Abstract
Histological observations in human pulmonary arterial hypertension (PAH) suggest a link between plexiform lesions and pulmonary supernumerary arteries. Pulmonary microvascular endothelial cells are characterized as hyperproliferative and progenitor-like. This study investigates the hypothesis that aneurysm-type plexiform lesions form in pulmonary supernumerary arteries because of their anatomical properties and endothelial characteristics similar to pulmonary microvascular endothelial cells. To induce PAH, rats were injected with Sugen5416, and exposed to hypoxia (10% O2) for 3 days (early stage) or 3 wk (mid-stage), or 3 wk of hypoxia with an additional 10 wk of normoxia (late-stage PAH). We examined morphology of pulmonary vasculature and vascular remodeling in lung serial sections from PAH and normal rats. Aneurysm-type plexiform lesions formed in small side branches of pulmonary arteries with morphological characteristics similar to supernumerary arteries. Over the course of PAH development, the number of Ki67-positive cells increased in small pulmonary arteries, including supernumerary arteries, whereas the number stayed consistently low in large pulmonary arteries. The increase in Ki67-positive cells was delayed in supernumerary arteries compared with small pulmonary arteries. In late-stage PAH, ~90% of small unconventional side branches that were likely to be supernumerary arteries were nearly closed. These results support our hypothesis that supernumerary arteries are the predominant site for aneurysm-type plexiform lesions in Sugen5416/hypoxia/normoxia-exposed PAH rats partly because of the combination of their unique anatomical properties and the hyperproliferative potential of endothelial cells. We propose that the delayed and extensive occlusive lesion formation in supernumerary arteries could be a preventive therapeutic target in patients with PAH.
Keywords: cell proliferation, dilatation lesion, occlusive lesion, plexiform lesions, supernumerary arteries
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a diverse group of diseases with poor prognosis, characterized by severe and progressive increase in pulmonary vascular resistance and extensive vascular remodeling (18, 27). It is generally thought that vascular remodeling and subsequent occlusion of small pulmonary arteries plays a significant role in the development and progression of PAH (7). We have recently proposed a concept that vasoconstriction and a consequent hemodynamic stress-dependent occlusive vascular remodeling create a vicious cycle to autonomously and progressively worsen PAH (1, 32).
The plexiform lesion, the pathognomonic hallmark of severe PAH, is a complex, disorganized proliferative lesion, featuring the plexus channel formation lined by endothelial marker-positive cells and separated by “core” cells (2, 22). Previously, we reported 2 morphologically different types of plexiform lesions in the Sugen5416/hypoxia/normoxia (SU/Hx/Nx)-exposed rat model of PAH. The stalk-type plexiform lesion is found within the arterial lumen, and the aneurysm-type plexiform lesion appears to project out of the artery into lung parenchyma (2), and similar plexiform lesion types are observed in human PAH patients as well (19, 33). The aneurysm type is the originally described plexiform lesion by Heath and Edwards in 1958 (8) and is likely the predominant type over the stalk type in human PAH, although no comparison or even clear description of these types has ever been made in human samples. One consistent and distinctive histological observation in the literature is that plexiform lesions form within aneurysm-like dilatations of small pulmonary arterial branches close to their origin from the parent vessels (aneurysm type) (8, 19). The branches containing this type of plexiform lesions have been suggested to be supernumerary arteries (5, 19, 33). Supernumerary arteries are unusual pulmonary arteries that do not have accompanying airway structures (4). They are characterized by acute, ~90° branching angles and smaller diameters compared with conventional arterial branches (11, 24). These structural properties, in addition to the sphincter-like structure at the orifice of these branches (4, 26), would discourage blood flow into supernumerary arteries. The branching pattern-dependent flow difference is indirectly supported by the absence of endothelial cell flow alignment in acutely branching arteries (16). It is speculated that plexiform lesions in aneurysm-like sacs are preferentially formed in supernumerary arteries because these arteries are prone to damage (19).
The genesis of occlusive pulmonary neointimal lesions in PAH is attributed to phenotypically different subtypes of endothelial cells that are apoptosis resistant, hyperproliferative, and angiogenic (25, 30). These phenotypes occur in endothelial cells in pulmonary microcirculation (15, 21), where neointimal occlusive lesions, including plexiform lesions, develop in severe PAH (3). Therefore, phenotypic heterogeneity in pulmonary endothelium may be critical to understanding the pathogenesis of neointimal occlusive lesions. Although endothelial cells in supernumerary arteries have not been characterized, those endothelial cells could conceivably possess similar phenotypes to pulmonary microvascular endothelial cells based on the small diameter of supernumerary arteries and their increased frequency in distal lung (4).
Based on this background, we hypothesize that aneurysm-type plexiform lesions originate in supernumerary arteries where endothelial cells have similar characteristics to those of the pulmonary microcirculation, specifically hyperproliferative potential, which leads to progressive proliferation when they are exposed to high hemodynamic stress. We investigated this hypothesis by histological examination of lungs from SU/Hx/Nx-exposed rats at three different time points in PAH progression. We examined early-stage PAH [3 days after the induction of PAH when right ventricular systolic pressure (RVSP) increases minimally but significantly], mid-stage PAH (3 wk after the induction of PAH when RVSP is near its maximum but severe PAH is not yet established and mature plexiform lesions are not observed), and late-stage PAH (~13 wk after initiation when mature plexiform lesions are frequently found) (2, 29).
MATERIALS AND METHODS
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of South Alabama. Adult male Sprague Dawley rats weighing 160–200 g were used for the study. PAH was induced in rats by single subcutaneous injection of the VEGF receptor blocker, Sugen5416 (20 mg/kg, Cayman), suspended in carboxymethyl cellulose, and subsequent exposure to normobaric hypoxia (10% O2) for 3 days or 3 wk, as previously described (2). For late-stage PAH rats, after 3-wk hypoxia, animals were then returned to normoxia (21% O2) for an additional 10 wk. Control rats were kept in normobaric normoxia.
Hemodynamic Measurements
Animals were anesthetized with pentobarbital sodium (40 mg/kg, i.p.) and placed on a controlled heating pad. RVSP was measured with a polyvinyl catheter connected to a disposable blood pressure transducer and bridge amplifier (FE224, ADInstruments). The polyvinyl catheter (PV-1, internal diameter: 0.28 mm) was inserted into the right ventricle via the right jugular vein. Signals were routed through an analog to digital converter (ML785, ADInstruments) and recorded on a computer using data acquisition software (LabChart Pro v7, ADInstruments) to measure and record RVSP. We ensured that the animals were anesthetized by the lack of toe pinch reflex throughout the experimental procedure, and, if needed, additional pentobarbital was given to animals.
Preparation of Tissue Sections
Lungs were inflated with 10% formalin plus 0.5% agarose via gravitational airway instillation at 20 cm H2O and submerged overnight in 10% formalin. The tissue blocks were prepared from left lung around the hilar region, and embedded in paraffin. Sections of 5 μm thickness were cut from each animal and stained with H&E. One hundred consecutive sections were prepared from control and late-stage PAH rat lungs (n = 5 each) at 5-μm thickness each, with total tissue loss of less than 10 μm. Therefore, ~0.5 mm of lung thickness (100 sections × 5 μm) was examined from each animal.
Immunohistochemistry
Sections were stained immunohistochemically with either von Willebrand factor (vWF) or Ki67 for morphological examination. Immunohistochemical staining was performed as previously described (2). Briefly, the histological sections were rehydrated and antigens were retrieved with citrate buffer (pH 6) and heat. The endogenous peroxide activity was inhibited by incubation with 3% hydrogen peroxide. The sections were incubated with primary antibodies against vWF (Affinity Biological, SARTW-IG) or Ki67 (Thermo Scientific, RM-9106-S1) overnight at 4°C. Biotinylated secondary antibodies were applied, and the target antigens were visualized with 3,3′-diaminobenzidine peroxide substrate system (Thermo Scientific, TA-125-HDX). The sections were counterstained with hematoxylin (Thermo Scientific, 7211) and dehydrated and mounted.
Histomorphological Evaluation
Aneurysm-type plexiform lesions and their distally connected vessels.
Aneurysm-type plexiform lesion was defined as a vascular lesion that projected out of a parent artery into the lung parenchyma (as opposed to stalk type that forms within the vessel lumen) and had a complex, disorganized morphology. The lesions had nonconcentric organization of cellular components, with the channel (plexus) formations lined by vWF-positive cells that were separated by oval-shaped and chromatin-rich core cells (2). We randomly identified 20 mature aneurysm-type plexiform lesions in lung serial sections from five late-stage PAH rats. We then examined each of the lesions serially concerning topological relationships among the plexiform lesion and its parent artery and structural characterization of the distal vessel connected to the lesion.
Serial evaluation of pulmonary arterial branches and occlusive lesions.
We next assessed types of pulmonary arterial branches and pathological lesions that occluded them. Pulmonary arteries with associated airways were followed through 100 serial sections. The arterial segments with an external diameter less than 150 μm were chosen to compare the similar range of arteries that develop pathological remodeling in pulmonary hypertension (29). Side branches of 19 arterial segments (0.5 mm in length) from five late-stage PAH rats (3–4 segments per animal) were examined.
classification of arterial side branches.
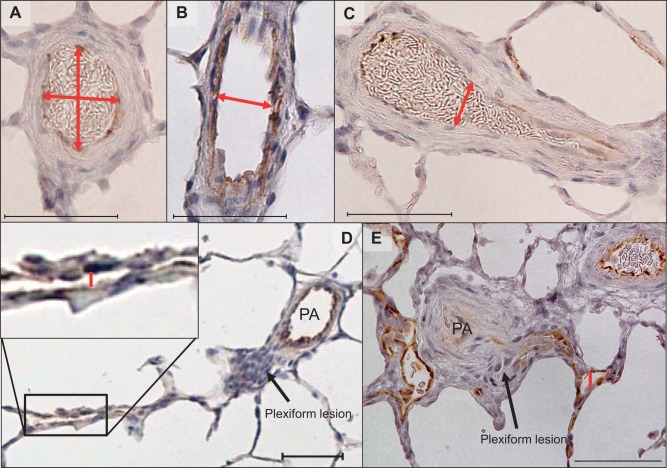
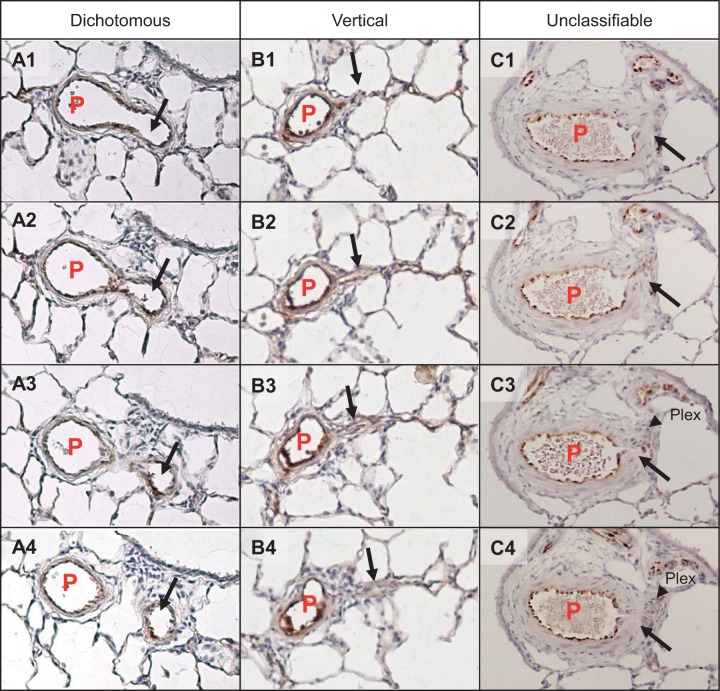
We attempted to identify supernumerary branches by the original defining criterion, which is the absence of accompanying airway, but this was found impossible due to the airway transitions into alveolar ducts in distal areas where this study was focused. It was also found difficult to accurately determine the angle of side branches (~90° for supernumerary arteries), particularly when they were severely remodeled. We thus identified supernumerary branches based on the combination of diameter ratio of branches to their parent arteries (size criterion) and approximate branching angles estimated by branching patterns (angle criterion) described below. In limited literature on supernumerary arteries, it has been reported that the mean diameter of supernumerary arteries in human fetal lungs is ~20% that of conventional pulmonary arteries (11), and our previous report has shown that the diameter ratio in rat lungs is ~28% (55.6 + 3.4 versus 15.5 + 0.8 μm; parent versus supernumerary arteries) (20). The diameters of dichotomous branches ranged from 20% to 100% of those of parent pulmonary arteries in bovine and rat lungs (12, 26). Thus, for the size criterion, we considered side branches as dichotomous when their diameter exceeded 20% of that of the parent artery and as supernumerary when their ratio was less than 20%. This stringent criterion might have misidentified some unusually large supernumerary arteries but should have excluded dichotomous branches. Inner diameters of parent and branch arteries were measured as follows (Fig. 1): For cross-sectional parent arteries (aspect ratio < 2), the mean of the longest and shortest diameters was used (Fig. 1A); for longitudinally cut parent arteries (aspect ratio > 2) (Fig. 1B), the width of the arteries at the widest axis was used; for longitudinal cut branches without severe occlusions at the branching point, the width of the branch at the branching point was used (Fig. 1C); and for many cases when the branch appeared collapsed after the occlusion with plexiform/plexiform-like lesions, the widest point of its downstream open part was used (Fig. 1D), except when there was a focal abnormally dilated part of the vessel (dilatation lesion) that was excluded from the diameter measurement (Fig. 1E). For the angle criterion, we assessed the pulmonary arterial branching pattern by morphology to roughly determine branch angles as follows: dichotomous type: two cross-sectional vessel lumens were clearly identified right below the branching point (Fig. 2, A1–A4); vertical type: only one cross and attaching longitudinal arterial luminal sections were identified (Fig. 2, B1–B4); and unclassifiable type: morphology of the branching point was unclear because of severe arterial remodeling (Fig. 2, C1–C4). Based on these criteria, branches having both of the following characteristics, i.e., diameter ratio > 20% and dichotomous-type branching pattern, were classified as “dichotomous,” and those having both diameter ratio ≤ 20% and vertical-type branching pattern were classified as “supernumerary.” For branches identified that had diameter ratio ≤ 20% but unclear branching pattern because of severe occlusive lesions, we further examined structural characteristics (vessel length and wall thickness) of their distal vessels connected to the lesions to see whether their characteristics agree with those of supernumerary arteries.
Fig. 1.
Examples of diameter measurements for parent and arterial branches. Red double-headed arrows/lines indicate the measured axes. A: cross-sectional parent arteries. B: longitudinally cut parent arteries. C: longitudinally cut branches without severe occlusive lesion. D: longitudinally cut branches with severe occlusive (plexiform) lesion with a continuously open part (red line). PA, parent artery. E: longitudinally cut branches with severe occlusive (plexiform) and dilatation (red line) lesions. Scale bars, 50 μm.
Fig. 2.
Examples of branching patterns observed in serial sections. A1–4: dichotomous type. B1–4: vertical type. C1–4: unclassifiable type. Images shown are serial sections. P, parent artery; black arrow, daughter branch; black arrowhead, plexiform lesion.
occlusion degree.
The degree of occlusions was scored as follows: no evidence of neointimal formation (Grade 0); partial (<50%) luminal occlusion (Grade I); severe-luminal occlusion (50%–90%, Grade II); and near closure (>90%, Grade III).
Cellular Proliferation
To determine whether vascular cells in small pulmonary arteries, including supernumerary arteries, have a hyperproliferative potential in response to hemodynamic stress, we evaluated the number of Ki67-positive cells in the intimal region of supernumerary and small (<100 μm) and large (>100 μm) pulmonary arteries (at least 5 vessels from each lung) from normal, early-stage (3 day), mid-stage (3 wk), and late-stage (13 wk) PAH rats (n = 4–5 each). Ki67-positive cells were counted and standardized per total number of innermost layer of cells. Obvious blood components (white blood cells) that appeared positive for Ki67 were excluded from quantifications.
Statistical Analysis
Measurements are expressed as means ± SE. Comparisons between the groups were made with one-way ANOVA with Bonferroni’s multiple comparison test, with GraphPad Prism version 4.0. Differences were considered significant at P < 0.05.
RESULTS
Evidence of Pulmonary Hypertension
All SU/Hx/Nx-exposed rats developed elevated RVSP. At the 3-day time point, rats had a mild but significant increase in RVSP (37 ± 2 mmHg, n = 5) compared with normal rats (28 ± 1 mmHg, n = 8; P < 0.05). Consistent with the previous report (29), at the 3-wk time point, rats showed a marked increase in RVSP (79 ± 6 mmHg, n = 5), and it further increased at the 13-wk time point (114 ± 4 mmHg, n = 5).
Development of the Aneurysm-Type Plexiform Lesion
In agreement with our previous reports (2, 29), mature aneurysm-type plexiform lesions were found only in the late-stage PAH rats, while concentric intimal lesions (Heath and Edwards’ grade 2) (8) were already abundant in the mid-stage (3-wk) PAH rat lungs (data not shown). The aneurysm-type lesions exhibited the distinguishing features of plexiform lesions, i.e., a complex and disorganized plexus-like structure comprising clusters of large, oval, and chromatin-rich, so-called “core” cells, which were separated by channels lined by vWF-positive cells. They were growing inside the aneurysm-like sac structures that were attached to thick-walled parent pulmonary arteries (Fig. 3, A and B). Figure 3, C and C’, shows a small aneurysm-type “plexiform-like” lesion growing inside a thin-walled small artery having almost all features of a plexiform lesion (a cluster of core cells wrapped with endothelial marker-positive cells), except for the typical slit-like channel formation.
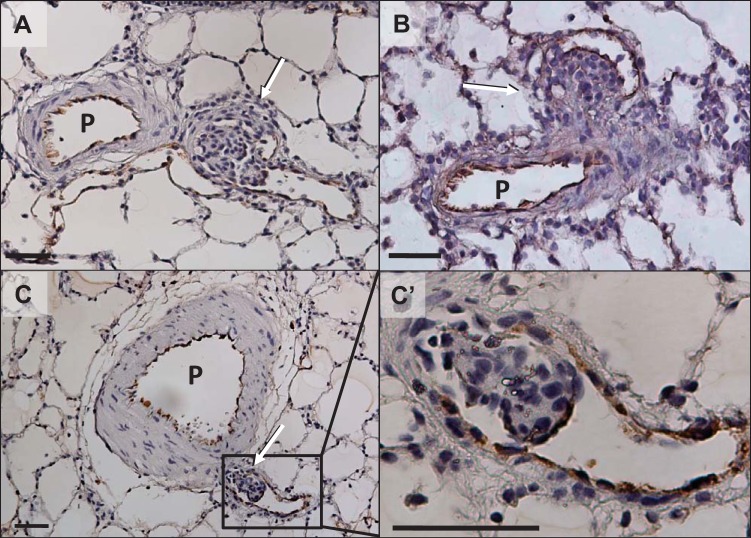
Fig. 3.
Representative photomicrographs of von Willebrand factor-stained aneurysm-type plexiform lesions in late-stage (13-wk time point) pulmonary arterial hypertension (PAH) rat lungs. A and B: typical mature lesions. C: small cellular lesion growing inside a thin-walled small branch artery with almost all plexiform cellular features except for the typical slit-like channel formation (plexiform-like lesion). C’: magnified area outlined in C. P, parent pulmonary artery; arrows indicate the complex lesion. Scale bars, 50 μm.
Detailed Structural Examination of the Aneurysm-Type Plexiform Lesion in Relation to Its Distally Connected Vessel
Serial section analyses of 20 randomly identified aneurysm-type plexiform lesions in 5 late-stage PAH lungs revealed that all lesions had direct connection with their parent arteries. We found two types of vessels distally connected to the complex lesion, all of which eventually connected to capillaries: 1) a short vessel without (n = 9/20, Fig. 4A) and with (n = 7/20, Fig. 4B) a thin-walled dilated lesion and 2) a long veinlike vessel with and without dilation (n = 4/20, Fig. 4C). In some cases, independent of the types, we found dilated thin-walled vessel structures (dilatation lesions) in close vicinity to the plexiform lesion that do not appear to be connected to the complex lesion (Fig. 4B, red arrowheads).
Fig. 4.
Representative photomicrographs of von Willebrand factor-stained different types of vessels connected distally to aneurysm-type plexiform lesions in the late-stage (13-wk time point) pulmonary arterial hypertension (PAH) rat lungs. A and B: very short types of distal vessels without (A) and with (B) a dilation lesion. C: a long type of distal vessel with veinlike structure. P, parent pulmonary artery; white arrows with black shade, complex lesion; white arrow heads with black shade, capillaries; red arrows and arrowheads, dilatation lesions connected and unconnected to the complex lesion, respectively; blue arrowheads, veinlike vessel. Scale bars, 50 μm.
Serial Analysis of Hypertensive Pulmonary Arterial Branches
Classification of arterial side branch.
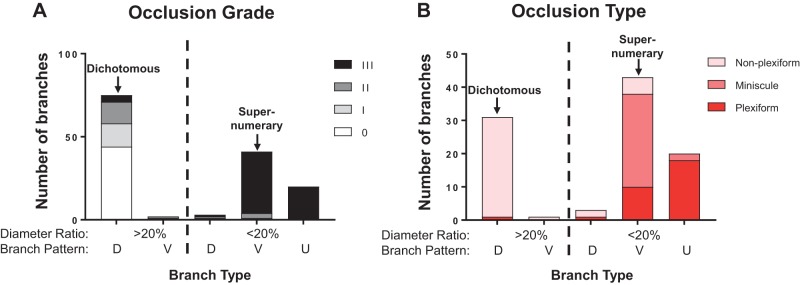
We identified 141 side branches in 19 pulmonary arterial segments from 5 late-stage PAH rats. As shown in Fig. 5A, 77 and 64 branches had diameter ratio > 20% (large) and ≤20% (small), respectively. Seventy-five large diameter ratio branches with a dichotomous-type branching pattern were classified as dichotomous, and 42 small diameter ratio branches with a vertical-type branching pattern were classified as supernumerary. In addition, there were branches we could not classify as either dichotomous or supernumerary by our definition using the size and angle criteria; i.e., two having a small diameter ratio with dichotomous-type branching pattern, and three having a large diameter ratio with vertical-type branching pattern, and 20 with a small diameter ratio whose branching patterns were unclassifiable because of severe remodeling. We further examined the 20 small diameter ratio branches with unidentifiable branching pattern in detail for structural characteristics of their distal vessels in an attempt to determine whether they were dichotomous or supernumerary branches. Analyses showed that their distal vessels were very thin walled, and, as described in the previous paragraph, there were 2 length types of distal vessels: short (15/20 = 75%) and long veinlike vessels (5/20 = 25%).
Fig. 5.
Quantification of occlusion grades and types and branch patterns of pulmonary arterial hypertension (PAH) rat (n = 5) pulmonary arteries. A: quantification of occlusion grades in relation to branch types. B: occlusion types in relation to branch types. Grade 0 lesions were not included in this bar graph. D, dichotomous; V, vertical; U, unclassified.
Occlusion type and degree.
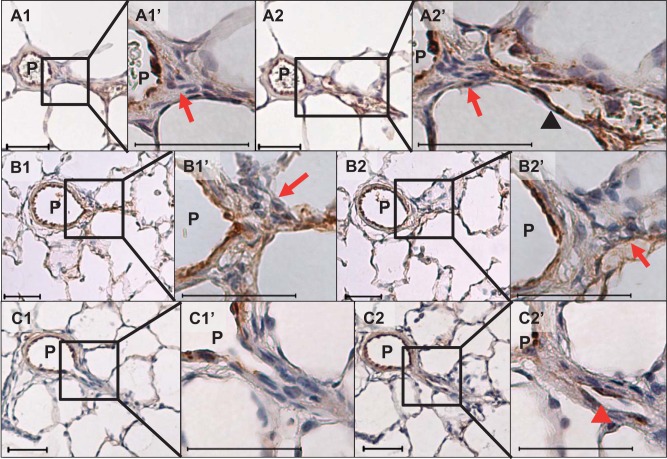
In our data set, we identified three morphological occlusion types: The first type was associated with a mature aneurysm-type plexiform lesion (Fig. 4, A–C). The second type appeared to be caused by a minuscule/small lesion (minuscule type) with an accumulation of “core cell”-like cells, but the occlusion was always severe (Grade III) and the branches were short and, almost disappeared/collapsed, and connected directly to capillaries (Fig. 6, A and B). For the third type, nonplexiform/concentric laminar, in contrast to other types, the wall of its distal segment was remodeled with concentric laminar thickening that appeared similar to what is reported in Heath and Edwards’ grade 2/3 vessels (8) (Fig. 6C). As shown in Fig. 5, A and B, a majority of branches classified as dichotomous had no intimal thickening (occlusion grade 0), and the rest had only mild to moderate (grade I or II) occlusions with nonplexiform/concentric laminar lesions, except for three branches that were found to have Grade III occlusion (3/77 = 5%; Fig. 5A). Among them, while two occlusions showed nonplexiform-type lesions, we observed one severe occlusion associated with a complex and disorganized lesion, which we classified as a stalk-like plexiform lesion because it apparently formed inside the lumen of a pulmonary artery with a thickened wall (Supplemental Fig. S1; Supplemental Material is available at https://doi.org/10.6084/m9.figshare.7820339), in contrast to the complex lesion formation inside a thin-walled sac-like structure in aneurysm-type plexiform lesions. In stark contrast, all but 6 (58/64 = 91%) small branches (diameter ratio ≤ 20%), including supernumerary branches, were severely occluded (Grade III), mostly with either plexiform or minuscule-type lesions (Fig. 5B).
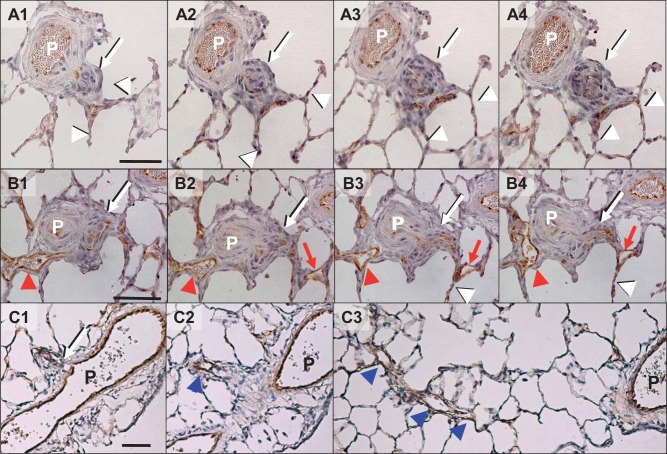
Fig. 6.
Representative photomicrographs of two additional types of von Willebrand factor-stained occlusive lesions at the branching point of supernumerary arteries of the late-stage (13-wk time point) pulmonary arterial hypertension (PAH) rat lungs. A and B: serial sections of a minuscule type of occlusive lesion with longer veinlike distal branch [A1 and A2 and their respective high magnification of the branching point (A1’ and A2’)] and disappearing/collapsed branches [B1 and B2 and their respective high magnification of the branching point (B1’ and B2’)]. Note that there was accumulation of “core cell”-like cells at the branching points (red arrows). C: serial section of nonplexiform type of occlusive lesion [C1 and C2 and their respective high magnification of the branching point (C1’ and C2’)]. Note the contrast of thin veinlike (black arrowhead in A2’) and thickened walls of the branches (red arrowhead in C2’). Scale bars, 50 μm.
Temporal Changes in Ki67-Positive Cell Number in Pulmonary Arteries
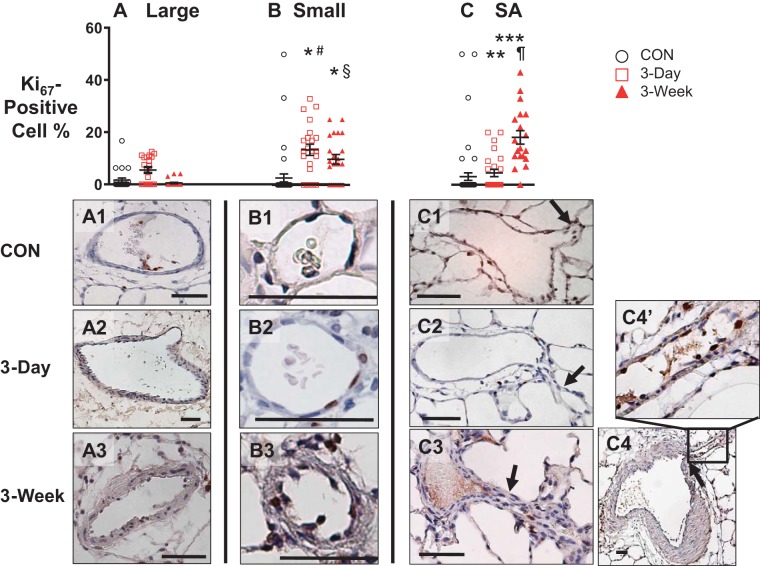
As shown in Fig. 7, the temporal Ki67-positive cell numbers among intimal cells were different depending on the pulmonary arterial size and type. In large pulmonary arteries, the percent of Ki67-positive cells stayed low over time (Fig. 7A). In contrast, the percentage of positive cells in small pulmonary arteries increased by the 3-day time point and stayed high (Fig. 7B), whereas in supernumerary arteries, the percentage did not increase until the 3-wk time point (Fig. 7C). A stark contrast in Ki67 expression between a large parent pulmonary artery and its supernumerary branch at the 3-wk time point is shown in Fig. 7, C4 and C4’. In contrast to a few Ki67-positive cells in the large parent pulmonary artery, many innermost layer cells in a supernumerary branch were positively stained with Ki67 (Fig. 7, C4 and C4’).
Fig. 7.
Top: time course of changes in percentages of Ki67-positive cells in innermost layer of large (Large, A) and small conventional pulmonary (Small, B) and supernumerary arteries (SA, C) at before (CON, black circle), 3 days after (red square), and 3 wk (red triangle) after Sugen5416/hypoxia-exposure. One-way ANOVA with Bonferroni’s multiple comparison test. *P < 0.05 vs. small CON, **vs. SA CON, ***vs. SA 3-day, #vs. large 3-day, §vs. large 3-wk, ¶vs. SA 3-day; n = 4–5. Each data point represents a vessel. Bottom: representative photomicrographs of Ki67-stained large (A1–A3) and small (B1–B3) pulmonary arteries, and supernumerary and its parent arteries (C1–C4 and C4’). Black arrow, supernumerary branch. Scale bars indicate 50 μm.
DISCUSSION
This study described the development of aneurysm-type plexiform lesions in pulmonary supernumerary arteries and demonstrated that most of the supernumerary arteries are occluded in severe PAH.
One of the unsettled major controversies regarding the plexiform lesions is where they form. There have been multiple hypotheses, including involvement of supernumerary arteries (5, 14, 33), branching points (3), and the bronchial circulation (6). We examined 20 mature plexiform lesions and their distally connecting vessels in detail with an animal model that develops PAH arteriopathy in an attempt to determine where the lesion originates, focusing on the aneurysm-type plexiform lesion (2). We found that there were two types of vessels distal to plexiform lesions, very short (16/20 = 80%) and long (4/20 = 20%) segments, both of which eventually drained into capillaries. In fact, nine of the very short type had almost no connecting vessel length between the complex lesions and capillaries (Fig. 2A). The rest had only short dilated thin-walled vessel structures between lesion and capillaries (Fig. 2B), and they thus appeared too short to be conventional pulmonary arterial branches or other type of arteries, including bronchial arteries. The long type had a thin-walled, veinlike structure with a much smaller (<20%) diameter than their parent arteries (Fig. 2C). These observations, especially the smaller size of the connecting vessels, suggest that these branches are likely to be supernumerary rather than conventional dichotomous branches. We speculate that the very short and long types of vessels account for the previously described accessory (short) and aberrant (long) supernumerary arteries, respectively (24). In addition, we observed no aneurysm-type plexiform lesion formation in conventional branches classified by the size criteria and, in agreement with a majority of previous observations in human PAH (14, 19, 33), no clear interactions between plexiform lesions and the bronchial circulation.
A recent review article summarizing a session at the latest World Symposium on pulmonary hypertension has highlighted the possibility that the bronchial circulation might contribute significantly to the pathogenesis of the plexiform lesions (13). It is true that there are a few case report-type publications (unfortunately without any detailed analytical data of multiple lesions) showing a connection between the plexiform lesion and the bronchial circulation in human PAH, including a recent one (6, 10). There are, however, numerous articles that counter these limited reports. It should be noted that at least two of the studies had performed detailed extensive morphological analyses on multiple plexiform lesions and their connecting vessels and denied the direct relationship between plexiform lesions and bronchial circulation (14, 33). It appears clear from the literature, therefore, that although there may be limited interactions between the plexiform lesions and the bronchial circulation, most of the lesions have no direct connection with the bronchial circulation in human PAH. Our study, for the first time, experimentally supports the majority of those observations made in human PAH.
We frequently observed abnormally dilated, thin-walled, short vascular segments connecting the plexiform lesions to capillaries. These dilated vessel segments may be equivalent to the dilatation lesions (Heath and Edwards’ grade 5) (8) often seen in human PAH lungs. We also found in some cases thin-walled dilated vessel structures close to the plexiform lesions that did not appear to have any direct connections to the complex lesion. It is unclear how those limited parts of thin-walled vessels dilate and what the relationship is between the complex lesions and the unconnected dilatation lesions.
In our serial examination of pulmonary arterial segments, we classified 75 (53%) and 42 (30%) branches as dichotomous (large diameter ratio with dichotomous-type branching pattern) and supernumerary (small diameter ratio with vertical-type branching pattern) by the definition, respectively. Two large diameter ratio branches with a vertical-type branching pattern could be unusually large supernumerary arteries. Although their branching patterns were unclear because of extending complex lesions at the branching points in 20 small diameter ratio branches, our additional analyses of the branches distal to the lesion revealed that their characteristics agreed with those of supernumerary arteries, indicating these small branches could be supernumerary, but not other types of branches. The three small diameter ratio branches that appeared to have dichotomous-type branching pattern by our criteria could not be dichotomous because their diameter ratios were way too small, and they could also be supernumerary or an unknown type of a small branch. Additionally, unlike the uncertainties in identifying supernumerary branches, our identification of 75 dichotomous branches (75/141 = 53% of total branches) should be accurate because of its solid definition. Therefore, based on a previous report that ~50% of pulmonary arterial branches in rats are supernumerary (12), it is highly unlikely that there were more dichotomous branches in the rest (47%), supporting our assessment that most, if not all, small diameter ratio branches were supernumerary. We acknowledge that there is a possible concern of distal dichotomous branches being degenerated because of obstruction, appearing like supernumerary branches. However, there are three cases in which large dichotomous branches were nearly closed but maintained relatively large diameters and thick walls (an example is shown in Supplemental Fig. S1), and therefore, we think it is unlikely that we mistakenly classified such vessels as supernumerary arteries.
Our serial examination also revealed that while occlusions of large diameter ratio branches were only mild to moderate, if any, except for three branches (3/77 = 4%), most small diameter ratio branches (58/64 = 91%) were nearly closed in the setting of severe established PAH. We found, in addition to the plexiform/plexiform-like lesions, two more morphologically different types of occlusive lesions in small diameter ratio branches. One was “minuscule lesions” that always occluded vessels severely and appeared to be caused by an accumulation of a small number of “core cell”-like cells. It is not clear whether these minuscule lesions were very early plexiform or unrelated lesions, but the involvement of “core cell”-like cells suggests the former. The other lesion was a nonplexiform or laminar intimal thickening type, which we found less frequently and caused less severe occlusions. It should be noted that, in agreement with results of our first morphological assessment that all vessels distal to typical aneurism-type plexiform lesions possessed characteristics similar to those of a supernumerary artery (2), our current serial examination also found aneurysm-type plexiform lesions that were observed exclusively and abundantly in small supernumerary branches. In the case of dichotomous branches, in contrast, plexiform lesions were extremely rare, and only one stalk type but no aneurysm-type plexiform lesion was observed.
There are at least two kinds of morphologically distinct lumen obliterating intimal lesions in PAH, i.e., the laminar intimal thickening (Heath and Edwards’ grade 2 and 3) that distributes widely in peripheral small conventional arteries and the disorganized cellular proliferation (Heath and Edwards’ grade 4 or plexiform lesion) that develops predominantly in small supernumerary branches (33). The reason why these occlusive lesions develop exclusively in small pulmonary arteries of PAH patients is unknown. It has been proposed that phenotypically distinct endothelial characteristics, specifically, the hyperproliferative potential of pulmonary microvascular endothelial cells, may play a role in this unexplained phenomenon (15). The phenotype of pulmonary supernumerary endothelial cells has never been characterized. This study investigated the hypothesis that supernumerary arterial endothelial cells have a hyperproliferative potential similar to that of the microvascular endothelial cells by examining endothelial Ki67 expression as a cell proliferation marker during the development of PAH. We found that, in contrast to consistent low expression of Ki67 in large pulmonary arteries, its expression was elevated in both small conventional and supernumerary arterial endothelial cells over the course of PAH development. These results support the idea that pulmonary supernumerary endothelial cells also have a hyperproliferative potential. An interesting observation is that while Ki67 expression increased from the early time point in small conventional pulmonary arteries when PAH was mild, its elevation in supernumerary arteries was delayed until more severe PAH developed. We speculate that increased hemodynamic stress induced Ki67 expression and that this increase was delayed in supernumerary arteries where blood flow/hemodynamic stress was limited until severe PAH developed. This speculation is supported by our recent findings that hemodynamic stress is essential to the development of the pulmonary plexogenic arteriopathy in pulmonary hypertension (1), and blood flow to pulmonary supernumerary arteries is limited during the moderate pulmonary hypertension induced by chronic hypoxia (20). Further experiments are needed to investigate whether endothelial cellular characteristics of pulmonary microvascular vessels and supernumerary arteries are the same or different and why, in response to hemodynamic stress, different types of lesions develop in these two types of small pulmonary arteries, i.e., laminar intimal thickening in small conventional arteries versus aneurysm-type plexiform lesions in supernumerary arteries, and whether those factors contribute to formation of different types of occlusive lesions.
The number of pulmonary supernumerary arteries is indeed abundant. In human lungs, there are more supernumerary than dichotomous branches, which accounts for a significant surface area of arterial side branches, up to 40% (24). Although the functional roles of supernumerary arteries are poorly understood, one possibility is that they serve as a recruitment/collateral pathway when distal pulmonary arterial obstruction or elevated pulmonary blood flow/pressure occurs (17, 23, 26, 31). Our findings that nearly all of these arteries are functionally lost in late PAH would indicate that an innate, effective mechanism to reduce abnormally increased pulmonary blood flow/pressure is lost in severe PAH. This may have a potential major therapeutic significance. It is well established that plexiform lesions are formed in most PAH cases at the time of autopsy or lung transplantation (28). Although it is unknown whether these complex lesions have developed at the time of diagnosis, limited reports of human PAH cases (9) suggest they are not observed at earlier stages of the disorder. Additionally, we have shown that no significant numbers of plexiform-type occlusions are observed until the late-stage PAH (13 wk) in the SU/Hx/Nx-exposed PAH rats, in which the 3~5-wk time point is reported to correspond to the time of diagnosis in humans (29). Taken together, it is reasonable to assume that in many cases, plexiform lesions may not have developed at the time of diagnosis. Therefore, based on our finding of the delayed development of the occlusive lesions in supernumerary arteries until PAH becomes severe, we speculate that these lesions may be a realistic therapeutic target to prevent, but not necessarily reverse, vascular remodeling, if the diagnosis is made with early symptoms. It therefore appears very important to fully elucidate the pathogenesis of occlusive lesions in supernumerary arteries over the course of PAH development.
In conclusion, our observations support the hypothesis that aneurysm-type plexiform lesions form in supernumerary arteries in SU/Hx/Nx-exposed PAH rats due, in part, to their unique anatomical properties and the hyperproliferative potential of their endothelial cells. We further hypothesize that the delayed and extensive occlusive lesion formation in supernumerary arteries could be a preventive therapeutic target at the time of diagnosis in patients with PAH. Our results warrant further investigation of the currently understudied vessels in the pulmonary circulation: the supernumerary arteries.
GRANTS
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute Program Project Grant HL-66299 and the Department of Pharmacology and the Center for Lung Biology, University of South Alabama.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.O. and M.O. conceived and designed research; K.O., S.R.J., J.M.M., Y.M., and K.A. performed experiments; K.O., E.S.C., I.F.M., and M.O. analyzed data; K.O. interpreted results of experiments; K.O., E.S.C., and M.O. prepared figures; K.O. and M.O. drafted manuscript; K.O., E.S.C., S.R.J., J.M.M., Y.M., I.F.M., and M.O. edited and revised manuscript; K.O., E.S.C., S.R.J., J.M.M., Y.M., I.F.M., K.A., and M.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the histological assistance from the Department of Pathology at Kyushu University and the technical assistance from Akiko Ando at Kyushu University.
Part of the data was presented previously in the form of an abstract (Am J Respir Crit Care Med 187: A5388, 2013).
REFERENCES
- 1.Abe K, Shinoda M, Tanaka M, Kuwabara Y, Yoshida K, Hirooka Y, McMurtry IF, Oka M, Sunagawa K. Haemodynamic unloading reverses occlusive vascular lesions in severe pulmonary hypertension. Cardiovasc Res 111: 16–25, 2016. doi: 10.1093/cvr/cvw070. [DOI] [PubMed] [Google Scholar]
- 2.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 3.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 155: 411–419, 1999. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott FM, Reid L. Some new facts about the pulmonary artery and its branching pattern. Clin Radiol 16: 193–198, 1965. doi: 10.1016/S0009-9260(65)80042-3. [DOI] [PubMed] [Google Scholar]
- 5.Fishman AP. Changing concepts of the pulmonary plexiform lesion. Physiol Res 49: 485–492, 2000. [PubMed] [Google Scholar]
- 6.Galambos C, Sims-Lucas S, Abman SH, Cool CD. Intrapulmonary bronchopulmonary anastomoses and plexiform lesions in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 193: 574–576, 2016. doi: 10.1164/rccm.201507-1508LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guignabert C, Tu L, Girerd B, Ricard N, Huertas A, Montani D, Humbert M. New molecular targets of pulmonary vascular remodeling in pulmonary arterial hypertension: importance of endothelial communication. Chest 147: 529–537, 2015. doi: 10.1378/chest.14-0862. [DOI] [PubMed] [Google Scholar]
- 8.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 18: 533–547, 1958. doi: 10.1161/01.CIR.18.4.533. [DOI] [PubMed] [Google Scholar]
- 9.Heath D, Smith P, Gosney J, Mulcahy D, Fox K, Yacoub M, Harris P. The pathology of the early and late stages of primary pulmonary hypertension. Br Heart J 58: 204–213, 1987. doi: 10.1136/hrt.58.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heath D, Smith P, Harris P, Yacoub M. Plexiform lesion in bronchopulmonary anastomosis. Br J Dis Chest 82: 294–299, 1988. doi: 10.1016/0007-0971(88)90072-1. [DOI] [PubMed] [Google Scholar]
- 11.Hislop A, Reid L. Intra-pulmonary arterial development during fetal life-branching pattern and structure. J Anat 113: 35–48, 1972. [PMC free article] [PubMed] [Google Scholar]
- 12.Hislop A, Reid L. Normal structure and dimensions of the pulmonary arteries in the rat. J Anat 125: 71–83, 1978. [PMC free article] [PubMed] [Google Scholar]
- 13.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 53: 1801887, 2019. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamison BM, Michel RP. Different distribution of plexiform lesions in primary and secondary pulmonary hypertension. Hum Pathol 26: 987–993, 1995. doi: 10.1016/0046-8177(95)90088-8. [DOI] [PubMed] [Google Scholar]
- 15.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67: 139–151, 2004. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Lane BP, Zeidler M, Weinhold C, Drummond E. Organization and structure of branches in the rat pulmonary arterial bed. Anat Rec 205: 397–403, 1983. doi: 10.1002/ar.1092050405. [DOI] [PubMed] [Google Scholar]
- 17.Laurie SS, Elliott JE, Goodman RD, Lovering AT. Catecholamine-induced opening of intrapulmonary arteriovenous anastomoses in healthy humans at rest. J Appl Physiol (1985) 113: 1213–1222, 2012. doi: 10.1152/japplphysiol.00565.2012. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Lindner JR, Moliterno DJ, Mukherjee D, Pohost GM, Rosenson RS, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ; ACCF/AHA . ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 119: 2250–2294, 2009. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 19.Mooi WJ, Grünberg K. Histopathology of pulmonary hypertensive diseases. Curr Diagn Pathol 12: 429–440, 2006. doi: 10.1016/j.cdip.2006.07.003. [DOI] [Google Scholar]
- 20.Oshima K, McLendon JM, Wagner WW Jr, McMurtry IF, Oka M. Chronic hypoxia does not cause wall thickening of intra-acinar pulmonary supernumerary arteries. Physiol Rep 4: e12674, 2016. doi: 10.14814/phy2.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parra-Bonilla G, Alvarez DF, Alexeyev M, Vasauskas A, Stevens T. Lactate dehydrogenase a expression is necessary to sustain rapid angiogenesis of pulmonary microvascular endothelium. PLoS One 8: e75984, 2013. doi: 10.1371/journal.pone.0075984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Levy PS, Reid LM, Vreim CE, Williams GW. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 80: 1198–1206, 1989. doi: 10.1161/01.CIR.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 23.Recavarren S. The preterminal arterioles in the pulmonary circulation of high-altitude natives. Circulation 33: 177–180, 1966. doi: 10.1161/01.CIR.33.2.177. [DOI] [PubMed] [Google Scholar]
- 24.Reid L. Structural and functional reappraisal of the pulmonary artery system. Sci Basis Med Annu Rev 1968: 289–307, 1968. [PubMed] [Google Scholar]
- 25.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19: 1178–1180, 2005. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 26.Shaw AM, Bunton DC, Fisher A, McGrath JC, Montgomery I, Daly C, MacDonald A. V-shaped cushion at the origin of bovine pulmonary supernumerary arteries: structure and putative function. J Appl Physiol (1985) 87: 2348–2356, 1999. doi: 10.1152/jappl.1999.87.6.2348. [DOI] [PubMed] [Google Scholar]
- 27.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62, Suppl 25: D34–D41, 2013. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 261–272, 2012. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toba M, Alzoubi A, O’Neill KD, Gairhe S, Matsumoto Y, Oshima K, Abe K, Oka M, McMurtry IF. Temporal hemodynamic and histological progression in Sugen5416/hypoxia/normoxia-exposed pulmonary arterial hypertensive rats. Am J Physiol Heart Circ Physiol 306: H243–H250, 2014. doi: 10.1152/ajpheart.00728.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994. [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner WW, Weir EK. The Pulmonary Circulation and Gas Exchange, edited by Wagner WW Jr, Kenneth Weir E. Armonk, NY: Futura Publishing, 1994. [Google Scholar]
- 32.Wu D, Archer SL. Pulmonary hypertension begets pulmonary hypertension: mutually reinforcing roles for haemodynamics, inflammation, and cancer-like phenotypes. Cardiovasc Res 111: 1–4, 2016. doi: 10.1093/cvr/cvw110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaginuma G, Mohri H, Takahashi T. Distribution of arterial lesions and collateral pathways in the pulmonary hypertension of congenital heart disease: a computer aided reconstruction study. Thorax 45: 586–590, 1990. doi: 10.1136/thx.45.8.586. [DOI] [PMC free article] [PubMed] [Google Scholar]