Abstract
Retinal degeneration from inherited gene mutation(s) is a common cause of blindness because of structural and functional alterations in photoreceptors. Accordingly, various approaches are being tested to ameliorate or even cure neuroretinal blinding conditions in susceptible patients by employing neuroprotective agents, gene therapeutics, optogenetics, regenerative therapies, and retinal prostheses. The FVB/NJ mouse strain inherently has a common Pde6b rd1 homozygous allele that renders its progeny blind by the time pups reach weaning age. To study the role matrix metalloproteinase-9 (MMP-9) in retinal structure and function, we examined a global MMP-9 knockout (KO) mouse model that has been engineered on the same FVB/NJ background to test the hypothesis whether lack of MMP-9 activity diminishes neuroretinal degenerative changes and thus helps improve the vision. We compared side-by-side various aspects of the ocular physiology in the wild-type (WT) C57BL/6J, FVB/NJ, and MMP-9 KO strains of mice. The results suggest that MMP-9 KO mice display subdued changes in their retinae as reflected by both structural and functional enhancement in the overall ocular neurophysiological parameters. Altogether, the findings appear to have clinical relevance for targeting conditions wherein MMPs and their overactivities are suspected to play dominant pathophysiological roles in advancing neurodegenerative retinal diseases.
Keywords: blindness, extracellular matrix, retina, vascular remodeling, vision loss
INTRODUCTION
Vision impairment and subsequent loss of eyesight are global health concerns. It is estimated that ~285 million people are currently suffering from vision disorders affecting their physical and mental health. Unfortunately, vision disability is rising at a rapid pace since current therapies are either limited or nonexistent (38). The rise is attributed to an increase in the aging population and life expectancy. Common to almost all retinal degenerative diseases are pathological alterations to photoreceptor cells, which malfunction as the respective disease(s) progress(es). The most common retinal degenerative diseases are age-related macular degeneration and retinitis pigmentosa. Both have genetic components as the driver toward disease causation. Inherited retinal degenerations affect both rod and cone photoreceptors and constitute one of the causes of incurable blindness in the world. Cyclic guanosine monophosphate (cGMP) is crucial in the phototransduction, and mutations in genes related to its metabolism are responsible for retinal degenerative pathologies. The cGMP-degrading phosphodiesterase 6 (PDE6) mutations cause around 4–5% of retinitis pigmentosa, a rare form of retinal degeneration. Given the magnitude and severity of the problem, new treatment strategies are needed to solve the problem (28). Mutation of the PDE6b gene leads to dysfunction of PDE that results in failure of the hydrolysis of cGMP molecule. The rd1 mouse is a well-characterized animal model of retinitis pigmentosa caused by mutation of the PDE6b gene (9, 15). In rd1 animals, rod photoreceptor cells begin degenerating, and by ~3 wk no rod photoreceptors remain. Degeneration is preceded by accumulation of cGMP in the retina and is correlated with deficient activity of the rod photoreceptor cGMP-PDE; however, the cone photoreceptors undergo a slower degeneration that causes the mutants to go blind (4).
Previously, we have shown that increased matrix metalloproteinase-9 (MMP-9) activity leads to degradation of extracellular matrix (ECM) (33, 34, 40). Others too have confirmed activation of ECM degradation in the eyes by MMPs, including differential vascular remodeling via zinc and calcium ion-dependent MMPs’ proteolytic enzymes (6, 18, 19, 43). Based upon these developments we hypothesized that imbalance of the “MMP-9–tissue inhibitor of matrix metalloproteinase 1 (TIMP-1)” axis plays crucial role(s) in neuroretinal remodeling affecting both the visual and cognitive functions by structure-function alterations in the ocular compartment. Vascular remodeling can result in a dynamic change in the retinal architecture, leading to leakage of the plasma contents into the ocular cavity that in turn sets off pathological changes causing vision loss via several mechanisms, including elevation of intraocular pressure (IOP). It is known that structural and functional heterogeneity in vessels determine the degree of vascular remodeling and that MMPs facilitate the process of new vessel growth through dissolution of basement membrane (BM); however, MMPs are kept in check by their respective TIMPs preventing the overdigestion of the BM (30, 42).
MMP-9 is an important endopeptidase and has been studied for its ECM modifying and remodeling activities in the vasculature (24, 32). It was demonstrated that MMP-9 is necessary for neovascularization since MMP-9 inhibition decreased angiogenesis (21). In addition, MMP-9 is upregulated in the presence of vascular endothelial growth factor (VEGF) (7). Furthermore, MMP-9 has been found in the secretory vesicles of invading endothelial cells, which are shed in response to local VEGF production. All of these facilitate a feed-forward mechanism toward inducing other MMP members that are implicated in the release of molecules such as basic fibroblast growth factors, which are involved in angiogenesis including the proliferative form of diabetic retinopathy (49). Therefore, MMPs and TIMPs play important roles in maintaining the integrity and functionality of the vascular network. Furthermore, gap junctions and intercellular communication are essential for cell survival and maintenance of tissues, thus making them vital for regulation of growth, differentiation, and development (41, 53). Lapses in gap-junction activity could result in the loss of retinal endothelial cells and pericytes or apoptosis (25, 45).
The MMP-9 knockout (KO) mouse model has served as an excellent system for investigating ECM and related gap-junction changes in various disorders. To dissect out the role of MMP-9 in retinal homeostasis we employed this model to test our hypothesis that lack of MMP-9 activity slows down the overall neuroretinal degenerative process and thus helps preserve or improve the retinal physiology in comparison with the FVB/NJ background mouse, which is homozygous for the Pde6b rd1 allele (which renders the FVB/NJ strain blind). The MMP-9 KO, FVB/NJ, and C57BL/6J mice strains were individually bred, genotyped, and monitored for their vision-guided behavior and IOP along with biochemical, fluorescence imaging, and histological analyses. In total, the results suggest that physiological balance between MMP-9 and TIMP-1 plays a crucial role in the retinovascular homeostasis.
MATERIALS AND METHODS
Antibodies and reagents.
Antibodies for TIMP-1 and zonula occludens-2 (ZO-2) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was from Boster Biological Technology (Pleasanton, CA).
Animal handling and care.
Male and female 10–12 wk old mice, C57BL/6J, MMP-9 KO, and FVB/NJ were purchased, bred, and maintained as per procedures from the Jackson Laboratory (Bar Harbor, ME). Animal experiments were performed in compliance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. The animal care and guidelines of the National Institutes of Health (NIH) were also adhered to, and we followed procedures and protocols that were reviewed and subsequently approved by the Institutional Animal Care and Use Committee of the University of Louisville School of Medicine. The mice were maintained in a 12:12 h light-dark cycle environment, and they all had free access to food and water.
Genotyping of MMP-9 KO and FVB/NJ mice.
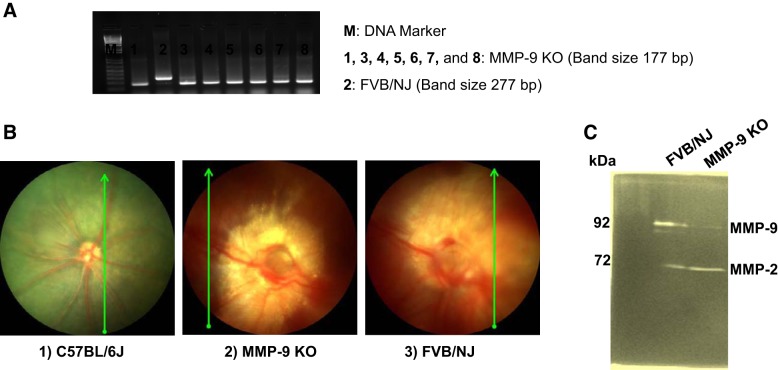
Again, the mice were weaned and genotyped according to the Jackson Laboratory’s recommendations as previously reported by our group (3). For example, tail biopsies were collected, and genotypic analyses were performed by PCR, as shown in Fig. 1. MMP-9 KO -mice presented a band at 177 bp and FVB/NJ at 277 bp. A total of three to five mice were used in each group for all the experiments. At the end of the experiment, animals were euthanized by using 2× tribromoethanol (TBE). Mice were grouped as follows: 1) C57BL/6J, 2) MMP-9 KO, and 3) FVB/NJ.
Fig. 1.
Genotypic and phenotypic features of the mice strains. Genotyping analysis of the mice from their tail clips was performed via DNA electrophoretic gel depicting pups positive for the missing matrix metalloproteinase-9 (MMP-9) alleles [MMP-9 knockout (KO)] with staining of DNA band at 177 bp, while FVB/NJ line showing a band at 277 bp (A). The noninvasive in vivo imaging of the retinal microvasculature by fundoscopy (B2) wherein deficiency of MMP-9 shows less degenerative changes of retinal arteries, veins, or the whole vasculature with the preservation of major vessels in the retina in comparison to the C57BL/67 (B1) and the background FVB/NJ mouse (B3) strains (B) and the MMP-9 activity as assessed by gelatin zymography (C).
Gelatin zymography analysis for MMPs.
Gelatinolytic activity of MMP-9 and MMP-2 was examined by substrate gelatin zymography. Equal amounts of proteins obtained from retinal lysates were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel containing 0.1% gelatin. The gel was washed twice with an interval of 1 h in 2.5% Triton X-100 washing buffer and then incubated in incubation buffer containing 50 mM Tris·HCl, 10 mM CaCl2, 1 M ZnCl2, and 200 mM NaCl, pH 7.5 at 37°C for 18–20 h. Then the gel was stained with Coomassie solution (0.05% Coomassie brilliant blue R-250 in 40% methanol and 10% acetic acid) and partially destained with destaining solution (20% methanol and 10% acetic acid) to visualize the clear zone of gelatin lysis against the blue background stain indicating the presence of MMPs. The zymography gel was imaged for the lysis zones in every lane for MMP-2 and -9 activities.
Western blot analysis.
Preparation of mouse retinal tissue samples were carried out as per the standard protocol. Briefly, the retinal samples were sonicated in ice with 1× RIPA buffer (Tris·HCl 50 mM, pH 7.4; NP-40, 1%; 0.25% Na-deoxycholate, 150 mM NaCl; 1 mM EDTA; 1 mM PMSF; 1 µg/ml each of aprotinin, leupeptin, pepstatin; 1 mM Na3VO4; 1 mM NaF) containing 1 mM PMSF and 1 µg of complete protease inhibitor (Sigma-Aldrich Corp., St. Louis, MO). After centrifugation, the supernatants were collected; the protein amount for all samples was quantified by the Bradford’s method (Bio-Rad, Hercules, CA) and stored at −80°C until further use. Western blot analysis pertaining to the TIMP-1 and the tight junction protein (TJP) ZO-2 was performed as in a previously reported protocol (11). Equal amounts of total protein (50 µg) were resolved on SDS-PAGE and transferred to polyvinylidene fluoride membranes. The membranes were probed overnight at 4°C with primary antibodies followed by 2 h incubation in secondary antibodies. The signal capturing was done with the Bio-Rad ChemiDoc XRS+ system and Image Lab software (Bio-Rad, Hercules, CA). The relative optical density of protein bands was analyzed with gel software Image Lab 3.0, and finally the membranes were stripped and reprobed with GAPDH as a loading control.
Measurement of IOP.
IOP was monitored with a model TV02 Tonovet tonometer (iCare, Raleigh, NC) according to the manufacturer’s instructions. Briefly, mice were anesthetized intraperitoneally (i/p) with TBE at 5 mg/kg body weight. Animals were placed in the prone position, and IOP was registered by keeping the probe horizontal and maintaining a probe-cornea distance ~3–5 mm with an angle of 25° limit relative to the visual axis at the corneal apex for IOP recording (31). Only measurements that were judged by the data analytical system to be within acceptable parameters were recorded as being valid. After effectively measuring a few times, the tonometer generated average values that were considered as appropriate measurements for each eye. Every time, a new disposable probe was used for recording the pressure measurements.
Barium sulfate angiography and microvascular leakage imaging.
Barium sulfate angiography was undertaken in mice as described in our previous work (19). Briefly, barium sulfate at 0.1 g/mL was dissolved in 50 mM Tris buffer (pH 5.0) and infused slowly at a constant flow and pressure with a syringe pump via carotid artery post-TBE anesthesia. The mouse eye globes from each of the experimental group were dissected out and placed in the X-ray chamber Kodak 4000 MM image station (Kodak, Rochester, NY), and angiograms were captured with the high penetrative phosphorous screen by 31 KVP X-ray exposures for 3 min. Vessel density was quantified with the Ves Seg tool (Institute for Signal Processing, University of Lübeck, Lübeck, Germany) as previously reported (27). Vascular permeability was measured in anesthetized mice with a modification of our previously described procedure (11). Briefly, bovine serum albumin conjugated fluorescein isothiocyanate (BSA-FITC) was injected via tail vein and allowed to circulate in the system for up to 20–30 min. After that mice were euthanized, and eye globes were harvested for the retinal mount preparations. The retinal vascular imaging was performed with the help of the BX61WI fluorescent microscope (Olympus, Tokyo, Japan). Subsequently, the data were interpreted with the software provided with the instrument and Image-J software.
Electroretinography analysis.
Electroretinography (ERG) analysis was performed to measure photoreceptor function and dark adaptation (54, 55). Prior to these tests, mice were transferred to a light-proof cabinet with water and food and dark-adapted for at least 18 h. For in vivo ERG experiments, dark-adapted mice were anesthetized by i/p injection of a mixture of ketamine (70 mg/kg) and xylazine (10 mg/kg) and placed on a heating pad with thermal feedback to avoid hypothermia. Pupils were dilated with a single drop of 0.5% tropicamide/0.5% phenylephrine hydrochloride and proper contact with the electrodes was ensured. Contact-lens electrodes were then carefully attached to the cornea for recording the transretinal voltage signals relative to a needle electrode inserted under the scalp. After stabilizing the baseline in darkness for 15 min, we triggered ERG light responses and recorded them by using Ganzfeld ERG (Phoenix Research Laboratories, Pleasanton, CA).
Optical coherence tomography and fundoscopy.
The integrity of the retina was assessed in vivo by optical coherence tomography (OCT) according to a previous protocol (51). In brief, mice were anesthetized with a rodent anesthesia cocktail containing 100 mg/mL ketamine and 12 mg/mL xylazine (Sigma-Aldrich Corp., St. Louis, MO). Pupils were dilated with 1% tropicamide (Bausch & Lomb, Tampa, FL) followed by application of GenTeal Lubricant Eye Gel (Alcon, Ft. Worth, TX). Systane lubricant eye drops (Alcon) were applied throughout the procedure to keep the cornea moist. OCT images were obtained by using OCT 2 (Phoenix Research Laboratories). Imaging included averaged single B scan and volume intensity scans with images centered on the optic nerve head. We measured total retinal thickness (TRT), which included nerve fiber layer, inner plexiform layer, inner nuclear layer (INL), outer plexiform layer, outer nuclear layer, inner/outer segment, and the retinal pigment epithelium, and the TRT was plotted after calculating the averaged value for each group of the mice strains. The fundoscopy analysis was made using Micron IV: Retinal Imaging Microscope (Phoenix Research Laboratories).
Assessment of vision-guided behavior.
To assess the vision-guided behavior in mice, we relied upon the protocol for performing the novel object recognition test (NORT). In brief, mice were subjected to a training session involving two identical objects in an open-field arena for 10 min. After completion of the training, mice underwent a testing session wherein they were presented with one familiar stimulus (i.e., object 1) and with a novel stimulus for 5 min. The amount of time spent exploring the stimuli during the training and test sessions was recorded by a computerized algorithm. The relative exploration time during the test trial was expressed as a discrimination index [D.I. = (time novel − time familiar)/(time novel + time familiar)]. Similarly, we performed light-dark box test (LDBT) analyses in which the latency of the initial time taken from the light (illuminated) to the dark (nonilluminated) areas was recorded after the initial 20 s of exploration. All groups of animals were subjected individually to NORT and LDBT assessments respectively.
Statistical analyses.
Data analyses and graphical presentations were performed with the help of GraphPad InStat 3 and GraphPad Prism, version 6.07 (GraphPad Software, Inc., La Jolla, CA). Data are represented as mean values ± standard error (SE) from five independent experiments in all cases. The experimental groups were compared by one-way analyses of variance (ANOVA) if the values were sampled from Gaussian distributions. For a set of data, if ANOVA indicated a significant difference (P < 0.05); Tukey-Kramer multiple-comparison tests were used to compare group means. Posttest was only performed if P < 0.05. If the value of Tukey-Kramer “q” is < 4.046, then the P value is <0.05 and considered statistically significant.
RESULTS
Genotypic screening, funduscopic imaging, and gelatin zymography.
MMP-9 KO mice presented a band at 177 bp, while FVB/NJ showed a 277 bp band size (Fig. 1A). Funduscopic imaging of the retina and its microvasculature revealed a distinguishable vasculature pattern that clearly reflected alterations in the retinal anatomy. A deficiency of MMP-9 exhibited to a lesser extent the degenerative processes for retinal arteries, veins, or even the entire vasculature with distinct preservation of some vessels in the retinal parenchyma as compared with C57BL/6J and its original background strain, FVB/NJ (Fig. 1B1–3). Increased activity of MMP-9 was observed in FVB/NJ retinal lysate with no appreciable detection in the MMP-9 KO retinal sample (Fig. 1C).
Retinal angiography and OCT.
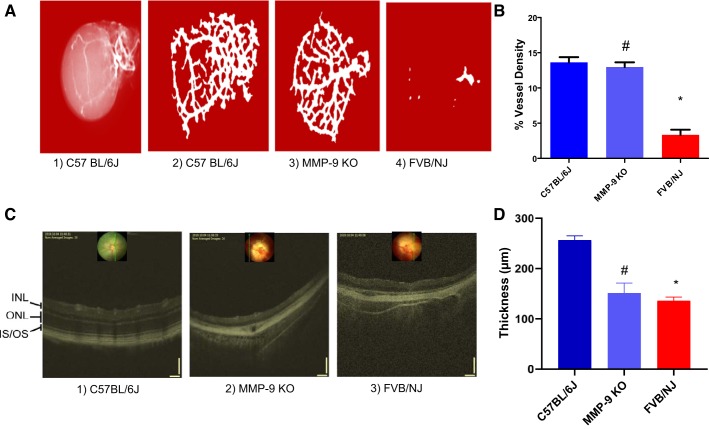
Barium sulfate angiographic imaging performed individually to examine the retinal vasculature depicted changes in retinal microvasculature wherein vessels were less developed and discrete in their appearance in the FVB/NJ mice in contrast to the MMP-9 KO mice, indicating that MMP-9 deficiency was able to halt degradation of the vascular network in the retina (Fig. 2A). The difference can be appreciated on quantification of the blood vessel density in the retina (Fig. 2B). Furthermore, there was an almost complete absence of vascularization in FVB/NJ mice (Fig. 2A4) as compared with C57BL/6J (Fig. 2A2) and MMP-9 KO (Fig. 2A3), again confirming degeneration of the retinal matrix in the eyes of the FVB mouse strain. When in vivo imaging was carried out on these animals via the OCT to observe fundus and the retina, it enabled us to visualize the degeneration and thinning of the various retinal layers in the back of the eyes. The retinal layers were completely disrupted/altered in FVB/NJ mice compared with the MMP-9 KO and C57BL/6J strains (Fig. 2C).
Fig. 2.
Barium sulfate angiography and the optical coherence tomographic (OCT) imaging of the mice eyes. Barium sulfate images showing the retinal microvasculature changes (A) and respective quantification of their vessels’ density (B). Absence of MMP-9 prevents the degeneration of the retinal vessels (A3). Almost complete absence of vascularization is visible in FVB/NJ mice (A4, and C3) as compared with C57BL/6J (A2, C1) and MMP-9 KO (A3, C2), confirming retinal degeneration. A progressive retinal degeneration is the hallmark in the FVB/NJ mouse as shown in the quantitative vessel density analysis (B). Also, the degeneration and thinning of the various retinal layers were successfully captured via OCT analysis along with demonstration of retinal layers’ separation/distortion occurring more in the FVB/NJ strain than in C57BL/6J, and MMP-9 KO in the back of the eyes. Although outer retinal layers run into each other in both MMP-9 KO and FVB/NJ retinae, disruption of the retinal architecture is more prominent, along with a significant loss of vasculature, in the FVB/NJ strain. Also, the layers are more severely disrupted/altered in FVB/NJ than the MMP-9 KO and C57BL/6J strains (C). Estimation of the total thickness of the retinal layers is depicted. *P < 0.0001 when compared with C57BL/6J, #P < 0.05 when compared with FVB/NJ (D). INL, inner nuclear layer; ONL, outer nuclear layer; IS, inner segment; OS, outer segment.
Ex vivo visualization of retinal vessels and the inner architecture.
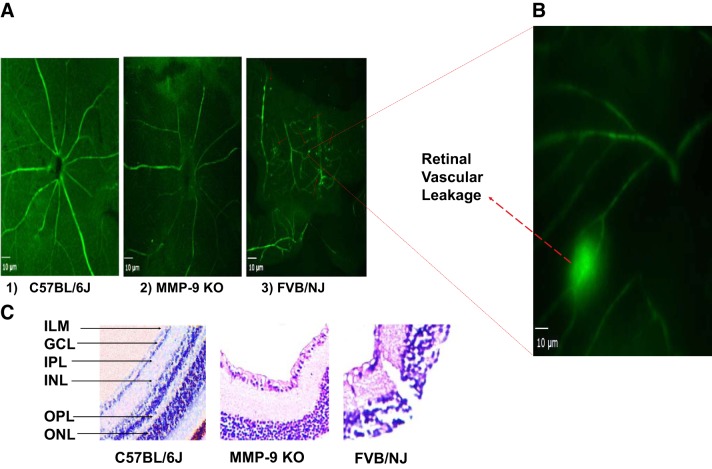
To determine whether diminished MMP-9 activity can mitigate blood-retinal barrier (BRB) permeability in MMP-9 KO mice and thus improve BRB functions, we looked at the ex vivo flat mount prepared from MMP-9 KO mice, which showed appreciable improvement as seen in the micrographs exhibiting the vascular integrity of the blood vessels in the retina. Leakage of BSA-FITC in FVB/NJ is evident (Fig. 3A3, red arrows, and Fig. 3B) in the retinal flat-mount compared with C57BL/6J (Fig. 3A1) and MMP-9 KO (Fig. 3A2). Hematoxylin and eosin staining showed inner retinal layer alterations because of extensive degenerative changes in the FVB/NJ mouse eyes. This reflects the destruction of the retina in FVB/NJ mice in comparison with MMP-9 KO and C57BL/6J strains (Fig. 3C).
Fig. 3.
Ex vivo visualization of the BSA-FITC in retinal vessels and the architecture of inner retina in mice: representative micrographs depicting vascular integrity of the retina. The permeability or leakage in FVB/NJ is evident as shown by red arrows in A3 (and in an enlarged image shown in B) as observed on retinal flat-mount compared with C57BL/6J in A1, and MMP-9 KO in A2. Micrographs were prepared 1 h post-BSA-FITC injection through the tail vein in each strain of the mouse, scale bar = 10 μm. On further probing employing hematoxylin-eosin staining, the inner retinal layers show altered anatomy as a result of degenerative changes in FVB/NJ mice compared with MMP-9 KO and C57BL/6J. ILM, inner limiting membrane; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; and, ONL, outer nuclear layer (C, ×200).
Expression analysis of key target proteins in the retina.
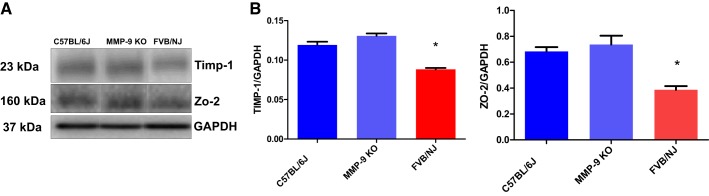
Immunoblotting of retinal lysates showed a decrease in the expression levels of the TIMP-1, and the tight junction protein ZO-2 in the FVB/NJ mouse strain in comparison with MMP-9 KO and C57BL/6J mice strains (Fig. 4A) as represented in their respective bar graphs after their quantitative estimations with respect to housekeeping GAPDH protein normalization (Fig. 4B).
Fig. 4.
Expression analysis of key target molecules in the retina of mice via Western blotting. The immunoblot data from the retinal lysates show decreased expression levels of tissue inhibitor of matrix metalloproteinase (TIMP)-1, and the component of the tight junction protein ZO-2 in the FVB/NJ when compared with MMP-9 KO and the C57BL/6J strains of the mice (A). The bar graphs show their quantitative estimations after normalization with the housekeeping GAPDH protein; the data are presented as means ± SE, *P < 0.05 when compared with C57BL/6J (B).
Electroretinogram and IOP assessment.
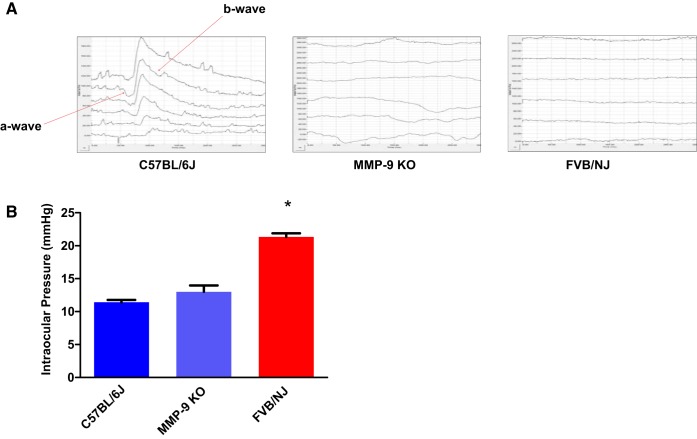
The in vivo dark-adapted ERG recordings revealed the response of the retina as observed by a- and b-wave components (Fig. 5A, red arrows). They were either markedly reduced in MMP-9 KO or completely missing in the FVB/NJ mice strain, indicating altered electrical responses in their respective retinae unlike C57BL/6J mice cones’ b-wave maximal response amplitudes. The IOP measurements were higher in FVB/NJ in comparison with the MMP-9 KO and C57BL/6J mice strains (Fig. 5B). These results suggest that MMP-9 activity affects the overall number of cone cells and their functions in the eyes.
Fig. 5.
Recordings of the electroretinogram (ERG) and the intraocular pressure (IOP) in mice strains. ERGs were recorded in response to 0.47 log cd·s/m2 strobe flashes presented under dark-adapted recording conditions. In comparison with C57BL/6J response, the a- and b-wave components (red arrows) were either markedly reduced in MMP-9 KO or completely missing in the FVB/NJ mouse strain, indicating altered electrical responses in their respective retinae (A). IOP measurements show enhanced pressure recording in FVB/NJ in comparison with the MMP-9 KO and C57BL/6J mice strains, *P < 0.0001 when compared with C57BL/6J (B).
Assessment of the vision-guided response.
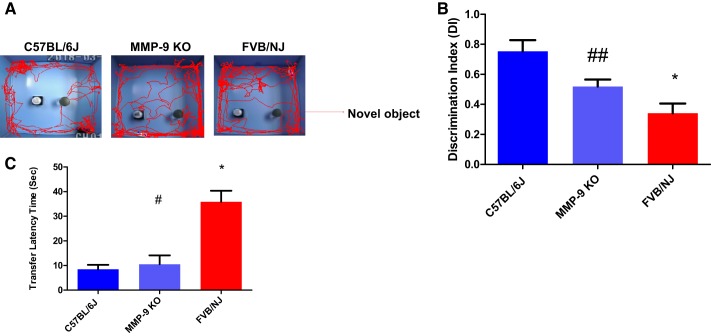
NORT and LDBT were performed on these animals to test vision-guided responses that revealed the individual mouse’s natural exploratory instincts. As displayed in the schematic representation, the mice in the FVB group exhibited significantly impaired novel object recognition performance during the task relative to age-matched control animals (C57 BL/6J; Fig. 6A). The discrimination index (DI) as calculated by the computerized algorithm was found to be significantly reduced in FVB/NJ mice in comparison with the C57BL/6J group; however, the MMP-9 KO mice exhibited improvement in their recognition preference between a novel and the familiar object when compared with the FVB/NJ mice (Fig. 6B). Similarly, the LDBT data showed an increased transfer latency time for the FVB/NJ group in comparison with C57BL/6J and mice and the MMP-9 KO group (Fig. 6C).
Fig. 6.
Improvement in the vision guided response in MMP-9 KO mice. Visual function tests were performed with the novel object recognition test (NORT) and the light-dark box test (LDBT) analyses on all three strains of the mice to evaluate their vision and thus ability to navigate their paths under different testing conditions. Representative images showing vision guided behavior as the discrimination index via NORT (A, B) and the transfer latency time via LDBT (C). Data values are means ± SE of 4–6 separate experiments; *P < 0. 0001 compared with C57BL/6J, #P < 0. 0001 compared with FVB/NJ, ## P < 0. 05 compared with FVB/NJ.
DISCUSSION
Declining vision or the fear of blindness leaves the patient with tortuous feelings of loss. Several eye diseases arising from retinal degeneration because of inherited gene mutation(s) are common causes of blindness. The resultant structural and functional alterations of the photoreceptors lead to complex pathophysiological changes in the eye that result in blindness since there are no available cures. Therefore, various therapeutic approaches are being tested to ameliorate or even cure these retinal conditions in susceptible patients. Suitable treatment options must be explored. Current therapeutic approaches that are in development include neuroprotective agents, gene therapeutics, optogenetics, regenerative therapies, and retinal prostheses (46). The rd1 mouse is a widely used model of retinal degeneration that carries a nonsense mutation in the photoreceptor cGMP PDE6b (Pde6b) gene. It is also a frequently employed model for studying the mechanisms of retinal degenerative changes in the eye. In brief, the defective gene encodes the β-subunit of rod PDE (β-PDE), an essential component of the phototransduction cascade responsible for the molecular signaling pathway that converts light into an electric signal in the outer segments of rod photoreceptors (39). In this mouse, the rod photoreceptors degenerate rapidly and are subsequently lost through a secondary unknown mechanism(s) (36).
MMPs are known to be highly regulated at the level of synthesis and during their subsequent activation. Like any other tissue, the vascular remodeling is also subjected to the mechanisms and regulatory dynamics of MMP molecules and their TIMPs (10, 13, 20, 26, 35, 37, 50). In fact, some of them have been shown to play crucial roles in vascular remodeling and ocular disorders. In that context, the balance of the MMP-9/TIMP-1 axis appears to be significant in maintaining normal blood vessel structure since the extracellular nature of the metalloproteinases and their interactions with regulators situate them as an important driver of the cells’ fate (14). It is also known that vascular beds have differential susceptibility to developing various vascular dysfunctions (22, 23). For example, TJPs maintain the structural integrity of the BRB in the eyes as shown via a schematic representation in Fig. 7. Previous studies have indicated that alterations in TJPs are associated with increased vascular permeability and complications in serious retinal diseases such as diabetic retinopathy and other ocular disorders (2, 8, 17, 47, 52). Studies have also shown that genetic removal of MMP-9 ameliorated the symptoms of fragile X syndrome, and it was also observed that deficiency of MMP-9 attenuated glomerular injury (44, 48). During other pathophysiological conditions, MMP-9 has been shown to contribute to pathologies that involve inflammatory processes, including arthritis, diabetes, and cancer (12). Under these conditions, MMP-9 proteolytic properties stimulated pathogenesis and exacerbated disease progression. Also, we have previously reported that ablating MMP-9 helps induce cell survival and their differentiation (29). In this study we used the FVB/NJ background having the pde6b rd1 homozygous allele that leads to rod photoreceptor loss by weaning age; however, the cone photoreceptors are still present after the weaning period is over. We tested the hypothesis and present evidence that when MMP-9 is knocked out on the FVB/NJ background, there is comparatively less retinal damage and that lack of MMP-9 activity diminishes the neuroretinal degenerative processes, thus helping to preserve or even improve retinal functions. Based on our data and interpretation of the results, we observed that the absence of MMP-9 led to the upregulation of TJP (ZO-2), suggesting that interaction of MMP-9 and TJP expression plays a key role in BRB integrity, and any alteration can, in fact, lead to BRB dysfunction. In fact, ZO-2 has been found to localize with connexin-36 within individual neuronal gap junctions especially in the plexiform layers of the retina, suggesting an important scaffolding role played by it in the gap junction (5). Differential TIMP-1 expression is supposed to play an important role in ECM remodeling dynamics in the eye during retinal detachment and proliferative vitreoretinopathy (16). Interestingly, MMP-9 and TIMP-1 are predominantly localized in the interphotoreceptor matrix and are supposed to be secreted by RPE cells (1).
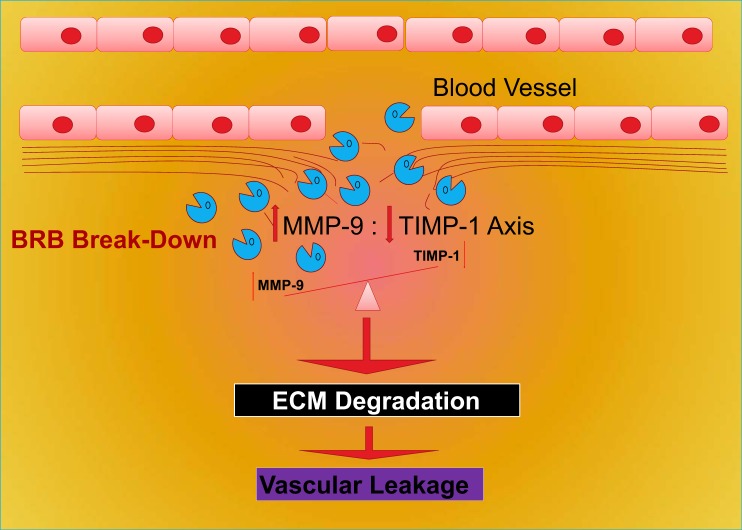
Fig. 7.
A schematic model showing the importance of the MMP-9/TIMP-1 axis. An imbalance of the MMP-9 and TIMP-1 axis induces the degradation of ECM, which leads to the loss of BRB integrity and the retinal vascular permeability (BRB breakdown). BRB, blood retinal barrier; ECM, extracellular matrix; TIMP-1, tissue inhibitor of metalloproteinase-1; MMP-9, matrix metalloproteinase-9.
As revealed by fluorescence microscopy analysis the increased retinal vascular permeability in FVB/NJ as compared with MMP-9 KO and C57BL/6J mice, together with maintenance of the vessel density in the MMP-9-deficient animals, emphasizes the importance of the balance between MMP-9 and TIMP-1. This, in fact, could be developed as a potential therapeutic target for several vasculopathies, including conditions affecting the eyes such as retinovascular thrombosis, retinitis pigmentosa, and retinal neovascularization. The physiologic importance of MMPs in vascular remodeling can be ascribed to their unique structures as well as to their matrix components. Understanding them further may lead to better insights in advancing therapeutic modalities based on structural domain knowledge and their intimate cross talk with host-derived inflammatory factors that are responsible for retinal vascular homeostasis.
Results from our study suggest that MMP-9 activity affects retinal cells and their functions in the eyes. OCT and fundoscopy imaging revealed a disturbed nature of the retinal layers/vasculature in FVB/NJ mice in comparison with MMP-9 deficient mice. NORT findings exhibited significantly impaired novel object recognition performance in the FVB group relative to the control C57BL/6J and the MMP-9 mice groups as demonstrated via the discrimination indices. Likewise, LDBT data showed an increased transfer latency time in the FVB/NJ group in comparison with C57BL/6J mice, but there was significant improvement in the MMP-9 KO group. Similarly, FVB/NJ mice showed increased IOP compared with the MMP-9 KO and C57BL/6J groups, again indicating the degeneration of cell types that help maintain pressure homeostasis in the ocular compartment. It is noteworthy to mention that MMP-9 inhibition promoted survival of the neurons, again highlighting a deleterious role for MMP-9 in regulating neuronal survival in the central nervous system (24). The eye, being the most metabolically active organ in the human body, requires strict maintenance of retinal architecture to perform its functions properly. MMP activity or their physiological dysregulation can thus lead to disruption of the ECM, causing ocular damage and malfunctioning of various cell types in the eyes. Since the FVB/NJ is a blind strain and not much has been studied with respect to the potential role of MMP-9 in vision, we believe that our study opens up new avenue(s) in exploring further the contribution of ECM in toward the maintenance of neurovascular homeostasis that specifically pertains to neuroretinal physiology.
GRANTS
The work reported here was supported by NIH Grants HL-74815, HL-107640, and NS-084823.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.C.T. and M.S. conceived and designed research; A.K.G., R.P.H., and M.S. performed experiments; A.K.G., R.P.H., S.C.T., and M.S. analyzed data; A.K.G., R.P.H., A.M., S.C.T., and M.S. interpreted results of experiments; A.K.G., R.P.H., A.M., and M.S. prepared figures; A.K.G., S.C.T., and M.S. drafted manuscript; A.K.G., S.C.T., and M.S. edited and revised manuscript; A.K.G., R.P.H., A.M., S.C.T., and M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank all members of the laboratory for continued help and support.
Part of this study was presented at the joint hypertension scientific session of the American Heart Association and American Society of Hypertension, September 6–9, 2018, Hyatt Regency, Chicago, IL.
REFERENCES
- 1.Ahuja S, Ahuja P, Caffé AR, Ekstrom P, Abrahamson M, van Veen T. rd1 mouse retina shows imbalance in cellular distribution and levels of TIMP-1/MMP-9, TIMP-2/MMP-2 and sulfated glycosaminoglycans. Ophthalmic Res 38: 125–136, 2006. doi: 10.1159/000090533. [DOI] [PubMed] [Google Scholar]
- 2.Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol 14: 240–248, 1999. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- 3.Bhargava S, Pushpakumar S, Metreveli N, Givvimani S, Tyagi SC. MMP-9 gene ablation mitigates hyperhomocystenemia-induced cognition and hearing dysfunction. Mol Biol Rep 41: 4889–4898, 2014. doi: 10.1007/s11033-014-3425-x. [DOI] [PubMed] [Google Scholar]
- 4.Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res 42: 517–525, 2002. doi: 10.1016/S0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 5.Ciolofan C, Li XB, Olson C, Kamasawa N, Gebhardt BR, Yasumura T, Morita M, Rash JE, Nagy JI. Association of connexin36 and zonula occludens-1 with zonula occludens-2 and the transcription factor zonula occludens-1-associated nucleic acid-binding protein at neuronal gap junctions in rodent retina. Neuroscience 140: 433–451, 2006. doi: 10.1016/j.neuroscience.2006.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das A, McGuire PG. Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog Retin Eye Res 22: 721–748, 2003. doi: 10.1016/j.preteyeres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Di Y, Nie QZ, Chen XL. Matrix metalloproteinase-9 and vascular endothelial growth factor expression change in experimental retinal neovascularization. Int J Ophthalmol 9: 804–808, 2016. doi: 10.18240/ijo.2016.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis 10: 103–117, 2007. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- 9.Farber DB, Lolley RN. Enzymic basis for cyclic GMP accumulation in degenerative photoreceptor cells of mouse retina. J Cyclic Nucleotide Res 2: 139–148, 1976. [PubMed] [Google Scholar]
- 10.García-Moll X; Ready for Use in the Clinical Setting . [Inflammatory and anti-inflammatory markers in acute coronary syndromes. Ready for use in the clinical setting?]. Rev Esp Cardiol 58: 615–617, 2005. doi: 10.1016/S1885-5857(06)60246-6. [DOI] [PubMed] [Google Scholar]
- 11.George AK, Behera J, Kelly KE, Mondal NK, Richardson KP, Tyagi N. Exercise Mitigates Alcohol Induced Endoplasmic Reticulum Stress Mediated Cognitive Impairment through ATF6-Herp Signaling. Sci Rep 8: 5158, 2018. doi: 10.1038/s41598-018-23568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halade GV, Jin YF, Lindsey ML. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 139: 32–40, 2013. doi: 10.1016/j.pharmthera.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanaoka M, Droma Y, Ota M, Ito M, Katsuyama Y, Kubo K. Polymorphisms of human vascular endothelial growth factor gene in high-altitude pulmonary oedema susceptible subjects. Respirology 14: 46–52, 2009. doi: 10.1111/j.1440-1843.2008.01420.x. [DOI] [PubMed] [Google Scholar]
- 14.Hojilla CV, Mohammed FF, Khokha R. Matrix metalloproteinases and their tissue inhibitors direct cell fate during cancer development. Br J Cancer 89: 1817–1821, 2003. doi: 10.1038/sj.bjc.6601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeler CE. The geotropic reaction of rodless mice in light and in darkness. J Gen Physiol 11: 361–368, 1928. doi: 10.1085/jgp.11.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim B, Abdel-Rahman MH, Wang T, Pouly S, Mahmoud AM, Cebulla CM. Retinal MMP-12, MMP-13, TIMP-1, and TIMP-2 expression in murine experimental retinal detachment. Invest Ophthalmol Vis Sci 55: 2031–2040, 2014. doi: 10.1167/iovs.13-13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Kim CS, Lee YM, Jo K, Shin SD, Kim JS. Methylglyoxal induces hyperpermeability of the blood-retinal barrier via the loss of tight junction proteins and the activation of matrix metalloproteinases. Graefes Arch Clin Exp Ophthalmol 250: 691–697, 2012. doi: 10.1007/s00417-011-1912-5. [DOI] [PubMed] [Google Scholar]
- 18.Korshunov VA, Solomatina MA, Plekhanova OS, Parfyonova YV, Tkachuk VA, Berk BC. Plasminogen activator expression correlates with genetic differences in vascular remodeling. J Vasc Res 41: 481–490, 2004. doi: 10.1159/000081804. [DOI] [PubMed] [Google Scholar]
- 19.Kowluru RA, Zhong Q, Santos JM. Matrix metalloproteinases in diabetic retinopathy: potential role of MMP-9. Expert Opin Investig Drugs 21: 797–805, 2012. doi: 10.1517/13543784.2012.681043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar M, Tyagi N, Moshal KS, Sen U, Kundu S, Mishra PK, Givvimani S, Tyagi SC. Homocysteine decreases blood flow to the brain due to vascular resistance in carotid artery. Neurochem Int 53: 214–219, 2008. doi: 10.1016/j.neuint.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakka SS, Gondi CS, Dinh DH, Olivero WC, Gujrati M, Rao VH, Sioka C, Rao JS. Specific interference of urokinase-type plasminogen activator receptor and matrix metalloproteinase-9 gene expression induced by double-stranded RNA results in decreased invasion, tumor growth, and angiogenesis in gliomas. J Biol Chem 280: 21882–21892, 2005. doi: 10.1074/jbc.M408520200. [DOI] [PubMed] [Google Scholar]
- 22.Langille BL, Bendeck MP, Keeley FW. Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol 256: H931–H939, 1989. doi: 10.1152/ajpheart.1989.256.4.H931. [DOI] [PubMed] [Google Scholar]
- 23.Langille BL, O’Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231: 405–407, 1986. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Hörbelt M, Mang HE, Knipe NL, Bacallao RL, Sado Y, Sutton TA. MMP-9 gene deletion mitigates microvascular loss in a model of ischemic acute kidney injury. Am J Physiol Renal Physiol 301: F101–F109, 2011. doi: 10.1152/ajprenal.00445.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li AF, Roy S. High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Invest Ophthalmol Vis Sci 50: 1400–1407, 2009. doi: 10.1167/iovs.07-1519. [DOI] [PubMed] [Google Scholar]
- 26.Libby P, Lee RT. Matrix matters. Circulation 102: 1874–1876, 2000. doi: 10.1161/01.CIR.102.16.1874. [DOI] [PubMed] [Google Scholar]
- 27.Machens HG, Grzybowski S, Bucsky B, Spanholtz T, Niedworok C, Maichle A, Stöckelhuber B, Condurache A, Liu F, Egana JT, Kaun M, Mailänder P, Aach T. A technique to detect and to quantify fasciocutaneous blood vessels in small laboratory animals ex vivo. J Surg Res 131: 91–96, 2006. doi: 10.1016/j.jss.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Fernández de la Cámara C, Sequedo MD, Gómez-Pinedo U, Jaijo T, Aller E, García-Tárraga P, García-Verdugo JM, Millán JM, Rodrigo R. Phosphodiesterase inhibition induces retinal degeneration, oxidative stress and inflammation in cone-enriched cultures of porcine retina. Exp Eye Res 111: 122–133, 2013. doi: 10.1016/j.exer.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Mishra PK, Chavali V, Metreveli N, Tyagi SC. Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: a role of extracellular matrix. Can J Physiol Pharmacol 90: 353–360, 2012. doi: 10.1139/y11-131. [DOI] [PubMed] [Google Scholar]
- 30.Mohammad G, Siddiquei MM. Role of matrix metalloproteinase-2 and -9 in the development of diabetic retinopathy. J Ocul Biol Dis Infor 5: 1–8, 2012. doi: 10.1007/s12177-012-9091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison JC, Jia L, Cepurna W, Guo Y, Johnson E. Reliability and sensitivity of the TonoLab rebound tonometer in awake Brown Norway rats. Invest Ophthalmol Vis Sci 50: 2802–2808, 2009. doi: 10.1167/iovs.08-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muhs BE, Plitas G, Delgado Y, Ianus I, Shaw JP, Adelman MA, Lamparello P, Shamamian P, Gagne P. Temporal expression and activation of matrix metalloproteinases-2, -9, and membrane type 1-matrix metalloproteinase following acute hindlimb ischemia. J Surg Res 111: 8–15, 2003. doi: 10.1016/S0022-4804(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 33.Munjal C, Tyagi N, Lominadze D, Tyagi SC. Matrix metalloproteinase-9 in homocysteine-induced intestinal microvascular endothelial paracellular and transcellular permeability. J Cell Biochem 113: 1159–1169, 2012. doi: 10.1002/jcb.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muradashvili N, Tyagi R, Metreveli N, Tyagi SC, Lominadze D. Ablation of MMP9 gene ameliorates paracellular permeability and fibrinogen-amyloid beta complex formation during hyperhomocysteinemia. J Cereb Blood Flow Metab 34: 1472–1482, 2014. doi: 10.1038/jcbfm.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, Fayad Z, Budoff MJ, Rumberger J, Naqvi TZ, Shaw LJ, Faergeman O, Cohn J, Bahr R, Koenig W, Demirovic J, Arking D, Herrera VL, Badimon J, Goldstein JA, Rudy Y, Airaksinen J, Schwartz RS, Riley WA, Mendes RA, Douglas P, Shah PK; SHAPE Task Force . From vulnerable plaque to vulnerable patient--Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol 98, 2A: 2H–15H, 2006. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Nishiguchi KM, Carvalho LS, Rizzi M, Powell K, Holthaus SM, Azam SA, Duran Y, Ribeiro J, Luhmann UF, Bainbridge JW, Smith AJ, Ali RR. Gene therapy restores vision in rd1 mice after removal of a confounding mutation in Gpr179. Nat Commun 6: 6006, 2015. doi: 10.1038/ncomms7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ota R, Kurihara C, Tsou TL, Young WL, Yeghiazarians Y, Chang M, Mobashery S, Sakamoto A, Hashimoto T. Roles of matrix metalloproteinases in flow-induced outward vascular remodeling. J Cereb Blood Flow Metab 29: 1547–1558, 2009. doi: 10.1038/jcbfm.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 96: 614–618, 2012. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 39.Pittler SJ, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci USA 88: 8322–8326, 1991. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pushpakumar SB, Kundu S, Metreveli N, Tyagi SC, Sen U. Matrix Metalloproteinase Inhibition Mitigates Renovascular Remodeling in Salt-Sensitive Hypertension. Physiol Rep 1: e00063, 2013. doi: 10.1002/phy2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy S, Bae E, Amin S, Kim D. Extracellular matrix, gap junctions, and retinal vascular homeostasis in diabetic retinopathy. Exp Eye Res 133: 58–68, 2015. doi: 10.1016/j.exer.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Roy S, Ha J, Trudeau K, Beglova E. Vascular basement membrane thickening in diabetic retinopathy. Curr Eye Res 35: 1045–1056, 2010. doi: 10.3109/02713683.2010.514659. [DOI] [PubMed] [Google Scholar]
- 43.Sahay P, Rao A, Padhy D, Sarangi S, Das G, Reddy MM, Modak R. Functional Activity of Matrix Metalloproteinases 2 and 9 in Tears of Patients With Glaucoma. Invest Ophthalmol Vis Sci 58: BIO106–BIO113, 2017. doi: 10.1167/iovs.17-21723. [DOI] [PubMed] [Google Scholar]
- 44.Sakamaki Y, Sasamura H, Hayashi K, Ishiguro K, Takaishi H, Okada Y, D’Armiento JM, Saruta T, Itoh H. Absence of gelatinase (MMP-9) or collagenase (MMP-13) attenuates adriamycin-induced albuminuria and glomerulosclerosis. Nephron, Exp Nephrol 115: e22–e32, 2010. doi: 10.1159/000312883. [DOI] [PubMed] [Google Scholar]
- 45.Sato T, Haimovici R, Kao R, Li AF, Roy S. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes 51: 1565–1571, 2002. doi: 10.2337/diabetes.51.5.1565. [DOI] [PubMed] [Google Scholar]
- 46.Scholl HP, Strauss RW, Singh MS, Dalkara D, Roska B, Picaud S, Sahel JA. Emerging therapies for inherited retinal degeneration. Sci Transl Med 8: 368rv6, 2016. doi: 10.1126/scitranslmed.aaf2838. [DOI] [PubMed] [Google Scholar]
- 47.Shin ES, Sorenson CM, Sheibani N. Diabetes and retinal vascular dysfunction. J Ophthalmic Vis Res 9: 362–373, 2014. doi: 10.4103/2008-322X.143378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidhu H, Dansie LE, Hickmott PW, Ethell DW, Ethell IM. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J Neurosci 34: 9867–9879, 2014. doi: 10.1523/JNEUROSCI.1162-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol 160: 673–680, 2002. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tardif JC, Heinonen T, Orloff D, Libby P. Vascular biomarkers and surrogates in cardiovascular disease. Circulation 113: 2936–2942, 2006. doi: 10.1161/CIRCULATIONAHA.105.598987. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Saul A, Cui X, Roon P, Smith SB. Absence of Sigma 1 Receptor Accelerates Photoreceptor Cell Death in a Murine Model of Retinitis Pigmentosa. Invest Ophthalmol Vis Sci 58: 4545–4558, 2017. doi: 10.1167/iovs.17-21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Tong J, Chang B, Wang B, Zhang D, Wang B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol Med Rep 9: 2352–2356, 2014. doi: 10.3892/mmr.2014.2126. [DOI] [PubMed] [Google Scholar]
- 53.Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol 20: 811–838, 2004. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- 54.Xue Y, Shen SQ, Corbo JC, Kefalov VJ. Circadian and light-driven regulation of rod dark adaptation. Sci Rep 5: 17616, 2015. doi: 10.1038/srep17616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue Y, Shen SQ, Jui J, Rupp AC, Byrne LC, Hattar S, Flannery JG, Corbo JC, Kefalov VJ. CRALBP supports the mammalian retinal visual cycle and cone vision. J Clin Invest 125: 727–738, 2015. doi: 10.1172/JCI79651. [DOI] [PMC free article] [PubMed] [Google Scholar]