicating moderate and good reproducibility, and the inter-observer ICC ranged from 0.814 to 0.977 for single measures and from 0.901 to 0.988 for average measures, thereby showing good to excellent reproducibility.
Table 2. Intra-and inter-observer intraclass correlation coefficient (ICC) of the elastographic parameters and cervical length.
| Intra-observer | Inter-observer | |||
|---|---|---|---|---|
| Single measures ICC (95% CI) | Average measures ICC (95% CI) | Single measures ICC (95% CI) | Average measures ICC (95% CI) | |
| Cervical length | 0.955 (0.951–0.959) | 0.985 (0.983–0.986) | 0.903 (0.871–0.928) | 0.949 (0.932–0.962) |
| IOS | 0.660 (0.635–0.685) | 0.854 (0.839–0.867) | 0.977 (0.969–0.983) | 0.988 (0.984–0.991) |
| EOS | 0.633 (0.605–0.659) | 0.838 (0.822–0.853) | 0.928 (0.903–0.947) | 0.963 (0.951–0.973) |
| Ratio (IOS/EOS) | 0.644 (0.618–0.670) | 0.845 (0.829–0.859) | 0.883 (0.845–0.913) | 0.936 (0.913–0.953) |
| ECI | 0.723 (0.700–0.744) | 0.887 (0.875–0.897) | 0.814 (0.756–0.860) | 0.901 (0.867–0.927) |
| Hardness ratio | 0.663 (0.638–0.688) | 0.855 (0.841–0.869) | 0.976 (0.968–0.983) | 0.988 (0.984–0.991) |
ICC, intraclass correlation coefficient; CI, confidence interval
IOS, internal os of the cervix; EOS, external os of the cervix; Ratio (IOS/EOS), ratio of the strain of the internal and external os; ECI, elasticity contrast index; CI, confidence interval.
3. Effect of baseline clinical factors on the measurement of cervical elastographic parameters
In this analysis, a total of 216 women (32 short cervix, 21 PTL, 22 twin, 7 LEEP and 134 normal group) were were finally used after exclusion of inadequate images. Twenty-two women in the progesterone group also belonged to the short cervix group. Patients' characteristics and delivery outcomes are presented in Table 3. First, we performed correlation analysis and found that EOS, ECI, and the hardness ratio were significant but had weak correlations with maternal BMI on elastography (Table 4). There were no significant correlations between the elastographic parameters and other baseline clinical factors, including maternal blood pressure, heart rate, and uterine artery Doppler indices. Next, we performed regression analysis to determine the association between elastographic parameters and baseline clinical factors, including fetal cephalic presentation (Table 5). Although the hardness ratio was slightly affected by maternal BMI as per unadjusted regression analysis, it became nonsignificant after adjusting for maternal age, parity, and gestational age at examination by regression analysis. All other elastographic parameters were unaffected by unadjusted and adjusted regression analysis.
Table 3. Patients characteristics and delivery outcomes of the study population for the analysis of baseline clinical factors that affect the measurement of elastographic parameters.
| Variables | Short cervixa) (n=32) | PTL (n=21) | Twin (n=22) | LEEP (n=7) | Normal (n=134) |
|---|---|---|---|---|---|
| Age (yr) | 33.0 (30.0–36.0) | 34.0 (30.0–36.0) | 35.5 (34.0–37.0) | 33.5 (32.3–36.8) | 34.0 (31.0–36.0) |
| Parous women (%) | 21 (53.1) | 5 (23.8) | 6 (27.3) | 4 (57.1) | 52 (38.8) |
| BMI before pregnancy (kg/m2) | 20.9 (19.8–23.2) | 20.9 (19.5–25.4) | 21.7 (20.8–23.2) | 20.7 (18.6–26.3) | 20.8 (19.7–23.5) |
| BMI at elastography (kg/m2) | 23.1 (21.9–25.5) | 24.7 (22.1–26.9) | 23.1 (22.0–26.3) | 22.5 (20.8–26.9) | 22.9 (21.3–25.4) |
| Prior sPTB (%) | 1 (3.1) | 0 (0.0) | 1 (4.5) | 1 (14.3) | 3 (2.2) |
| Overt DM (%) | 2 (6.3) | 1 (4.8) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| Chronic hypertension (%) | 0 (0.0) | 0 (0.0) | 1 (4.5) | 1 (14.3) | 7 (5.2) |
| GA at elastography (wk) | 25.2 (21.4–28.4) | 31.1 (28.4–32.3) | 23.1 (22.0–26.8) | 21.0 (18.5–26.1) | 21.0 (20.4–21.3) |
| Cervical length (mm) | 21.0 (15.7–23.9) | 24.0 (18.5–32.7) | 33.0 (24.7–41.2) | 26.0 (13.0–31.4) | 37.3 (32.3–42.3) |
| GA at delivery (wk) | 38.4 (36.5–39.5) | 38.0 (34.3–35.5) | 36.6 (35.0–37.1) | 36.1 (29.4–39.6) | 38.5 (38.0–39.4) |
| Neonatal birth weight (g) | 2,935 (2,652–3,422) | 2,880 (2,008–3,196) | 2,388 (2,062–2,708) | 3,040 (1,310–3,130) | 3,154 (2,890–3,464) |
| PTB <37 wk (%) | 7 (21.9) | 6 (28.6) | 13 (59.1) | 4 (57.1) | 15 (11.1) |
| sPTB <37 wk (%) | 5 (15.6) | 5 (23.8) | 6 (27.3) | 3 (42.9) | 8 (5.9) |
Data are shown as median (interquartile range) and number (%).
BMI, body mass index; sPTB, spontaneous preterm birth; DM, diabetes mellitus; GA, gestational age; PTB, preterm birth; PTL, preterm labor; LEEP, loop electrosurgical excision procedure.
a)The short cervix group included 22 women from the progesterone group.
Table 4. Correlation analysis between elastographic parameters and baseline clinical factors.
| Clinical factors | No. | IOS | EOS | Ratio (IOS/EOS) | ECI | Hardness ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spearman's rho | P-value | Spearman's rho | P-value | Spearman's rho | P-value | Spearman's rho | P-value | Spearman's rho | P-value | ||
| BMI | 216 | 0.13 | 0.054 | 0.14 | 0.042 | −0.02 | 0.778 | 0.14 | 0.038 | −0.16 | 0.023 |
| MAP | 216 | 0.09 | 0.176 | 0.07 | 0.310 | 0.05 | 0.443 | 0.04 | 0.564 | −0.09 | 0.170 |
| Heart rate | 216 | 0.08 | 0.283 | 0.09 | 0.206 | 0.02 | 0.733 | 0.01 | 0.940 | −0.10 | 0.164 |
| UAPI | 114 | −0.03 | 0.754 | 0.08 | 0.367 | −0.11 | 0.217 | −0.02 | 0.854 | 0.01 | 0.884 |
| UARI | 114 | −0.06 | 0.516 | 0.05 | 0.600 | −0.12 | 0.169 | −0.08 | 0.397 | 0.05 | 0.555 |
P-values <0.05 are shown in bold.
IOS, strain of internal os of the cervix; EOS, strain of external os of the cervix; Ratio (IOS/EOS), ratio of the strain of the internal and external os; ECI, elasticity contrast index; BMI, body mass index; MAP, mean arterial pressure; UAPI, uterine artery pulsatility index; UARI, uterine artery resistance index.
Table 5. Regression analysis for association between elastographic parameters and baseline clinical factors.
| Clinical factors | IOS | EOS | Ratio (IOS/EOS) | ECI | Hardness ratio | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda) | Unadjusted | Adjusteda) | Unadjusted | Adjusteda) | Unadjusted | Adjusteda) | Unadjusted | Adjusteda) | |||||||||||
| β±SE (β) | P-value | β±SE (β) | P-value | β±SE (β) | P-value | β±SE (β) | P-value | β±SE (β) | P-value | β±SE(β) | P-value | β±SE (β) | P-value | β±SE (β) | P-value | β±SE (β) | P-value | β±SE (β) | P-value | |
| BMI | 0.003±0.002 | 0.122 | 0.002±0.002 | 0.394 | 0.0004±0.001 | 0.611 | 0.001±0.001 | 0.351 | −0.003±0.002 | 0.244 | −0.001±0.003 | 0.769 | 0.006±0.010 | 0.539 | 0.041±0.024 | 0.093 | −0.844±0.323 | 0.010 | −0.511±0.296 | 0.085 |
| MAP | 0.001±0.001 | 0.332 | 0.001±0.001 | 0.347 | 0.0004±0.001 | 0.498 | 0.0003±0.001 | 0.619 | 0.001±0.002 | 0.379 | 0.001±0.002 | 0.407 | 0.007±0.007 | 0.338 | 0.006±0.007 | 0.392 | −0.120±0.102 | 0.240 | −0.105±0.094 | 0.269 |
| Heart rate | 0.001±0.001 | 0.428 | 0.0002±0.001 | 0.678 | 0.0004±0.0005 | 0.370 | 0.0002±0.0005 | 0.657 | 0.001±0.001 | 0.577 | 0.0001±0.001 | 0.948 | 0.002±0.006 | 0.720 | 0.001±0.006 | 0.924 | −0.112±0.079 | 0.157 | −0.057±0.082 | 0.492 |
| UAPI | 0.010±0.038 | 0.802 | 0.011±0.041 | 0.798 | 0.024±0.037 | 0.523 | 0.057±0.036 | 0.117 | −0.072±0.078 | 0.356 | −0.008±0.009 | 0.403 | 0.287±0.378 | 0.449 | 0.313±0.379 | 0.410 | −2.884±4.877 | 0.555 | −4.737±6.522 | 0.469 |
| UARI | −0.073±0.102 | 0.474 | -0.024±0.115 | 0.834 | 0.026±0.091 | 0.779 | 0.107±0.100 | 0.287 | −0.349±0.229 | 0.129 | −0.391±0.240 | 0.106 | −0.609±0.987 | 0.538 | −0.293±1.016 | 0.773 | 9.334±13.646 | 0.495 | −2.788±18.700 | 0.882 |
| Cephalic presentation | −0.007±0.015 | 0.651 | 0.001±0.013 | 0.931 | -0.011±0.014 | 0.464 | -0.005±0.015 | 0.718 | 0.015±0.039 | 0.687 | 0.012±0.045 | 0.798 | 0.283±0.177 | 0.113 | 0.352±0.184 | 0.058 | −0.358±2.442 | 0.883 | −2.262±2.215 | 0.308 |
P-values <0.05 are shown in bold.
IOS, strain of internal os of the cervix; EOS, strain of external os of the cervix; Ratio (IOS/EOS), ratio of the strain of the internal and external os; ECI, elasticity contrast index; BMI, body mass index; MAP, mean arterial pressure; UAPI, uterine artery pulsatility index; UARI, uterine artery resistance index; SE, standard error.
a)Adjusted for maternal age, parity, and gestational age at examination.
4. Correlation analysis of elastographic parameters with cervical length and gestational age at examination
Overall, 846 women (334 in the high-risk and 512 in the low-risk group) were finally available for this analysis. The hardness ratio showed a positive correlation with the cervical length (Spearman's rho=0.184, P<0.001), whereas ECI showed a negative correlation (Spearman's rho=−0.279, P<0.001). The strain value of IOS and IOS/EOS ratio also showed a weak negative correlation with the cervical length (IOS, Spearman's rho=−0.099, P=0.002; IOS/EOS ratio, Spearman's rho=−0.074, P=0.022), whereas that of EOS was not correlated (Spearman's rho=−0.057, P=0.075). Regarding the correlation with gestational age on examination, all elastographic parameters were significantly correlated. In detail, strain value of IOS, EOS, IOS/EOS ratio, and ECI showed positive correlation (IOS, Spearman's rho=0.216, P<0.001; EOS, Spearman's rho=0.080, P=0.013; IOS/EOS ratio, Spearman's rho=0.161, P<0.001; ECI, Spearman's rho=0.149, P<0.001), whereas the hardness ratio showed a negative correlation (Spearman's rho=−0.205, P<0.001), reflecting decreasing cervical stiffness with advancing gestational week.
Discussion
In this study, we established a standardized protocol for cervical strain elastography measurement using intrinsic compression (E cervix program). Indeed, protocol for the measurement of cervical elastography is similar to that normally recommended for the measurement of the cervical length [9]. Recently, instructions regarding obtaining and measuring cervical length were provided by the Maternal-Fetal Medicine Unit network from the United States, and proper performance of transvaginal measurement of cervical length was highlighted [10]. According to these instructions, at least 3 separate images should be taken, and the shortest distance of cervical length should be chosen. In addition, it was noted that one good way to obtain the “shortest best” cervical length is to measure the length repeatedly until the variation between measurements is <10%. In our protocol of cervical elastography, we also recommend that at least 3 separate images be obtained and the average value of each elastographic parameter be used. Despite such a protocol, we found that in about 10% of our high- as well as low-risk study population, a number of cervixes were considered inadequate for cervical elastography measurement due to several reasons, including cervical asymmetry. This is rather similar to that in an earlier study reporting that 12% of low-risk women were excluded from the assessment of the cervical consistency index due to the presence of a non-horizontal cervical canal before compression [11].
Several studies have demonstrated that the implementation of standardization improves inter-observer variability and reproducibility in the measurement of the cervical length [12]. Indeed, it was previously noted that at least 23 supervised ultrasound scans appear necessary for an operator with no experience in transvaginal ultrasound in measuring cervical length. They have shown that the inter-observer ICC was progressed from 0.43 in the first study to 0.77 in the third study [9]. Although we did not directly assess the effect of training of cervical elastography on reproducibility, we witnessed that elastographic images taken by operators with more experience in obtaining proper plane and measuring cervical elastography less require re-measurement by internal quality control monitoring. Therefore, it is important to adhere to the standardized protocol for performing cervical elastography, and supervised training time is necessary, as previously suggested in the measurement of the cervical length [10] and cervical consistency index [11].
According to a previous study that examined reproducibility using the E cervix program including 90 women with 2 repeated examinations (per measurement), the intra- and inter-observer ICC for single measures of hardness ratio were 0.737 and 0.775, respectively [13]. In this study, the intra-observer ICC was assessed using 895 women from 7 institutions who underwent 3 repeated examinations (per measurement) according to the study protocol. As a result, the intra-observer ICC for most elastographic parameters ranged from 0.633 to 0.723 for single measures and from 0.838 to 0.887 for average measures, showing moderate to good reproducibility and indirectly supporting our protocol that at least 3 repeated examinations should be performed in the measurement of cervical elastography. In contrast to the previous study in which inter-observer variance was assessed by 2 separate examinations by 2 operators [13], the inter-observer ICC in the present study was assessed by acquisition of the cervical elastography image itself by one operator and measurement of elastographic parameters by 2 operators. With this method, the inter-observer ICC for most elastographic parameters ranged from 0.814 to 0.977 for single measures and from 0.901 to 0.988 for average measures. However, it should be noted that this does not reflect the real reproducibility in a situation where a patient is examined by 2 different physicians, rather it reflects a situation where one operator acquires a cervical elastography image in front of patient examination, and the measurement of cervical elastographic parameters is assessed later by another examiner. This type of inter-observer variance with pre-stored image was also demonstrated by another investigator in assessing cervical consistency index [11]. Given that the overall rate of images requiring re-measurement using pre-captured elastographic images was 10.0% in our initial analysis, the measurement of elastographic parameters by applying the ROI accurately by a single experienced operator using a properly pre-captured cervical elastographic image can be a practical way to decrease inter-observer variation.
There are 2 methods of elastography, namely, shear-wave and strain elastography. Shear-wave elastography requires an acoustic force that creates a mechanical impulse following tissue displacement. Because shear-wave elastography provides a shear-wave speed in a given tissue, it is a more objective method of elastography [14], which is considered the main advantage. Because shear-wave elastography requires the basic assumption that the assessed tissue has anisotropic characteristics [15], it works well in relative homogenous tissues, such as liver [16]. In contrast, the application of shear-wave elastography in the cervix is likely problematic because transverse wave behavior is much more complicated in heterogeneous tissues, such as the cervix [17]. Fetal safety is also more of a concern in shear-wave elastography than in strain elastography [18]. Strain elastography is based on tissue displacement by external applied forces; the main disadvantage of strain elastography is that the external force exerting the cervix is hard to standardize. Some strain elastography uses intrinsic compression, which is intrinsically generated by fine vibration of the internal organs, such as arterial pulsation [19]. In fact, this type of intrinsic compression was initially implemented in thyroid elastography, which uses carotid artery pulsation as a compression source [20], and later investigated in several studies [21,22,23,24]. This method is less operator-dependent than external compression elastography in thyroid assessment [23]. However, such an intrinsic compression is also difficult to quantify for ideal standardization. In our study, we used cervical elastography using intrinsic compression; therefore, it was theoretically considered that intrinsic factors, including maternal blood pressure, may affect the measurement of elastographic parameters as expected. In this study, we found that none of the elastographic parameters were significantly affected by baseline clinical factors, including arterial pressure, heart rate, BMI, and uterine artery Doppler indices. However, all elastographic parameters were correlated with gestational age at examination, reflecting that the cervix becomes softer as the gestational age increases. In our practical experiences, the intra-observer variability in breech presentation was higher than that in head presentation, although there was no statistical significance (data not shown). Therefore, caution is advised to avoid active fetal movement when measuring cervical elastography, as indicated in the protocol (Table 1).
In summary, cervical elastography has emerged as a promising ancillary tool to measure the cervical length in the prediction of preterm birth [25,26,27,28]. Meanwhile, the standardization of cervical elastography measurements is an indispensable component that requires to be established prior to clinical studies and following practical application. We presented the standardization, reproducibility, and analysis of baseline clinical factors that potentially affect the measurement of elastographic parameters of the cervix while conducting an on-going study, which is intended to investigate the clinical implication of cervical elastography in prediction of preterm birth in low- and high-risk pregnant patients and is supposed to be completed in December 2020.
Acknowledgements
We would like to thank maternal-fetal medicine fellows, research nurses, and statisticians for their dedicated and technical assistance with this research and manuscript as followings; Seung Yeon Pyeon, MD, Hyun-Min Jung, RN, Soo Jin Kim (Statistics support office) (Kyung Hee University Hospital at Gangdong), Bong Seon Lim, RN (Kyungpook National University Hospital), Yoon Sun Kim, RN (Dongguk University Ilsan Hospital), Seung Woo Yang, MD (Konkuk University Medical Center), Min Hye Kwon, RN (Yonsei University Severance Hospital), Yoo-Min Kim MD, Seo-yeon Kim, MD, Do Youn Kwon, MD, Hyea Park, MD, Hye Won Ha, RN (Samsung Medical Center), and Sook-Young Woo (Statistics and Data Center, Samsung Medical Center, Seoul, Korea). We also acknowledge the technical support from Samsung Medison, specifically for lending ultrasound equipment (WS80A Ultrasound System, Samsung Medison, Seoul, Korea), in 3 institutions (Kyung Hee University Hospital at Gangdong, Dongguk University Ilsan Hospital, and Konkuk University Medical Center) among the 7 institutions during the study period. This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C1696).
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
Ethical approval: This study was approved by the Institutional Review Board from all participating institutions (IRB No. Kyung Hee University Hospital at Gangdong 2018-03-002, Kangbuk Samsung Hospital 2018-06-006, Kyungpook National University Hospital 2018-08-005-002, Dongguk University Ilsan Hospital 2017-12-019-007, Konkuk University Medical Center 1040070, Yonsei University Severance Hospital 1-2018-0022, Samsung Medical Center 2018-03-073-015).
Patient consent: Written informed consent was collected from all subjects.
SUPPLEMENTARY MATERIAL
Supplementary Table associated with this article can be found online at https://doi.org/10.5468/ogs.2020.63.1.42.
Cervical length and elastographic parameters in cases of short cervix with funneling: a comparison of 2 methods
References
- 1.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 2.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks' gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902–907. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 3.Committee on Practice Bulletins—Obstetrics, The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120:964–973. doi: 10.1097/AOG.0b013e3182723b1b. [DOI] [PubMed] [Google Scholar]
- 4.Barr RG, Nakashima K, Amy D, Cosgrove D, Farrokh A, Schafer F, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 2: breast. Ultrasound Med Biol. 2015;41:1148–1160. doi: 10.1016/j.ultrasmedbio.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Cosgrove D, Barr R, Bojunga J, Cantisani V, Chammas MC, Dighe M, et al. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: part 4. Thyroid. Ultrasound Med Biol. 2017;43:4–26. doi: 10.1016/j.ultrasmedbio.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 3: liver. Ultrasound Med Biol. 2015;41:1161–1179. doi: 10.1016/j.ultrasmedbio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Park HS, Kwon H, Kwak DW, Kim MY, Seol HJ, Hong JS, et al. Addition of cervical elastography may increase preterm delivery prediction performance in pregnant women with short cervix: a prospective study. J Korean Med Sci. 2019;34:e68. doi: 10.3346/jkms.2019.34.e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vayssière C, Morinière C, Camus E, Le Strat Y, Poty L, Fermanian J, et al. Measuring cervical length with ultrasound: evaluation of the procedures and duration of a learning method. Ultrasound Obstet Gynecol. 2002;20:575–579. doi: 10.1046/j.1469-0705.2002.00854.x. [DOI] [PubMed] [Google Scholar]
- 10.Iams JD, Grobman WA, Lozitska A, Spong CY, Saade G, Mercer BM, et al. Adherence to criteria for transvaginal ultrasound imaging and measurement of cervical length. Am J Obstet Gynecol. 2013;209:365.e1–365.e5. doi: 10.1016/j.ajog.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baños N, Murillo-Bravo C, Julià C, Migliorelli F, Perez-Moreno A, Ríos J, et al. Mid-trimester sonographic cervical consistency index to predict spontaneous preterm birth in a low-risk population. Ultrasound Obstet Gynecol. 2018;51:629–636. doi: 10.1002/uog.17482. [DOI] [PubMed] [Google Scholar]
- 12.Burger M, Weber-Rössler T, Willmann M. Measurement of the pregnant cervix by transvaginal sonography: an interobserver study and new standards to improve the interobserver variability. Ultrasound Obstet Gynecol. 1997;9:188–193. doi: 10.1046/j.1469-0705.1997.09030188.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim M, Kwak D, Oh S, Sajin K, Yang S, Choi E, Kim M, Park J, Kim K, editors. Intra- and interobserver variance of elastographic parameters measurement of uterine cervix during pregnancy; 26th World Congress on Ultrasound in Obstetrics and Gynecology; 2016 Sep 24–28; Rome, Italy. Hoboken: Wiley; 2016. Sep, p. 346. [Google Scholar]
- 14.Feltovich H, Carlson L. New techniques in evaluation of the cervix. Semin Perinatol. 2017;41:477–484. doi: 10.1053/j.semperi.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126–1147. doi: 10.1016/j.ultrasmedbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Feltovich H, Hall TJ, Berghella V. Beyond cervical length: emerging technologies for assessing the pregnant cervix. Am J Obstet Gynecol. 2012;207:345–354. doi: 10.1016/j.ajog.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feltovich H, Hall TJ. Quantitative imaging of the cervix: setting the bar. Ultrasound Obstet Gynecol. 2013;41:121–128. doi: 10.1002/uog.12383. [DOI] [PubMed] [Google Scholar]
- 18.Issaoui M, Debost-Legrand A, Skerl K, Chauveau B, Magnin B, Delabaere A, et al. Shear wave elastography safety in fetus: a quantitative health risk assessment. Diagn Interv Imaging. 2018;99:519–524. doi: 10.1016/j.diii.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Swiatkowska-Freund M, Preis K. Elastography of the uterine cervix: implications for success of induction of labor. Ultrasound Obstet Gynecol. 2011;38:52–56. doi: 10.1002/uog.9021. [DOI] [PubMed] [Google Scholar]
- 20.Bae U, Dighe M, Dubinsky T, Minoshima S, Shamdasani V, Kim Y. Ultrasound thyroid elastography using carotid artery pulsation: preliminary study. J Ultrasound Med. 2007;26:797–805. doi: 10.7863/jum.2007.26.6.797. [DOI] [PubMed] [Google Scholar]
- 21.Dighe M, Bae U, Richardson ML, Dubinsky TJ, Minoshima S, Kim Y. Differential diagnosis of thyroid nodules with US elastography using carotid artery pulsation. Radiology. 2008;248:662–669. doi: 10.1148/radiol.2482071758. [DOI] [PubMed] [Google Scholar]
- 22.Dighe M, Kim J, Luo S, Kim Y. Utility of the ultrasound elastographic systolic thyroid stiffness index in reducing fine-needle aspirations. J Ultrasound Med. 2010;29:565–574. doi: 10.7863/jum.2010.29.4.565. [DOI] [PubMed] [Google Scholar]
- 23.Lim DJ, Luo S, Kim MH, Ko SH, Kim Y. Interobserver agreement and intraobserver reproducibility in thyroid ultrasound elastography. AJR Am J Roentgenol. 2012;198:896–901. doi: 10.2214/AJR.11.7009. [DOI] [PubMed] [Google Scholar]
- 24.Dighe M, Luo S, Cuevas C, Kim Y. Efficacy of thyroid ultrasound elastography in differential diagnosis of small thyroid nodules. Eur J Radiol. 2013;82:e274–e280. doi: 10.1016/j.ejrad.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Andrade E, Garcia M, Ahn H, Korzeniewski SJ, Saker H, Yeo L, et al. Strain at the internal cervical os assessed with quasi-static elastography is associated with the risk of spontaneous preterm delivery at ≤34 weeks of gestation. J Perinat Med. 2015;43:657–666. doi: 10.1515/jpm-2014-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swiatkowska-Freund M, Preis K. Cervical elastography during pregnancy: clinical perspectives. Int J Womens Health. 2017;9:245–254. doi: 10.2147/IJWH.S106321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H, Hwang HS. Elastographic measurement of the cervix during pregnancy: current status and future challenges. Obstet Gynecol Sci. 2017;60:1–7. doi: 10.5468/ogs.2017.60.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oturina V, Hammer K, Möllers M, Braun J, Falkenberg MK, de Murcia KO, et al. Assessment of cervical elastography strain pattern and its association with preterm birth. J Perinat Med. 2017;45:925–932. doi: 10.1515/jpm-2016-0375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cervical length and elastographic parameters in cases of short cervix with funneling: a comparison of 2 methods