Abstract
Several lines of evidence have implicated long interspersed nuclear element-1 (LINE-1) retroelement in the onset and progression of lung cancer. Retrotransposition-dependent mechanisms leading to DNA mobilization give rise to insertion mutations and DNA deletions, whereas retrotransposition-independent mechanisms disrupt epithelial programming and differentiation. Previous work by our group established that tobacco carcinogens such as benzo(a)pyrene (BaP) reactivate LINE-1 in bronchial epithelial cells through displacement of nucleosome remodeling and deacetylase (NuRD) corepressor complexes and interference with retinoblastoma-regulated epigenetic signaling. Whether LINE-1 in coordination with other genes within its regulatory network contributes to the in vivo genotoxic response to BaP remains largely unknown. Evidence is presented here that intratracheal instillation of ORFeusLSL mice with BaP alone or in combination with adenovirus (adeno)-CRE recombinase is genotoxic to the lung and associated with activation of the human LINE-1 transgene present in these mice. LINE-1 reactivation modulated the expression of genes involved in oncogenic signaling, and these responses were most pronounced in female mice compared with males and synergized by adeno-CRE recombinase. This is the first report linking LINE-1 and genes within its oncogenic regulatory network with early sexually dimorphic responses of the lung in vivo.
Keywords: benzo(a)pyrene, LINE-1, lung, oncogenic reprogramming, ORFeusLSL
INTRODUCTION
Long interspersed nuclear elements (LINEs) are a group of non-long terminal repeat (non-LTR) retrotransposons widely distributed within the genome of eukaryotes (25, 51). LINEs account for ~20% of the human genome sequence (30), with most retroelements identified as molecular fossils devoid of activity (19, 30). About 100 full-length copies of human LINE-1 remain retrotransposition competent and capable of mobilization through RNA intermediates (4, 9). Full-length LINE-1 is ~6 kb in length, contains an internal bidirectional promoter, two open reading frames (ORFs) encoding ORF1 and ORF2, and a 3′ poly(A) tail. ORF1 is a 40-kDa protein with RNA-binding activity, whereas ORF2 is a 150-kDa protein with reverse transcriptase and endonuclease activities (16, 35). Both proteins are required for retrotransposition (35). The LINE-1 retrotransposition cycle entails three critical steps: 1) epigenetic reactivation and transcription of LINE-1, 2) cytoplasmic export of the mRNA for translation into proteins that bind the LINE-1 (L1) transcript to form ribonucleoprotein complexes that return to the nucleus, and 3) cleavage of genomic DNA by ORF2p to create a free 3′-end for reverse transcription as well as new sites for genome integration (43).
The majority of LINEs are silenced by DNA methylation and histone covalent modifications (11, 38, 55). Although human LINE-1 and mouse LINE-1 exhibit structural differences, benzo(a)pyrene (BaP) mediates transcriptional activation via comparable mechanisms involving disruption of epigenetic silencing, interference with retinoblastoma-regulated macromolecular interactions, and downregulation of DNA (cytosine-5)-methyltransferase 1 (DNMT1) expression (38, 55). Several lines of evidence in vitro established the ability of LINE-1 to retrotranspose to new genomic locations where it compromises genome stability through altered gene splicing, disruption of gene function, and increased recombination (7, 15, 53). LINEs may also control gene expression by providing regulatory sequences that direct expression of other genes (24, 56). The activity of LINE-1 in vivo has not been widely studied, and to date, only two active human LINEs, L1RP (10) and L1LRE3 (13), have been described. These LINEs exhibit extremely low retrotransposition frequencies when introduced into mice in their native form, and genetic manipulation is required to optimize expression (2).
BaP is a by-product of the incomplete combustion of organic matter (47) and the principal constituent of tobacco smoke implicated in lung carcinogenesis (28). The parent hydrocarbon and its oxidative metabolites bind to the aryl hydrocarbon receptor (AhR) to induce nuclear translocation and dimerization with the AhR nuclear translocator to form a liganded complex that regulates several genes, including members of the cytochrome P450 (CYP) superfamily (36). The relative abundance of CYP and AhR proteins in the lung is high, with CYPs readily catalyzing the conversion of BaP to (±)-anti-benzo(a)pyrene-7,8-diol-9,10-epoxide (BPDE) and other metabolites that form the DNA adducts detected in the lung tissue of smokers (40). BaP activates LINE-1 expression in various cell types and multiple mammalian species (33, 34, 54), and AhR signaling participates in the LINE-1 reactivation cascade (54).
The present study evaluated profiles of LINE-1 reactivation and genetic reprogramming following genotoxic lung injury by BaP. The ORFeusLSL murine transgenic model containing a single copy of an optimized human LINE-1 transgene was employed to define exogenous activation. We report that the genotoxicity of BaP alone or in combination with adenovirus (adeno)-CRE recombinase was associated with activation of the LINE-1 transgene and a sexually dimorphic profile of genetic reprogramming linked to early oncogenic signaling.
MATERIALS AND METHODS
Animals.
Founder ORFeusLSL mice, a genetically modified conditional model of LINE-1 retrotransposition under control of CRE recombinase, were provided by Dr. W. An (South Dakota State University). All procedures were reviewed and approved by the University of Arizona Institutional Animal Care and Use Committee.
Reactivation of ORFeusLSL transgene by BaP alone or with adeno-CRE recombinase.
BaP dissolved in dimethyl sulfoxide (DMSO) was administered via instillation with or without adeno-CRE recombinase for conditional activation of a single copy of a human LINE-1 transgene (2). A BioLite Intubation System (Braintree Scientific) was used for intratracheal instillation of ORFeusLSL mice (29–38 g) anesthetized with ketamine and xylazine (100 and 10 mg/kg, respectively). All mice were genotyped to confirm genetic identity. Control mice were given 1 dose of 5 × 1010 plaque-forming units (PFU) adeno-CRE recombinase dissolved in H2O or DMSO. The optimal adenovirus dose was defined empirically as a dose that only modestly activated the transgene (data not shown). Treated mice were intubated with BaP (50 mg/kg) alone or in combination with 5 × 1010 PFU adeno-CRE virus. Cervical dislocation of mice was performed after euthanasia with CO2 1 wk after treatment. Tissues were collected and stored at 4°C in RNA later (Thermo Fisher Scientific).
Histological analysis.
Lung sections were snap-frozen in optimum cutting temperature compound. Tissues were fixed with cold acetone and stained with hematoxylin-eosin. Samples were evaluated by two pathologists blinded to the treatment given.
32P postlabeling.
The 32P-postlabeling assay for DNA adducts was performed as reported previously (39).
Quantitative real-time PCR.
RNA was extracted using the RNeasy Plus Mini Kit (Qiagen). An aliquot of 250 ng RNA was reverse transcribed into first-strand cDNA using SuperScript III (Invitrogen). Quantitative real-time PCR (RT-qPCR) was performed for LINE-1 and nine genes within its oncogenic regulatory network. These genes included AhR; chemokine (C-C motif) ligand 2 (CCL2); cytochrome P450, family 2, subfamily a, polypeptide 4 (CYP2A4); microsomal glutathione S-transferase 1 (MGST1); phenylalanine hydroxylase (PAH); periostin, osteoblast-specific factor (POSTN); protein tyrosine phosphatase, receptor type B (PTPRB); vascular cell adhesion molecule 1 (VCAM1); and transforming growth factor-β1 (TGF-β1; 8, 45). GAPDH was used as a control. Each reaction included 4.0 μL of cDNA, 0.5 μL of gene-specific primer pairs, and 5.0 μL SYBR Green dye, and double-distilled H2O to a final volume of 20 μL. Amplifications were completed using StepOnePlus RT-qPCR System and fold changes were calculated using the 2−∆∆Ct method (where Ct is threshold cycle).
Statistical analyses.
All normally distributed data were analyzed by ANOVA and Duncan’s multiple range test. Nonnormally distributed data were evaluated using the Mann–Whitney U test. Significance was defined at the P ≤ 0.05 level.
RESULTS
BPDE-deoxyguanine (dG) DNA adducts were detected in the lungs of both female and male ORFeusLSL mice given a single dose of 25 mg/kg BaP alone or in combination with adeno-CRE (Table 1). Only background signal was detected in control untreated, CRE-H2O, or CRE-DMSO mice. All major adducts were derived from BPDE, as evidenced by autoradiographic signals (3, 6). Adduct intensities were considerably higher in female mice compared with males, with significant differences between BaP alone and BaP plus adeno-CRE seen only in male mice. The occurrence of BPDE-dG DNA adducts 1 wk following carcinogen treatment established persistence of covalent binding following widespread DNA damage. This is significant given that the BaP dose given is well below the total cumulative dose range of 25–100 mg/kg administered daily over several months for human toxicological risk assessments (52). No dramatic toxic effects were observed at any time in mice treated with BaP alone or in combination with adeno-CRE recombinase.
Table 1.
DNA adduct signal intensity in the lungs of ORFeusLSL mice following treatment with benzo(a)pyrene alone or in combination with CRE recombinase
| Relative Adduct Labeling |
||||||
|---|---|---|---|---|---|---|
| Treatment | Adduct 1 | Adduct 2 | Adduct 3 | Adduct 4 | Adduct 5 | Total |
| Untreated control | 3.50 ± 1.91f | 1.69 ± 0.88n,o | 2.71 ± 1.65j | 1.39 ± 0.62q | 1.68 ± 0.34n,o | 2.19 ± 0.82E |
| CRE-DMSO | 2.82 ± 1.95i | 1.71 ± 1.23n | 1.92 ± 0.80m | 0.84 ± 0.35u,v | 1.18 ± 0.29s | 1.69 ± 0.70F |
| BaP (F) | 28.01 ± 17.49a | 2.95 ± 0.99h | 4.04 ± 1.74e | 1.44 ± 0.65p,q | 1.40 ± 0.83q | 7.57 ± 10.92A |
| BaP (M) | 10.35 ± 6.21d | 1.65 ± 0.30o | 1.48 ± 0.78p | 0.66 ± 0.23w | 1.25 ± 0.43r | 3.08 ± 3.78D |
| Cre-BaP (F) | 23.86 ± 3.99b | 2.39 ± 0.87k | 3.10 ± 0.66g | 0.89 ± 0.16u | 1.30 ± 0.55r | 6.31 ± 9.12B |
| Cre-BaP (M) | 17.61 ± 9.34c | 1.94 ± 0.35m | 2.29 ± 0.51l | 0.80 ± 0.28v | 1.05 ± 0.72t | 4.73 ± 6.68C |
| Total | 14.36 ± 9.89A | 2.06 ± 0.48B | 2.59 ± 0.86B | 1.0 ± 0.30C | 1.31 ± 0.22C | |
Values are means ± SD; n = 3 animals examined. Relative adduct labeling is equal to the number of adducts per 108 nucleotides. BaP, benzo(a)pyrene; F, female; M, male.
a–w, A–FSignificant differences between and among the different groups by sex × treatment.
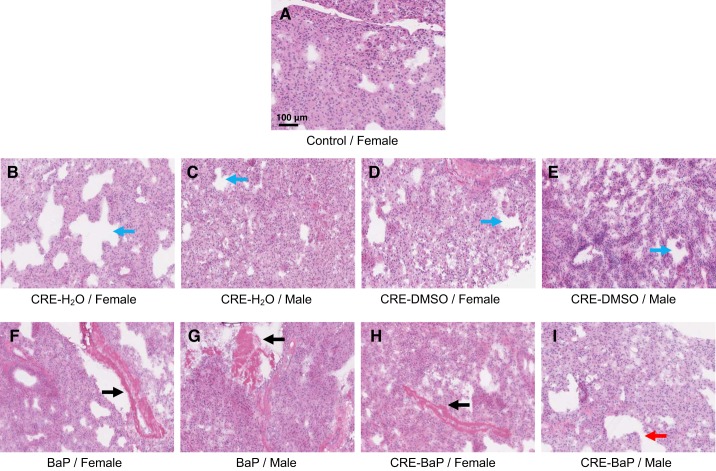
Control lung tissues from mice given water or DMSO alone, or in combination with adeno-CRE recombinase, showed normal histology, except for a modest degree of iatrogenic pulmonary edema evidenced by vascular dilation (Fig. 1, A–E). In contrast, significant leukocytic infiltration into the lungs was prominent in mice given BaP alone or combined with adeno-CRE. Female and male mice in both treatment groups showed pulmonary vessel congestion with focal-to-diffuse mononuclear cell infiltration into parenchyma. Lung tissues also showed emphysematous changes evidenced by large empty spaces with variable preservation of alveolar structures (Fig. 1, F–I).
Fig. 1.
Histology of lung tissues in ORFeusLSL mice treated with benzo(a)pyrene (BaP) alone or in combination with CRE recombinase. A–I: representative results for all treatment groups at ×20. Blue arrows indicate the pulmonary edema evidenced by vascular dilation, black arrows denote pulmonary vessel congestion, and the red arrow denotes emphysematous changes evidenced by large empty spaces with variable preservation of alveolar structures. Here, n = 3 animals examined.
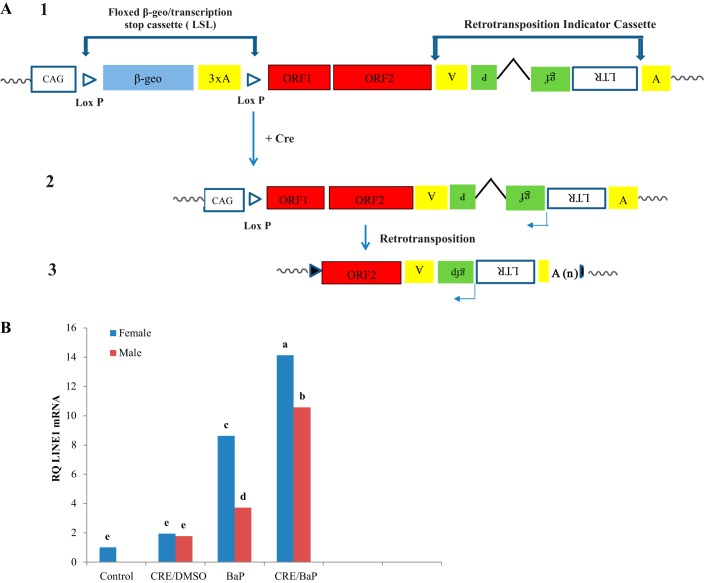
Measurements of ORFeus LINE-1 mRNA were completed to evaluate the ability of the carcinogen to activate the human transgene. A schematic of the ORFeusLSL transgene under control by loxP is presented in Fig. 2A. BaP alone significantly increased expression of the transgene, whereas adeno-CRE recombinase alone was only modestly active. Increased expression of ORFeus LINE-1 mRNA was observed in both male and female mouse lungs given the combined BaP-adeno-CRE treatment compared with BaP alone or vehicles. In keeping with patterns of DNA adduct formation and tissue damage, ORFeus LINE-1 mRNA levels were higher in female mouse lungs compared with male mouse lungs (Fig. 2B). The specificity of the lung induction response was confirmed in studies showing that only stomach, but not heart, kidney, liver, or intestine, showed activation of the LINE-1 transgene and that BaP treatment induced CYP gene expression in the lungs of ORFeus mice (data not shown).
Fig. 2.
A: schematic of the ORFeusLSL transgene: 1) the floxed allele of the ORFeusLSL transgene, 2) the excised allele of the ORFeusLSL transgene following Cre-mediated excision of the floxed β-geo-stop cassette (LSL), and 3) putative insertion upon retrotransposition. B: long interspersed nuclear element-1 (LINE-1) mRNA in lungs of ORFeusLSL mice treated with benzo(a)pyrene (BaP) alone or in combination with CRE recombinase. LINE-1 mRNA was measured by RT-qPCR using primers specific for ORFeusLSL. A (upright), polyadenylation signal; gfp, green fluorescent protein; LTR, long terminal repeat; ORF, open reading frame; RQ, relative quantification; n = 3 animals examined. Different letters denote differences between control, BaP-treated, and CRE-BaP-treated groups at P ≤ 0.05.
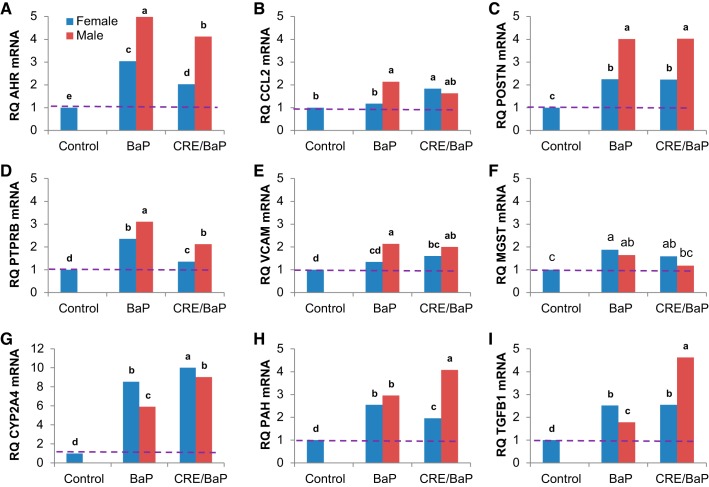
To investigate the biological consequences of LINE-1 activation in the lungs of ORFeus mice, we next examined genes within a LINE-1 regulatory network that is activated during oncogenic reprogramming of epithelial genomes (8, 45). The targets chosen constitute a regulatory network involved in early oncogenic signaling and included AhR, CCL2, CYP2A4, MGST1, PAH, POSTN, PTPRB, VCAM1, and TGF-β1. These genes were differentially regulated upon activation of the LINE-1 transgene, with notable differences between BaP alone or in combination with adeno-CRE seen for all but POSTN and PTPRB (Fig. 3, A–I). All mice were of comparable age to obviate potential age-related differences. Thus, induction of ORFeus LINE-1 was associated with genetic reprogramming in the murine lung in vivo, and this response was sustained for up to 1 wk after carcinogen treatment.
Fig. 3.
mRNA abundance of genes within the long interspersed nuclear element-1 oncogenic regulatory network in lungs of ORFeusLSL mice. A–I: results for different genes within the network: AhR, aryl hydrocarbon receptor; CCL2, chemokine (C-C motif) ligand 2; CYP2A4, cytochrome P450, family 2, subfamily a, polypeptide 4; MGST, microsomal glutathione S-transferase 1; PAH, phenylalanine hydroxylase; POSTN, periostin, osteoblast-specific factor; PTPRB, protein tyrosine phosphatase, receptor type B; TGFB1, transforming growth factor-β1; VCAM, vascular cell adhesion molecule 1. BaP, benzo(a)pyrene; RQ, relative quantification; n = 3 animals examined. Different letters denote differences between control, BaP-treated, and CRE-BaP-treated groups at P ≤ 0.05.
DISCUSSION
BaP is a procarcinogen metabolized by CYP1A1 and CYP1B1 to highly reactive electrophilic intermediates (17, 26). This metabolic activation mediates DNA adduct formation, including those associated with BPDE, the primary lung cancer etiologic agent (27, 50). The total cumulative dose of BaP examined in our study approximates the cumulative dose experienced by a smoker consuming 40 cigarettes per day for 2 yr. This is significant given that 1 yr in the life of a human is equivalent to 9 days in the mouse (14). As such, our findings reflect early changes associated with activation of the LINE-1 retrotransposon and genetic elements within its oncogenic regulatory network.
BaP is rapidly distributed throughout the body and detected in most tissues within minutes to hours after exposure. DNA adduction, a key event in the mutagenic and carcinogenic response, appears as early as 4 h after dosing of mice with the parent hydrocarbon (18). We present evidence for the first time that the genotoxic response of the mouse lung to BaP in vivo involves sustained activation of LINE-1 retrotransposon and this response exhibits a sexually dimorphic profile. These findings impact our present understanding of tobacco-induced carcinogenesis.
Sex differences in BaP metabolism and DNA repair capacity have been documented (49), with several studies indicating that relative lung cancer risk among females exceeds that of males by 2.5-fold (21, 48, 60). In our study, the levels of BaP-DNA adducts were higher in the lungs of female mice compared with males, a finding consistent with the enhanced female susceptibility to tobacco carcinogens and sex-related differences in CYP gene expression between women and men (20, 37). Increased lung cancer susceptibility in females may also be strongly influenced by interactions between estrogen receptors and proteins involved in the regulation of hydrocarbon metabolism (16). Estrogen receptors are expressed in normal lung tissue as well as tumors (5) and strongly implicated in lung development (22).
In most human and rodent somatic cells, LINEs are silenced epigenetically via DNA methylation, a process that functions in tandem with LINE-1 deamination by DNA dC->dU-editing enzyme APOBEC (APOBEC) proteins, degradation of LINE-1 mRNA by three-prime repair exonuclease 1 (TREX1) and Piwi-interacting RNAs, and transcriptional repression by epigenetic modifying proteins (29). As such, activation of a LINE-1 transgene engineered to operate under the regulatory control of CRE recombinase in vivo provides compelling evidence that BaP overrides regulatory control to persistently activate LINE-1 transcription and to activate its genetic regulatory network. Given the potentially devastating consequences of LINE-1 reactivation, these findings suggest that LINE-1 and genes within its oncogenic regulatory network play a major role in the lung pathologic response to tobacco carcinogens. Although the degree to which transcriptional activation of LINE-1 and/or retrotransposition couples with DNA damage in vivo to modulate carcinogenesis requires further investigation, LINE-1 is associated with insertion mutations, genetic deletions, and reprogramming of epithelial differentiation (29). As such, the observed responses in vivo raise important questions about the contributions of retrotransposition-dependent and -independent mechanisms to BaP carcinogenesis in vivo. In accord, oncogenic transformation is associated with elevated LINE-1 expression in human lung tumors in the absence of changes in neighboring tissues (46).
Previous work by our group discretized a LINE-1 genetic regulatory network that is linked to host regulation of LINE-1 and oncogenic signaling (8, 45). In vivo validation of the functional integrity of this regulatory network in the lungs of ORFeus mice indicates that genes within the network are involved in the acute and adaptive responses of the lung to BaP. For instance, AhR-dependent genetic regulation of lung tissue has been associated with inflammation, DNA adduct formation, epithelial-mesenchymal transition, and tumorigenesis (23, 31, 57). The diffuse infiltration of mononuclear cells in BaP-treated lung tissue is consistent with the upregulation of CCL2, a major chemoattractant involved in leukocyte homing to sites of inflammation (32). The upregulated expression of MGST1 and CYP2A4 is often secondary to disruption of cellular defenses by electrophilic compounds through conjugation of reduced glutathione and compensatory feedback control (42). BaP induces genes involved in epithelial reprogramming such as POSTN (59) and PTPRB, the latter being a protein tyrosine phosphatase involved in oncogenic transformation (12). VCAM1, a member of the immunoglobulin superfamily (61), is elevated in idiopathic pulmonary fibrosis by TGF-β1 (1), a known inducer of epithelial dedifferentiation (44). Thus, our findings advance our understanding of the role of LINE-1 during the early stages of oncogenic signaling activated by tobacco lung carcinogens in vivo and provide a platform for evaluation of treatment modalities for precision management of thoracic malignancies.
GRANTS
Funding for this work was provided in part by University of Arizona Health Sciences and the Texas Governor’s University Research Initiative to K. S. Ramos, an Egyptian Science and Technology Development Fund Junior Scientist Development visit grant, Cycle 17, to A. A. I. Hassanin, and NIH Grants R01 ES-029382 and R01 HL-129794 to B. Moorthy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A.I.H., M.T.-G., B.M., and K.S.R. conceived and designed research; A.A.I.H., M.T.-G., and G.-D.Z. performed experiments; A.A.I.H., B.M., G.-D.Z., and K.S.R. analyzed data; A.A.I.H., M.T.-G., B.M., G.-D.Z., and K.S.R. interpreted results of experiments; A.A.I.H. prepared figures; A.A.I.H. drafted manuscript; A.A.I.H., M.T.-G., B.M., and K.S.R. edited and revised manuscript; K.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors express our gratitude to Prof. Salah Eldin Mesalhy Aly and Dr. Sherif Moawed, Suez Canal University, for assistance with interpretation of histological images and statistical analyses, respectively. We thank Dr. Jiang Chang, Texas A&M Institute of Biosciences and Technology, for assistance with graphics. We thank Dr. W. An of South Dakota State University for providing the parental ORFeus mouse strain used in these studies.
REFERENCES
- 1.Agassandian M, Tedrow JR, Sembrat J, Kass DJ, Zhang Y, Goncharova EA, Kaminski N, Mallampalli RK, Vuga LJ. VCAM-1 is a TGF-β1 inducible gene upregulated in idiopathic pulmonary fibrosis. Cell Signal 27: 2467–2473, 2015. doi: 10.1016/j.cellsig.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An W, Han JS, Wheelan SJ, Davis ES, Coombes CE, Ye P, Triplett C, Boeke JD. Active retrotransposition by a synthetic L1 element in mice. Proc Natl Acad Sci USA 103: 18662–18667, 2006. doi: 10.1073/pnas.0605300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arif JM, Shappell N, Sikka HC, Kumar S, Gupta RC. 32P-postlabeling analysis of lipophilic DNA adducts resulting from interaction with (±)-3-hydroxy-trans-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Chem Biol Interact 118: 87–97, 1999. doi: 10.1016/S0009-2797(98)00116-1. [DOI] [PubMed] [Google Scholar]
- 4.Badge RM, Alisch RS, Moran JV. ATLAS: a system to selectively identify human-specific L1 insertions. Am J Hum Genet 72: 823–838, 2003. doi: 10.1086/373939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baik CS, Eaton KD. Estrogen signaling in lung cancer: an opportunity for novel therapy. Cancers (Basel) 4: 969–988, 2012. doi: 10.3390/cancers4040969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banasiewicz M, Nelson G, Swank A, Grubor N, Ross J, Nesnow S, Köfeler H, Small GJ, Jankowiak R. Identification and quantitation of benzo[a]pyrene-derived DNA adducts formed at low adduction level in mice lung tissue. Anal Biochem 334: 390–400, 2004. doi: 10.1016/j.ab.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Beck CR, Collier P, Macfarlane C, Malig M, Kidd JM, Eichler EE, Badge RM, Moran JV. LINE-1 retrotransposition activity in human genomes. Cell 141: 1159–1170, 2010. doi: 10.1016/j.cell.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bojang P Jr, Roberts RA, Anderton MJ, Ramos KS. Reprogramming of the HepG2 genome by long interspersed nuclear element-1. Mol Oncol 7: 812–825, 2013. doi: 10.1016/j.molonc.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, Kazazian HH Jr. Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci USA 100: 5280–5285, 2003. doi: 10.1073/pnas.0831042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson CM, Largaespada DA. Insertional mutagenesis in mice: new perspectives and tools. Nat Rev Genet 6: 568–580, 2005. doi: 10.1038/nrg1638. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Dahlstrom JE, Chandra A, Board P, Rangasamy D. Prognostic value of LINE-1 retrotransposon expression and its subcellular localization in breast cancer. Breast Cancer Res Treat 136: 129–142, 2012. doi: 10.1007/s10549-012-2246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du Y, Grandis JR. Receptor-type protein tyrosine phosphatases in cancer. Chin J Cancer 34: 61–69, 2015. doi: 10.5732/cjc.014.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupuy AJ, Fritz S, Largaespada DA. Transposition and gene disruption in the male germline of the mouse. Genesis 30: 82–88, 2001. doi: 10.1002/gene.1037. [DOI] [PubMed] [Google Scholar]
- 14.Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci 152: 244–248, 2016. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Faulkner GJ, Kimura Y, Daub CO, Wani S, Plessy C, Irvine KM, Schroder K, Cloonan N, Steptoe AL, Lassmann T, Waki K, Hornig N, Arakawa T, Takahashi H, Kawai J, Forrest AR, Suzuki H, Hayashizaki Y, Hume DA, Orlando V, Grimmond SM, Carninci P. The regulated retrotransposon transcriptome of mammalian cells. Nat Genet 41: 563–571, 2009. doi: 10.1038/ng.368. [DOI] [PubMed] [Google Scholar]
- 16.Feng Q, Moran JV, Kazazian HH Jr, Boeke JD. Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell 87: 905–916, 1996. doi: 10.1016/S0092-8674(00)81997-2. [DOI] [PubMed] [Google Scholar]
- 17.Gelboin HV. Benzo[α]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev 60: 1107–1166, 1980. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg GL, Atherholt TB. DNA adduct formation in mouse tissues in relation to serum levels of benzo(a)pyrene-diol-epoxide after injection of benzo(a)pyrene or the diol-epoxide. Cancer Res 50: 1189–1194, 1990. [PubMed] [Google Scholar]
- 19.Grimaldi G, Singer MF. Members of the KpnI family of long interspersed repeated sequences join and interrupt α-satellite in the monkey genome. Nucleic Acids Res 11: 321–338, 1983. doi: 10.1093/nar/11.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugen A. Women who smoke: are women more susceptible to tobacco-induced lung cancer? Carcinogenesis 23: 227–229, 2002. doi: 10.1093/carcin/23.2.227. [DOI] [PubMed] [Google Scholar]
- 21.Henschke CI, Miettinen OS. Women’s susceptibility to tobacco carcinogens. Lung Cancer 43: 1–5, 2004. doi: 10.1016/j.lungcan.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Hsu LH, Chu NM, Kao SH. Estrogen, estrogen receptor and lung cancer. Int J Mol Sci 18: 1713, 2017. doi: 10.3390/ijms18081713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikuta T, Kawajiri K. Zinc finger transcription factor Slug is a novel target gene of aryl hydrocarbon receptor. Exp Cell Res 312: 3585–3594, 2006. doi: 10.1016/j.yexcr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Jordan IK, Rogozin IB, Glazko GV, Koonin EV. Origin of a substantial fraction of human regulatory sequences from transposable elements. Trends Genet 19: 68–72, 2003. doi: 10.1016/S0168-9525(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 25.Jurka J. Repeats in genomic DNA: mining and meaning. Curr Opin Struct Biol 8: 333–337, 1998. doi: 10.1016/S0959-440X(98)80067-5. [DOI] [PubMed] [Google Scholar]
- 26.Kang ZC, Tsai SJ, Lee H. Quercetin inhibits benzo[a]pyrene-induced DNA adducts in human Hep G2 cells by altering cytochrome P-450 1A1 gene expression. Nutr Cancer 35: 175–179, 1999. doi: 10.1207/S15327914NC352_12. [DOI] [PubMed] [Google Scholar]
- 27.Kapitulnik J, Wislocki PG, Levin W, Yagi H, Jerina DM, Conney AH. Tumorigenicity studies with diol-epoxides of benzo(a)pyrene which indicate that (±)-trans-7β,8α-dihydroxy-9α,10α-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene is an ultimate carcinogen in newborn mice. Cancer Res 38: 354–358, 1978. [PubMed] [Google Scholar]
- 28.Kasala ER, Bodduluru LN, Barua CC, Sriram CS, Gogoi R. Benzo(a)pyrene induced lung cancer: Role of dietary phytochemicals in chemoprevention. Pharmacol Rep 67: 996–1009, 2015. doi: 10.1016/j.pharep.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Khalid M, Bojang P Jr, Hassanin AAI, Bowers EC, Reyes-Reyes EM, Ramos IN, Ramos KS. Line-1: implications in the etiology of cancer, clinical applications, and pharmacologic targets. Mutat Res 778: 51–60, 2018. doi: 10.1016/j.mrrev.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 30. Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann Y, Stojanovic N, Subramanian A, Wyman D, Rogers J, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, International Human Genome Sequencing Consortium, et al . Initial sequencing and analysis of the human genome. Nature 409: 860–921, 2001. [Errata in Nature 411: 720 and 412: 565–566, 2001]. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 31.Lin P, Chang H, Ho WL, Wu MH, Su JM. Association of aryl hydrocarbon receptor and cytochrome P4501B1 expressions in human non-small cell lung cancers. Lung Cancer 42: 255–261, 2003. doi: 10.1016/S0169-5002(03)00359-3. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Das AM, Seideman J, Griswold D, Afuh CN, Kobayashi T, Abe S, Fang Q, Hashimoto M, Kim H, Wang X, Shen L, Kawasaki S, Rennard SI. The CC chemokine ligand 2 (CCL2) mediates fibroblast survival through IL-6. Am J Respir Cell Mol Biol 37: 121–128, 2007. doi: 10.1165/rcmb.2005-0253OC. [DOI] [PubMed] [Google Scholar]
- 33.Lu KP, Ramos KS. Identification of genes differentially expressed in vascular smooth muscle cells following benzo[a]pyrene challenge: implications for chemical atherogenesis. Biochem Biophys Res Commun 253: 828–833, 1998. doi: 10.1006/bbrc.1998.9866. [DOI] [PubMed] [Google Scholar]
- 34.Lu KP, Ramos KS. Redox regulation of a novel L1Md-A2 retrotransposon in vascular smooth muscle cells. J Biol Chem 278: 28201–28209, 2003. doi: 10.1074/jbc.M303888200. [DOI] [PubMed] [Google Scholar]
- 35.Mathias SL, Scott AF, Kazazian HH Jr, Boeke JD, Gabriel A. Reverse transcriptase encoded by a human transposable element. Science 254: 1808–1810, 1991. doi: 10.1126/science.1722352. [DOI] [PubMed] [Google Scholar]
- 36.Meyer BK, Perdew GH. Characterization of the AhR-hsp90-XAP2 core complex and the role of the immunophilin-related protein XAP2 in AhR stabilization. Biochemistry 38: 8907–8917, 1999. doi: 10.1021/bi982223w. [DOI] [PubMed] [Google Scholar]
- 37.Mollerup S, Berge G, Baera R, Skaug V, Hewer A, Phillips DH, Stangeland L, Haugen A. Sex differences in risk of lung cancer: Expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer 119: 741–744, 2006. doi: 10.1002/ijc.21891. [DOI] [PubMed] [Google Scholar]
- 38.Montoya-Durango DE, Liu Y, Teneng I, Kalbfleisch T, Lacy ME, Steffen MC, Ramos KS. Epigenetic control of mammalian LINE-1 retrotransposon by retinoblastoma proteins. Mutat Res 665: 20–28, 2009. doi: 10.1016/j.mrfmmm.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moorthy B, Randerath K. Pentachlorophenol enhances 9-hydroxybenzo [a] pyrene-induced hepatic DNA adduct formation in vivo and inhibits microsomal epoxide hydrolase and glutathione S-transferase activities in vitro: likely inhibition of epoxide detoxication by pentachlorophenol. Arch Toxicol 70: 696–703, 1996. doi: 10.1007/s002040050330. [DOI] [PubMed] [Google Scholar]
- 40.Moorthy B, Chu C, Carlin DJ. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci 145: 5–15, 2015. doi: 10.1093/toxsci/kfv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nebert DW, Vasiliou V. Analysis of the glutathione S-transferase (GST) gene family. Hum Genomics 1: 460–464, 2004. doi: 10.1186/1479-7364-1-6-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostertag EM, Kazazian HH Jr. Biology of mammalian L1 retrotransposons. Annu Rev Genet 35: 501–538, 2001. doi: 10.1146/annurev.genet.35.102401.091032. [DOI] [PubMed] [Google Scholar]
- 44.Pickup M, Novitskiy S, Moses HL. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer 13: 788–799, 2013. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos KS, He Q, Kalbfleisch T, Montoya-Durango DE, Teneng I, Stribinskis V, Brun M. Computational and biological inference of gene regulatory networks of the LINE-1 retrotransposon. Genomics 90: 176–185, 2007. doi: 10.1016/j.ygeno.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rangasamy D, Lenka N, Ohms S, Dahlstrom JE, Blackburn AC, Board PG. Activation of LINE-1 retrotransposon increases the risk of epithelial-mesenchymal transition and metastasis in epithelial cancer. Curr Mol Med 15: 588–597, 2015. doi: 10.2174/1566524015666150831130827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rengarajan T, Rajendran P, Nandakumar N, Lokeshkumar B, Rajendran P, Nishigaki I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac J Trop Biomed 5: 182–189, 2015. doi: 10.1016/S2221-1691(15)30003-4. [DOI] [Google Scholar]
- 48.Risch HA, Howe GR, Jain M, Burch JD, Holowaty EJ, Miller AB. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol 138: 281–293, 1993. doi: 10.1093/oxfordjournals.aje.a116857. [DOI] [PubMed] [Google Scholar]
- 49.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97: 339–346, 2005. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 50.Shiizaki K, Kawanishi M, Yagi T. Modulation of benzo[a]pyrene-DNA adduct formation by CYP1 inducer and inhibitor. Genes Environ 39: 14, 2017. doi: 10.1186/s41021-017-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singer MF. SINEs and LINEs: highly repeated short and long interspersed sequences in mammalian genomes. Cell 28: 433–434, 1982. doi: 10.1016/0092-8674(82)90194-5. [DOI] [PubMed] [Google Scholar]
- 52.Singer MF, Thayer RE, Grimaldi G, Lerman MI, Fanning TG. Homology between the KpnI primate and BamH1 (M1F-1) rodent families of long interspersed repeated sequences. Nucleic Acids Res 11: 5739–5745, 1983. doi: 10.1093/nar/11.16.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Symer DE, Connelly C, Szak ST, Caputo EM, Cost GJ, Parmigiani G, Boeke JD. Human l1 retrotransposition is associated with genetic instability in vivo. Cell 110: 327–338, 2002. doi: 10.1016/S0092-8674(02)00839-5. [DOI] [PubMed] [Google Scholar]
- 54.Teneng I, Stribinskis V, Ramos KS. Context-specific regulation of LINE-1. Genes Cells 12: 1101–1110, 2007. doi: 10.1111/j.1365-2443.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 55.Teneng I, Montoya-Durango DE, Quertermous JL, Lacy ME, Ramos KS. Reactivation of L1 retrotransposon by benzo(a)pyrene involves complex genetic and epigenetic regulation. Epigenetics 6: 355–367, 2011. doi: 10.4161/epi.6.3.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thornburg BG, Gotea V, Makałowski W. Transposable elements as a significant source of transcription regulating signals. Gene 365: 104–110, 2006. doi: 10.1016/j.gene.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 57.Tsay JJ, Tchou-Wong KM, Greenberg AK, Pass H, Rom WN. Aryl hydrocarbon receptor and lung cancer. Anticancer Res 33: 1247–1256, 2013. [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshino I, Kometani T, Shoji F, Osoegawa A, Ohba T, Kouso H, Takenaka T, Yohena T, Maehara Y. Induction of epithelial-mesenchymal transition-related genes by benzo[a]pyrene in lung cancer cells. Cancer 110: 369–374, 2007. doi: 10.1002/cncr.22728. [DOI] [PubMed] [Google Scholar]
- 60.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 88: 183–192, 1996. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 61.Zhang D, Yuan D, Shen J, Yan Y, Gong C, Gu J, Xue H, Qian Y, Zhang W, He X, Yao L, Ji Y, Shen A. Up-regulation of VCAM1 relates to neuronal apoptosis after intracerebral hemorrhage in adult rats. Neurochem Res 40: 1042–1052, 2015. doi: 10.1007/s11064-015-1561-x. [DOI] [PubMed] [Google Scholar]