Abstract
Cyclic compressive loading (CCL) is a massage mimetic that improves muscle regrowth from atrophy in adult rats. Therefore, we tested if a single bout of CCL increases anabolic signaling and protein synthesis in muscle during normal, weight-bearing conditions in gastrocnemius muscle from adult and aged rats. Male Brown Norway/F344 rats at 10 (adult) and 30 (aged) months of age were assigned control or CCL (receiving a single bout of CCL). Twenty-four hours following a single bout of CCL there was no change in protein synthesis, Akt, or GSK3β signaling at either age, despite adult rats having higher abundance and activation of mechanosensitive pathways (integrins and integrin-linked kinase). Murf1 was elevated in response to CCL in both age groups, potentially indicating muscle remodeling. Muscle from aged rats exhibited an increase in heat shock protein (HSP) 25 and HSP70 and in the cold shock protein RNA-binding motif 3 (RBM3), demonstrating a unique stress response to CCL in aged muscle only. Finally, muscle from aged rats exhibited higher basal protein synthesis that was corroborated by elevated eIF2Bε and rpS6 signaling, without an additional effect of CCL. In summary, a single bout of CCL does not have anabolic effects on skeletal muscle during normal, weight-bearing conditions, even though it has previously been shown to improve regrowth from atrophy. These data demonstrate that interventions that may help recover from atrophy do not necessarily induce muscle hypertrophy in unperturbed conditions.
NEW & NOTEWORTHY Massage has been demonstrated to be an effective mechanotherapy to improve recovery from atrophy in adult skeletal muscle; however, this study shows that a single bout of massage fails to increase protein synthesis or anabolic signaling in adult or aged skeletal muscle during normal, weight-bearing conditions. Altogether, our data suggest massage is a useful mechanotherapy for preserving skeletal muscle when combined with other interventions but is not an anabolic stimulus on its own.
INTRODUCTION
Aging is associated with the gradual loss of muscle mass and strength, or sarcopenia, which has severe implications for overall function and health outcomes in older adults (36, 64). Sarcopenia is linked to reductions in quality of life and is highly predictive of mortality and morbidity in both older men and women (30, 62). Thus, it is clinically valuable to find treatments that may offset sarcopenia. This is especially true during times of muscle disuse, such as bed rest, injury, or sickness that may exacerbate sarcopenia and cause further muscle loss in older adults. However, aged muscle presents a unique challenge because it is now well established that muscle hypertrophy in response to anabolic cues is significantly reduced with aging (12, 78), and as such, aged muscle exhibits impaired rescue of muscle mass during recovery from atrophy (18, 43, 53, 77).
Our laboratory has previously shown that applying cyclic compressive loading (CCL), a massage mimetic, to skeletal muscle is an effective treatment for enhancing muscle regrowth following disuse atrophy in adult rats (49). The externally applied mechanical perturbation of muscle enhanced myofibrillar protein synthesis and even had positive anabolic effects on the non-massaged contralateral muscle, making it a potential mechanotherapy. This type of non-invasive, affordable, and easily administered mechanotherapy may provide a practical means for improving recovery from atrophy in patients who are unable to perform more intense physical activity or exercise. However, as previously mentioned, aged muscle exhibits an anabolic resistance that prevents effective recovery of muscle mass and function and impairs the utility of many therapeutic interventions. The inability of aged muscle to regrow/hypertrophy in response to an anabolic stimulus has been proposed to be a result of reduced activation of Akt/mammalian target of rapamycin (mTOR) signaling (22, 23, 59, 69), blunted ribosome biogenesis (34), reduced sensitivity to amino acids (9), reduced insulin sensitivity (63), and/or dampened rates of protein synthesis (22, 76) compared with younger muscle. However, some studies have shown no defects in anabolic signaling or mechanosensitivity in aged muscle in response to anabolic stimuli (31, 43, 51, 58, 77), leaving remaining questions of why the hypertrophic response of aged muscle does not approximate that of the adult muscle. Evidence from both animal (3, 48, 76) and human (45, 58, 73) studies show that aged muscles do not have deficits in basal protein synthesis, and the reduced muscle mass with aging is more likely a consequence of reduced anabolic responses to repeated mechanical and anabolic cues. The reasons driving the inability of aged muscle to regrow after atrophy are most likely a constellation of the aforementioned deficits, and it is important to find clinically viable and effective therapeutic interventions to overcome the limitations in aged muscle.
We propose that massage presents a potentially unique treatment option for aged muscle. Our previous work has shown that massage in the form of CCL is a useful mechanical perturbation to elevate myofibrillar protein synthesis in muscle during recovery from atrophy (49). In addition, we have shown that a single bout of massage increases satellite cell abundance in both adult and aged skeletal muscle from rats (32). Therefore, we hypothesized that massage is a useful intervention for combating age-related atrophy. The purpose of this study was to test if a single bout of massage is an effective stimulus to enhance anabolic and mechanosensitive signaling and protein synthesis in aged muscle (30-mo-old rats) compared with adult muscle (10-mo-old rats). Specifically, we measured rates of protein synthesis and mRNA and protein abundance related to protein translation, mechanotransduction, and stress response 24 h after one bout of CCL. This time point was chosen to determine if CCL would cause lasting elevations in protein synthesis and anabolic signaling similar to other mechanical stimuli (1, 11, 41, 61).
METHODS
Animals.
All procedures were approved by the University of Kentucky’s Institutional Animal Care and Use Committee. Male Brown Norway/F344 rats at 10 (adult) and 30 (aged) months of age (National Institute on Aging, Bethesda, MD) were used in this study. Rats were housed in regular-sized cages within the Division of Laboratory Animal Resources facility at the University of Kentucky, with access to food and water ad libitum. Rats from each age were randomly assigned into two groups: control (CON, n = 8) or CCL (receiving a single bout of CCL) (n = 8).
CCL of gastrocnemius muscle.
All animals were anesthetized for 30 min and rats in the CCL groups received one bout of CCL as a massage mimetic, whereas CON rats were anesthetized only. Anesthesia was induced using an induction chamber with 5% isoflurane and 1 L/min oxygen and maintained via a nose cone with 2% isoflurane and 500 mL oxygen. Rats were placed left lateral recumbent on a heated sling with the right hindlimb secured to a small platform by self-adherent Coban wrap encircling the talocrural joint/midfoot. This placed the lateral aspect of the right gastrocnemius muscle facing superiorly for the application of cyclic compressive loads by a custom fabricated CCL device as described previously (32, 49, 75). A force transducer enabled continuous, real-time measurement of the normal force applied to the muscle. For CCL application, the roller was placed on the skin overlying the gastrocnemius muscle immediately proximal to the lateral malleolus and cycled proximal and distal along the length of the gastrocnemius muscle, applying 4.5 N at a frequency of 0.5 Hz for 30 min. Rats in CON group were anesthetized and placed left lateral recumbent for 30-min without CCL. Afterwards, the rats were returned to their cages and allowed to recover with access to food and water ad libitum. To monitor protein synthesis, puromycin (0.04 μmol/g body weight) was administered via intraperitoneal injection 30 min before euthanasia (26), or 23.5 h post-CCL. Rats were euthanized 24 h post-CCL, and gastrocnemius muscles were harvested, weighed, and flash frozen in liquid nitrogen.
Western blotting analysis for determination of protein abundance.
A section of each gastrocnemius muscle was homogenized in RIPA buffer (Boston Bioproducts, Ashland, MA) with 10 μl/ml protease inhibitor cocktail (Roche, Indianapolis, IN), 5 mM benzamidine, 5 mM N-ethylmaleimide, 50 mM NaF, 25 mM B-glycerophosphate, 1 mM EDTA, and 1 mM PMSF added and centrifuged 5,000 g. Protein concentration of homogenates was determined using the bicinchoninic acid protein assay (Bio-Rad, Hercules, CA). For quantification of protein abundance, 30 μg total protein was loaded and separated on 4–15% acrylamide gradient gels (Bio-Rad, Hercules, CA), followed by transfer of proteins to PVDF membranes with 0.22 μm pore size (Millipore, Bedford, MA). Membranes were incubated in Odyssey blocking buffer (Li-Cor, Lincoln, NE) followed by incubation with the appropriate primary antibody overnight at 4°C. The following primary antibodies were used: HSP25 (1:1,000, Stressgen), Hsp70 (1:1,000), p-rpS6 (1:1,000), total 4EBP1 (1:1,000), p-Aktser473 (1:1,000), eIF2bε (1:1,000), p-GSK3β (1:1,000), total GSK3β (1:1,000), total rpS6 (1:1,000), total Akt (1:1,000), total integrin-linked kinase (ILK) (1:1,000) from Cell Signaling (Danvers, MA), MURF1 (1:1,000) from ECM Biosciences (Versailles, KY), puromycin clone 12D10 (1:5,000), and p-ILKSer246 (1:1,000) (Millipore, Bedford, MA). RNA-binding motif 3 (RMB3) primary antibody was a kind gift from Dr. Peter Vanderklish (15, 20). After primary antibody incubation, membranes were washed and further incubated with highly cross-absorbed infrared-labeled secondary antibodies for 1 h at room temperature [goat anti-rabbit (1:15,000, Li-Cor, Lincoln, NE), goat anti-mouse (1:15,000, Invitrogen, Omaha, NE) or for puromycin specifically, goat anti-mouse IgG2a (1:5,000, Invitrogen)]. Membranes were scanned using Odyssey infrared imaging system (Li-Cor) to detect specific antibody binding and to perform quantification. Ponceau S staining of the membranes was utilized to ensure equal loading.
RNA isolation and real-time RT-PCR.
Total RNA was isolated using mirVana miRNA Isolation Kit (Life Technologies) according to the manufacturer’s instructions. RNA integrity was determined using the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA), and RNA concentration was measured using a Nano Drop (Thermo Fisher Scientific). Quantitative real-time RT-PCR was performed using the protocols, chemistries, and the amplification and detection systems of Applied Biosystems (Applied Biosystems, Foster City, CA). For each sample, cDNA was synthesized from 1 μg of total RNA using iScript Reverse Transcriptase (Bio-Rad) according to the manufacturer’s instructions. cDNA samples were aliquoted and stored at −80°C. Primer sequences were selected from the accession numbers in NCBI database using the Primer Design function of the Primer Express v1.5 software (Applied Biosystems) and were as follows: 18S (M11188) forward, ttcggacgtctgccctatcaa, reverse, atggtaggcacggcgacta; NADH subunit 1 (X14848) forward, agaacggaaaatcctaggctacatac, reverse ccatatgggccttcgttgtt; tubulin (NM_022298) forward, ggcatggaggagggagagtt, reverse, ccaacctcctcataatccttctctag. Taqman Gene Expression Assay primers (Applied Biosystems) were as follows: Itgb5: Mm01169759_m1; Itga7: Mm00434400_m1; Itgb2: Rn01427948_m1; and Itgb1: RN00566727_m1. PCR reactions for 18S, NADH, and tubulin were assembled using the SYBR Green PCR Master Mix that required only the addition of cDNA template and primers as described previously. PCR reactions for Itgb5, Itga7, Itgb2, and Itgb1 were assembled using protocols from Applied Biosciences utilizing TaqMan Gene Expression Master Mix and protocol (Applied Biosystems). Data points for the standard curve were generated using fourfold serial dilutions of cDNA. The reactions were performed using the ABI PrismTM 7700 Sequence Detection System (Applied Biosystems) and the instrument’s universal cycling conditions. RNA abundance for each gene of interest was expressed as a ratio normalized to the geometric mean of NADH subunit 1, tubulin, and 18S.
Statistical analyses.
For the comparison of all groups a two-way ANOVA was performed using GraphPad Prism version 8.0.2 statistical analysis software. Main effects as well as interactions are reported as appropriate and in case of statistically significant differences the Holm-Sidak pairwise multiple comparison procedure was employed to determine group differences. All values reported are mean ± SE, and statistical significance was assumed at P < 0.05.
RESULTS
Muscle size and protein synthesis.
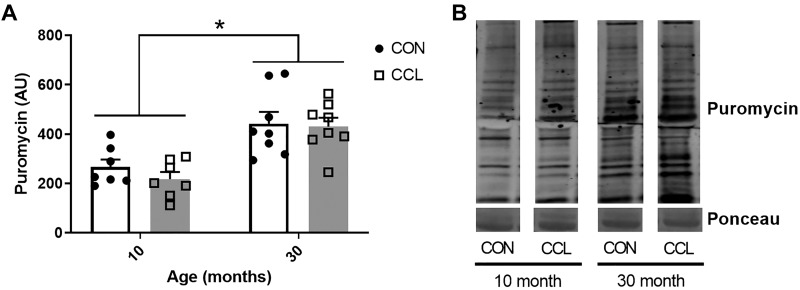
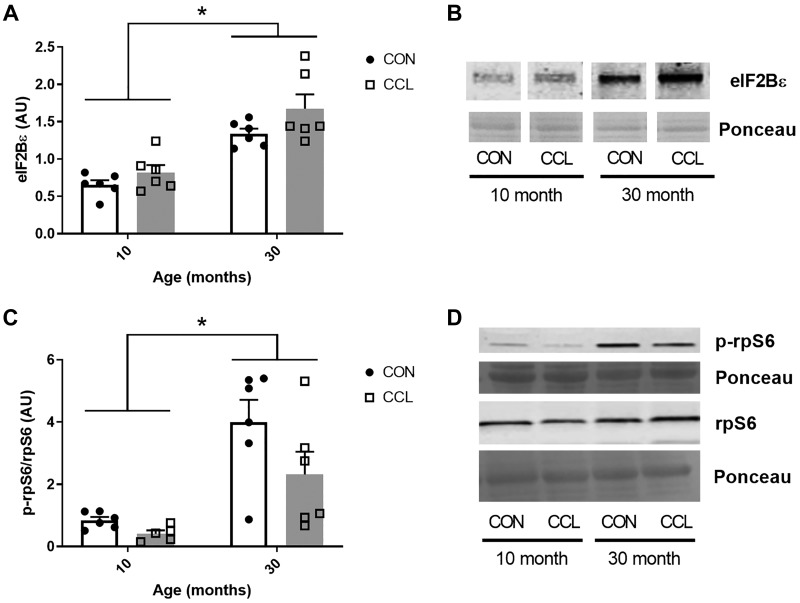
As expected, the 30-mo-old aged rats had significantly smaller gastrocnemius muscles compared with young, demonstrating sarcopenia (P < 0.0001; Table 1). Interestingly, despite clearly evident sarcopenia in the aged rat muscle, the rate of basal protein synthesis in the aged rat muscle was 49% higher when compared with adult. (P < 0.0001; Fig. 1, A and B). There was no impact of a single bout of massage on protein synthesis (P = 0.41) or muscle mass (P = 0.08) in the young or old rats. The elevated protein synthesis coincided with higher total eIF2Bε protein (P < 0.0001; Fig. 2, A and B) and higher activation of rpS6 (P = 0.0002; Fig. 2, C and D) in aged muscle compared with adult, but no effect of CCL was observed for these two variables.
Table 1.
Gastrocnemius muscle mass to body mass ratio
| 10 mo |
30 mo |
|||
|---|---|---|---|---|
| CON | CCL | CON | CCL | |
| MW (g) | 2.16 ± 0.04 | 2.23 ± 0.06 | 1.79 ± 0.05* | 1.87 ± 0.04* |
| MW/BW (mg/g) | 4.67 ± 0.07 | 4.73 ± 0.06 | 3.33 ± 0.07* | 3.04 ± 0.4* |
Data presented as mean ± SE. CCL, cyclic compressive loading; CON, control. MW, muscle weight; BW, body weight.
Significant (P < 0.05) main effect for age.
Fig. 1.
Age-associated elevation of protein synthesis is not affected by CCL. A: quantification of puromycin staining from 10-mo-old control (n = 7) or CCL-treated (n = 7) rats and 30-mo-old control (n = 7) and CCL-treated (n = 7) rats. B: representative image of puromycin staining. Data presented as mean ± SE. *Significant main effect for age (P < 0.05). CCL, cyclic compressive loading; CON, control.
Fig. 2.
Elevated levels of eIF2Bε and phosphorylated rpS6 in aged gastrocnemius muscle. A: quantification of eIF2Bε Western blot from 10-mo-old control (n = 6) or CCL-treated (n = 6) rats and 30-mo-old control (n = 6) or CCL-treated (n = 6) rats. B: representative image of eIF2Bε Western blot. C: quantification of the p-rpS6/rpS6 Western blot from 10-mo-old control (n = 6) or CCL-treated (n = 6) rats and 30-mo-old control (n = 6) and CCL-treated (n = 6) rats. D: representative image of p-rpS6/rpS6 Western blot. Data presented as mean ± SE. *Significant main effect for age (P < 0.05). CCL, cyclic compressive loading; CON, control.
Integrins and ILK.
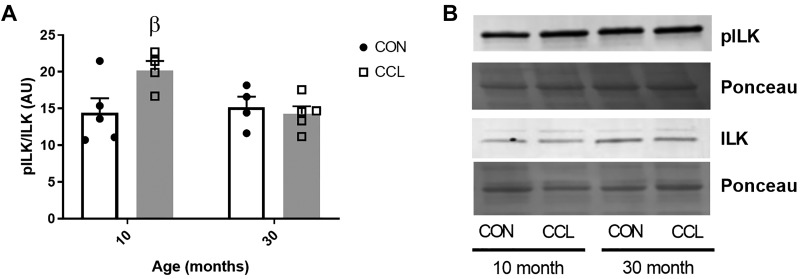
We measured key factors critical for translating mechanical cues into intracellular signals that modulate protein synthesis, which included integrins and ILK. The mRNA of all four integrins measured (ITGB1, ITGB2, ITGB5, ITGA7) were lower in aged muscle compared with adult (Table 2). There was elevated ITGB2 in response to CCL compared with CON in adult, but not aged rats. The higher abundance of integrins, which are the primary receptors at the membrane for transducing mechanical stimulus to intracellular signaling, in adult compared with aged muscle was also reflected in higher activation of ILK (higher phosphorylated ILK/total ILK ratio) in response to CCL in adult (P = 0.04), but not aged (P = 0.88) gastrocnemius muscle (Fig. 3).
Table 2.
Gene expression of integrins is lower in aged muscle
| 10 mo |
30 mo |
|||
|---|---|---|---|---|
| CON (n = 7) | CCL (n = 8) | CON (n = 7) | CCL (n = 8) | |
| ITGA7 | 3.5 ± 0.3 | 2.7 ± 0.3 | 2.2 ± 0.2* | 2.6 ± 0.4* |
| ITGB1 | 30.9 ± 1.7 | 26.7 ± 3.1 | 20.7 ± 3.4* | 20.4 ± 4.2* |
| ITGB2 | 20.7 ± 5.8 | 41.9 ± 6.4† | 12.2 ± 3.3* | 9.9 ± 4.0* |
| ITGB5 | 11.6 ± 3.0 | 5.7 ± 1.5 | 2.2 ± 0.7* | 2.1 ± 0.8* |
Data presented as mean ± SE. Values are expressed as arbitrary units normalized to geometric mean. CCL, cyclic compressive loading; CON, control.
Significant (P < 0.05) main effect for age;
significant effect (P < 0.05) of CCL within age.
Fig. 3.
Massage elevates mechanosensitive ILK signaling in muscle from adult but not aged rats. A: quantification of pILK/ILK Western blot from 10-mo-old control (n = 6) or CCL-treated (n = 6) rats and 30-mo-old control (n = 6) or CCL-treated (n = 6) rats. B: representative image of pILK and ILK Western blots. Data presented as mean ± SE. β denotes significant effect for CCL within age (P < 0.05). CCL, cyclic compressive loading; CON, control; ILK, integrin-linked kinase; pILK, phosphorylated integrin-linked kinase.
Akt and GSK3β signaling.
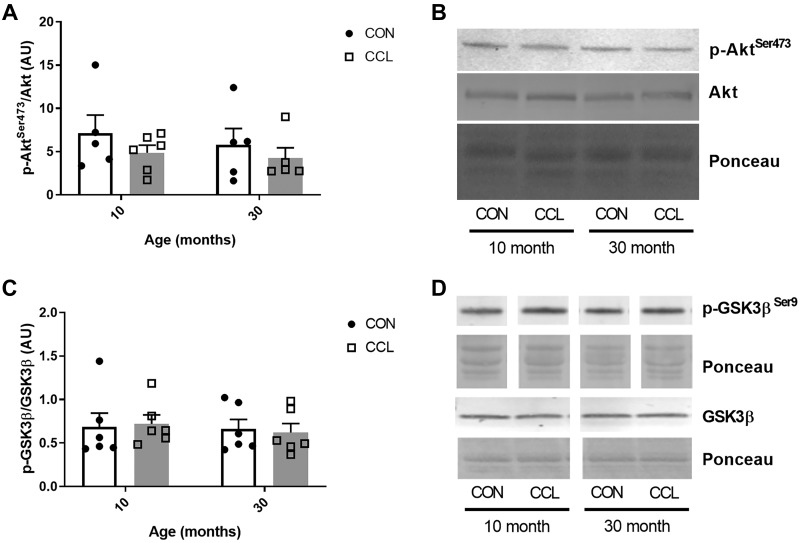
Akt signaling (p-Aktser473/Akt) was not different between adult and aged (P = 0.53) and was unaffected by CCL (P = 0.24) (Fig. 4, A and B). Similarly, GSK3β signaling (p-GSK3β/GSK3β) was unaffected by age (P = 0.61) or CCL (P = 0.97) (Fig. 4, C and D).
Fig. 4.
A single bout of massage does not affect Akt or GSK3β signaling. A: quantification of p-AktSer473/Akt Western blot from 10-mo-old control (n = 6) or CCL-treated (n = 6) rats and 30-mo-old control (n = 6) or CCL-treated (n = 6) rats. B: representative image of p-AktSer473/Akt Western blot. C: quantification of the p-GSK3β/GSK3β Western blot from 10-mo-old control (n = 6) or CCL-treated (n = 6) rats and 30-mo-old control (n = 6) and CCL-treated (n = 6) rats. D: representative image of p-GSK3β/GSK3β Western blot. Data presented as mean ± SE. CCL, cyclic compressive loading; CON, control.
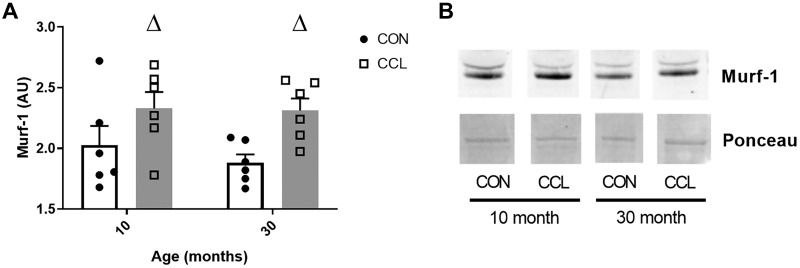
Murf1 abundance.
Protein abundance of Murf1 was elevated in both the adult and the aged rats by CCL application (P = 0.006) (Fig. 5, A and B) and no difference with age (P = 0.51) was observed.
Fig. 5.
A single bout of massage increases the protein abundance of Murf1. A: quantification of Murf1 Western blot from 10-mo-old control (n = 6) or CCL-treated (n = 6) rats and 30-mo-old control (n = 6) or CCL-treated (n = 6) rats. B: representative image of Murf1 Western blot. Data presented as mean ± SE. ΔSignificant main effect (P < 0.05) for CCL. CCL, cyclic compressive loading; CON, control.
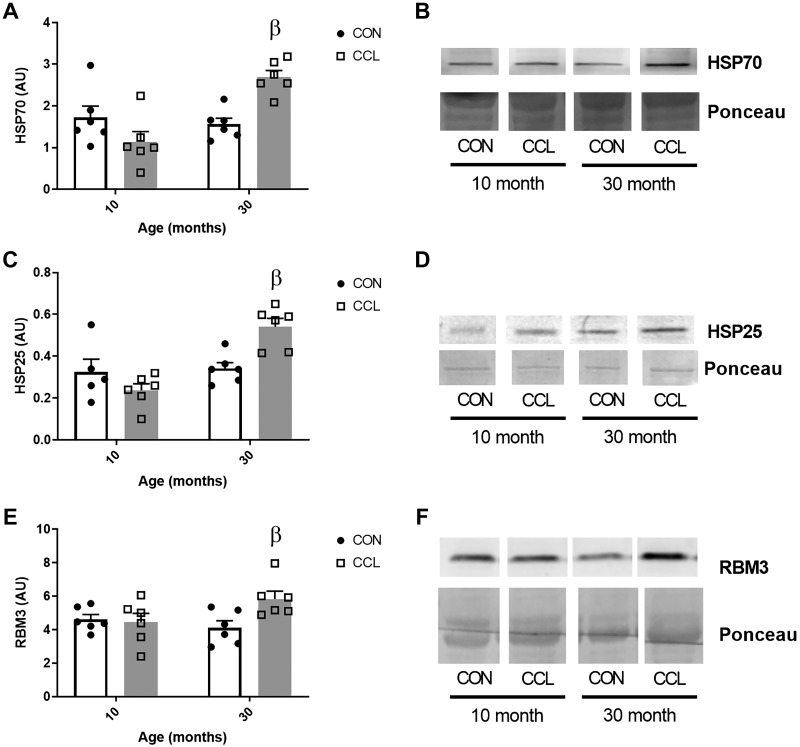
Stress protein response.
CCL caused an elevated protein abundance of heat shock protein (HSP) 70 (Fig. 6, A and B) and HSP25 (Fig. 6, C and D) in the aged rat gastrocnemius muscle (P = 0.002 and P = 0.004, respectively), but not in the adult (P = 0.13 and P = 0.28). Similarly, CCL caused an elevated protein abundance of the cold shock RNA-binding protein RBM3 in the aged rat gastrocnemius muscle (P = 0.02), but not in the adult (P = 0.95) (Fig. 6, E and F).
Fig. 6.
Aged muscle exhibits a stress response to a single bout of massage. Quantification of HSP70 (A), HSP25 (C), and RBM3 (E) Western blots from 10-mo-old control (n = 6) or CCL-treated (n = 6) rats and 30-mo-old control (n = 6) or CCL-treated (n = 6) rats. Representative images of HSP70 (B), HSP25 (D), and RBM3 (F) Western blots, respectively. Data presented as mean ± SE. βSignificant effect (P < 0.05) of CCL within age (P < 0.05). CCL, cyclic compressive loading; CON, control; HSP, heat shock protein; RBM3, RNA-binding motif 3.
DISCUSSION
The primary findings of this study were that gastrocnemius muscle from aged rats exhibited higher basal muscle protein synthesis compared with adult and a single bout of CCL, a massage mimetic, did not change protein synthesis in muscle from neither adult nor aged rats. These results demonstrate a discordant relationship between the basal rates of protein synthesis and the age-related atrophy apparent in the aged muscle. Despite the adult muscle showing greater mechanosensitive signaling via integrins and ILK compared with aged muscle, the adult muscle did not exhibit a higher anabolic response. Interestingly, higher levels of HSPs (HSP25 and HSP70) and the cold shock protein RBM3 in response to CCL occurred only in the aged muscle suggesting an elevated stress response to CCL compared with adult rats. Overall, under normal, weight-bearing conditions, one bout of CCL does not stimulate anabolic signaling or protein synthesis in adult and old muscle.
Our findings that basal protein synthesis is significantly higher in aged muscle corroborates growing evidence that there is no deficit in skeletal muscle basal protein synthesis with aging. Previous studies in rats have demonstrated elevated basal protein synthesis in the gastrocnemius (21, 33) and other hindlimb muscles (48) when compared with young or middle-aged rats. In support of these studies, human data from Volpi et al. (73) showed that basal protein synthesis tends to be higher in healthy older men compared with young men. Not all studies agree, though, as two studies that used the same puromycin labeling technique as our study recently showed no differences in basal protein synthesis in aged F344/BN rats compared with adult rats (3, 76). Elevated levels of the translation initiation factors eIF2β and rpS6 signaling supported the high protein synthesis in the aged muscle in our rats and highlights a potential mechanism dictating the puromycin data. A higher abundance of eIF2β has been linked to increased protein synthesis (28, 70) and skeletal muscle hypertrophy (47). Previous work from our laboratory in aged BN/F344 rats has shown elevated p70S6K activation in aged muscle compared with young adult muscle (77) and supports higher basal activation of the p70S6K/rpS6 axis in aged muscle. An examination of basal protein synthesis in a large cohort of young and old human subjects has also demonstrated elevated activation of muscle p70S6K phosphorylation in aged muscle, although basal rates of protein synthesis were not different between young and old in this study (45). Therefore, it is clear that reduced basal protein synthesis is not a primary mechanism leading to age-related atrophy.
Based on our previous findings that massage can improve muscle growth and augment recovery of muscle size during recovery from disuse atrophy (49), we hypothesized that massage may be a useful hypertrophic stimulus in unperturbed conditions. Specifically, it is suggested that the mechanical signals from massage would be sensed at the interface between the extracellular matrix and cell membrane and transduced to anabolic intracellular signals. However, the single bout of CCL failed to instigate lasting anabolic signaling in either the adult or aged muscle. Specifically, there were no changes in Akt/GSK3β signaling pathway, which can mediate muscle anabolic response and muscle hypertrophy (17, 72, 74, 77) and showed changes in response to loading in our previous work (49). Interestingly, muscles from adult rats exhibited overall higher mRNA expression levels of integrins compared with aged; integrins are the main receptors for transferring mechanical cues at the extracellular matrix with intracellular signaling pathways (7). Elevated levels of integrins are associated with an enhanced response to hypertrophy (82) and levels of β1 and β2 integrins are increased with resistance exercise (44, 56). It is known that the extracellular matrix changes with aging, exhibiting increased fibrosis and stiffness (8, 24, 79). Some studies report an increase in collagen (25, 79), whereas others do not (24), but it is well accepted that collagen cross-linking is increased, leading to altered force transmission (24, 79). Our recent work showed that aged muscle exhibited protection from elevated membrane permeability in response to a single bout of massage when compared with adult that we believe is a product of increased extracellular matrix (ECM) in the aged (32). The stiffer ECM in aged muscle is protective against the transmission of mechanical loads to the muscle fibers. Lower levels of integrins in aged muscle shown in this study may play a major role in dampening the mechanosensitivity of aged muscle in conjunction with the aforementioned alterations in ECM stiffness. Indeed, higher levels of integrins in the muscle of adult rats were associated with elevated activation of ILK in response to CCL, which was not observed in aged muscle. However, the increased mechanosensing ability of the adult muscle compared with the aged muscle did not manifest in a higher protein synthesis or anabolic signaling. It is possible that by waiting 24 h after CCL to harvest the muscle tissue we missed transient changes in anabolic signaling and protein synthesis that may have occurred earlier (i.e., activation of Akt and GSK3). Resistance exercise elevates mTOR pathway signaling and protein synthesis up to 48 h (1, 6, 11, 41, 59, 61); however, the mechanical stimulus in those studies is likely far more robust than CCL. Thus, CCL may have initiated a small anabolic signaling response that was returned to basal levels by the time the muscle tissue was harvested.
In contrast to the lack of signaling changes in the Akt/mTOR pathway, we observed a significant increase in the protein abundance of muscle ring finger 1 (Murf1). Murf1 is a muscle-specific ubiquitin ligase that directs the polyubiquination of proteins to target them for proteasomal degradation (4). As such, the expression of Murf1 is elevated in skeletal muscle during atrophic conditions and has been identified as a critical component in the proteasomal degradation that leads to skeletal muscle atrophy (5, 27). Elevated Murf1 expression and protein degradation also occur in response to hypertrophic stimuli, as exemplified by studies in rodents showing elevated Murf1 expression as an early response to muscle overload (2, 44) or during muscle regrowth after disuse (77). Multiple studies in humans have also shown a transient increase in Murf1 expression in response to a single bout of resistance training (19, 39). The observation that Murf1 protein abundance was upregulated in response to a single bout of CCL suggests that massage may transiently increase protein degradation similar to other hypertrophic stimuli, thereby initiating muscle remodeling. We posit that CCL induces muscle remodeling in both adult and aged muscle based on this finding, although future studies should directly measure proteasome activity following massage to confirm this hypothesis. Our previous work demonstrated that massage combined with reloading after hindlimb suspension in rats elevated protein degradation to higher levels compared with reloading alone (49), supporting that the beneficial effect of massage may not only include augmentation of protein synthesis but also enhanced muscle remodeling through elevated protein degradation. The elevated protein degradation in our previous work (49) was not mirrored by higher Murf1 abundance, but the measurement of Murf1 was at the mRNA level and made after 7 days of reloading and 24 h after the last of four bouts of massage, so transient elevations in Murf1 mRNA may have been missed. Altogether, despite a lack of lasting changes in the Akt/rpS6 axis there was a sustained increase in the protein abundance of Murf1 which continues to support that massage may elevate skeletal muscle remodeling.
The one unique adaptation of the aged muscle to CCL found in this study was evidence of an increased stress response compared with adult muscle. Aged muscle had increased protein content of HSP25 and HSP70 in response to CCL compared with adult muscle. HSPs function to restore cellular homeostasis in response to a variety of stimuli in skeletal muscle (37) and facilitate adaptation via their role in targeting and removal of denatured proteins (29), muscle contraction (55), and protein folding (29, 46). Both acute and chronic exercise causes an increase in the expression of HSPs in rodents and humans that may serve as an adaptation that provides protection for skeletal muscle structure and function (38, 42, 52, 54, 65). HSPs are typically downregulated during skeletal muscle atrophy (35, 57), and it has been speculated that a decrease in HSPs may contribute to the maladaptation that occurs in skeletal muscle with disuse. As such, transgenic mice overexpressing HSP70 demonstrated improved recovery of muscle mass and function after disuse atrophy compared with wild-type mice (50). In addition, overexpressing HSP70 or HSP25 via transfection in rats completely attenuated muscle fiber atrophy during hindlimb unloading by inhibiting FOXO3a and NF-κB activities (13, 14, 66, 67). CCL also increased the expression of the cold shock RNA-binding protein RBM3 in muscle from aged rats, which, similar to HSPs, is cell protective in many contexts (20, 40, 60, 71, 80). Elevated RBM3 may also promote beneficial adaptations in aged muscle because RBM3 enhances global rates of translation through its actions on RNA stability and translational programming (10, 15, 68, 81). RBM3 has been associated with muscle adaptation to atrophy (16), and Van Pelt et al. (71) showed that plasmid-mediated overexpression of RBM3 in vitro and in vivo attenuated atrophy and promoted muscle hypertrophy. The increase in protein content of HSPs and RBM3 in the aged muscle may reflect a higher stress response to CCL.
There are some limitations in this study that should be addressed. First, we measured the effect of CCL on the activation of various components of the Akt/mTOR signaling pathway 24 h after the last bout of CCL. Phosphorylation events and transient anabolic signaling that may have occurred in the immediate aftermath of the massage session may have recovered long before the tissue was harvested. However, our data suggest that even a small stimulus such as one bout of CCL can cause some lasting adaptations and signaling events in skeletal muscle. It has also been shown that some anabolic stimuli such as resistance exercise can stimulate an increase in protein synthesis for over 24 h (11, 41, 61), thus we chose to investigate whether massage produced similar lasting effects on protein synthesis. Second, we acknowledge that our assessment of muscle anabolic signaling is not all encompassing, but we have measured important regulatory nodes within anabolic signaling pathways that are both upstream and downstream of mTOR. It is, however, possible that we have missed changes in other important proteins within this pathway. Finally, although there is a relatively small amount of variability in our data, we acknowledge that small sample sizes for some measurements may have led to type 2 errors.
In summary, results from this study show that there are distinct age-related differences in response to application of a single bout of CCL, a massage mimetic, even though no anabolic response was observed in unperturbed muscle from adult or aged rats. Our previous work showed that CCL is effective at improving recovery of skeletal muscle mass and protein synthesis after disuse (49); therefore, it is concluded that interventions that may help recover from atrophy are not necessarily treatments that cause a hypertrophic stimulus under homeostatic conditions. It is also conceivable that massage may only be a useful intervention for promoting anabolism in muscle when combined other interventions, such as reloading, as seen in our previous work (49). Further work should be performed to investigate the impact of CCL applied during muscle disuse to investigate whether CCL as a mechanotherapy will attenuate atrophy in aged rats.
GRANTS
This study was supported by National Institutes of Health Grants AT-009268 and AG-042699.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.E.D.-V. and T.A.B. conceived and designed research; A.L.C., S.M.A., and E.R.H. performed experiments; D.W.V.P., A.L.C., S.M.A., and E.R.H. analyzed data; D.W.V.P., A.L.C., E.E.D.-V., and T.A.B. interpreted results of experiments; D.W.V.P. and A.L.C. prepared figures; D.W.V.P. and A.L.C. drafted manuscript; D.W.V.P., A.L.C., E.R.H., E.E.D.-V., and T.A.B. edited and revised manuscript; D.W.V.P., A.L.C., S.M.A., E.R.H., E.E.D.-V., and T.A.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Peter Vanderklish for generously sharing his RBM3 primary antibody.
REFERENCES
- 1.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276: C120–C127, 1999. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 2.Baehr LM, Tunzi M, Bodine SC. Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression. Front Physiol 5: 69, 2014. doi: 10.3389/fphys.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baehr LM, West DWD, Marshall AG, Marcotte GR, Baar K, Bodine SC. Muscle-specific and age-related changes in protein synthesis and protein degradation in response to hindlimb unloading in rats. J Appl Physiol (1985) 122: 1336–1350, 2017. doi: 10.1152/japplphysiol.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab 307: E469–E484, 2014. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 6.Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 553: 213–220, 2003. doi: 10.1113/jphysiol.2003.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boppart MD, Mahmassani ZS. Integrin signaling: linking mechanical stimulation to skeletal muscle hypertrophy. Am J Physiol Cell Physiol 317: C629–C641, 2019. doi: 10.1152/ajpcell.00009.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 9.Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev 41: 169–173, 2013. doi: 10.1097/JES.0b013e318292f3d5. [DOI] [PubMed] [Google Scholar]
- 10.Cok SJ, Acton SJ, Sexton AE, Morrison AR. Identification of RNA-binding proteins in RAW 264.7 cells that recognize a lipopolysaccharide-responsive element in the 3-untranslated region of the murine cyclooxygenase-2 mRNA. J Biol Chem 279: 8196–8205, 2004. doi: 10.1074/jbc.M308475200. [DOI] [PubMed] [Google Scholar]
- 11.Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie M. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab 290: E731–E738, 2006. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- 12.Degens H, Alway SE. Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve 27: 339–347, 2003. doi: 10.1002/mus.10314. [DOI] [PubMed] [Google Scholar]
- 13.Dodd S, Hain B, Judge A. Hsp70 prevents disuse muscle atrophy in senescent rats. Biogerontology 10: 605–611, 2009. doi: 10.1007/s10522-008-9203-1. [DOI] [PubMed] [Google Scholar]
- 14.Dodd SL, Hain B, Senf SM, Judge AR. Hsp27 inhibits IKKbeta-induced NF-kappaB activity and skeletal muscle atrophy. FASEB J 23: 3415–3423, 2009. doi: 10.1096/fj.08-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci USA 102: 1865–1870, 2005. doi: 10.1073/pnas.0409764102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont-Versteegden EE, Nagarajan R, Beggs ML, Bearden ED, Simpson PM, Peterson CA. Identification of cold-shock protein RBM3 as a possible regulator of skeletal muscle size through expression profiling. Am J Physiol Regul Integr Comp Physiol 295: R1263–R1273, 2008. doi: 10.1152/ajpregu.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 49: 59–68, 2014. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 13: 34–39, 2010. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Gonzalo R, Lundberg TR, Tesch PA. Acute molecular responses in untrained and trained muscle subjected to aerobic and resistance exercise training versus resistance training alone. Acta Physiol (Oxf) 209: 283–294, 2013. doi: 10.1111/apha.12174. [DOI] [PubMed] [Google Scholar]
- 20.Ferry AL, Vanderklish PW, Dupont-Versteegden EE. Enhanced survival of skeletal muscle myoblasts in response to overexpression of cold shock protein RBM3. Am J Physiol Cell Physiol 301: C392–C402, 2011. doi: 10.1152/ajpcell.00098.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fluckey JD, Vary TC, Jefferson LS, Evans WJ, Farrell PA. Insulin stimulation of protein synthesis in rat skeletal muscle following resistance exercise is maintained with advancing age. J Gerontol A Biol Sci Med Sci 51A: B323–M330, 1996. doi: 10.1093/gerona/51A.5.B323. [DOI] [PubMed] [Google Scholar]
- 22.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1: 11, 2011. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funai K, Parkington JD, Carambula S, Fielding RA. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 290: R1080–R1086, 2006. doi: 10.1152/ajpregu.00277.2005. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech 41: 465–469, 2008. doi: 10.1016/j.jbiomech.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldspink G, Fernandes K, Williams PE, Wells DJ. Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord 4: 183–191, 1994. doi: 10.1016/0960-8966(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 26.Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, Hornberger TA. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J 25: 1028–1039, 2011. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 43: 12–21, 2013. doi: 10.1007/s12020-012-9751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardt SE, Tomita H, Katus HA, Sadoshima J. Phosphorylation of eukaryotic translation initiation factor 2Bepsilon by glycogen synthase kinase-3beta regulates beta-adrenergic cardiac myocyte hypertrophy. Circ Res 94: 926–935, 2004. doi: 10.1161/01.RES.0000124977.59827.80. [DOI] [PubMed] [Google Scholar]
- 29.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295: 1852–1858, 2002. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 30.Hirani V, Blyth F, Naganathan V, Le Couteur DG, Seibel MJ, Waite LM, Handelsman DJ, Cumming RG. sarcopenia is associated with incident disability, institutionalization, and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. J Am Med Dir Assoc 16: 607–613, 2015. doi: 10.1016/j.jamda.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Hornberger TA, Mateja RD, Chin ER, Andrews JL, Esser KA. Aging does not alter the mechanosensitivity of the p38, p70S6k, and JNK2 signaling pathways in skeletal muscle. J Appl Physiol (1985) 98: 1562–1566, 2005. doi: 10.1152/japplphysiol.00870.2004. [DOI] [PubMed] [Google Scholar]
- 32.Hunt ER, Confides AL, Abshire SM, Dupont-Versteegden EE, Butterfield TA. Massage increases satellite cell number independent of the age-associated alterations in sarcolemma permeability. Physiol Rep 7: e14200, 2019. doi: 10.14814/phy2.14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimball SR, O’Malley JP, Anthony JC, Crozier SJ, Jefferson LS. Assessment of biomarkers of protein anabolism in skeletal muscle during the life span of the rat: sarcopenia despite elevated protein synthesis. Am J Physiol Endocrinol Metab 287: E772–E780, 2004. doi: 10.1152/ajpendo.00535.2003. [DOI] [PubMed] [Google Scholar]
- 34.Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA, McCarthy JJ. Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol (1985) 119: 321–327, 2015. doi: 10.1152/japplphysiol.00296.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ku Z, Yang J, Menon V, Thomason DB. Decreased polysomal HSP-70 may slow polypeptide elongation during skeletal muscle atrophy. Am J Physiol Cell Physiol 268: C1369–C1374, 1995. doi: 10.1152/ajpcell.1995.268.6.C1369. [DOI] [PubMed] [Google Scholar]
- 36.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol Rev 99: 427–511, 2019. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Gampert L, Nething K, Steinacker JM. Response and function of skeletal muscle heat shock protein 70. Front Biosci 11: 2802–2827, 2006. doi: 10.2741/2011. [DOI] [PubMed] [Google Scholar]
- 38.Locke M, Noble EG, Atkinson BG. Exercising mammals synthesize stress proteins. Am J Physiol Cell Physiol 258: C723–C729, 1990. doi: 10.1152/ajpcell.1990.258.4.C723. [DOI] [PubMed] [Google Scholar]
- 39.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol (1985) 103: 1744–1751, 2007. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 40.Ma R, Zhao LN, Yang H, Wang YF, Hu J, Zang J, Mao JG, Xiao JJ, Shi M. RNA binding motif protein 3 (RBM3) drives radioresistance in nasopharyngeal carcinoma by reducing apoptosis via the PI3K/AKT/Bcl-2 signaling pathway. Am J Transl Res 10: 4130–4140, 2018. [PMC free article] [PubMed] [Google Scholar]
- 41.MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol 20: 480–486, 1995. doi: 10.1139/h95-038. [DOI] [PubMed] [Google Scholar]
- 42.Maglara AA, Vasilaki A, Jackson MJ, McArdle A. Damage to developing mouse skeletal muscle myotubes in culture: protective effect of heat shock proteins. J Physiol 548: 837–846, 2003. doi: 10.1113/jphysiol.2002.034520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magne H, Savary-Auzeloux I, Vazeille E, Claustre A, Attaix D, Anne L, Véronique SL, Philippe G, Dardevet D, Combaret L. Lack of muscle recovery after immobilization in old rats does not result from a defect in normalization of the ubiquitin-proteasome and the caspase-dependent apoptotic pathways. J Physiol 589: 511–524, 2011. doi: 10.1113/jphysiol.2010.201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marino JS, Tausch BJ, Dearth CL, Manacci MV, McLoughlin TJ, Rakyta SJ, Linsenmayer MP, Pizza FX. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol 295: C1026–C1036, 2008. doi: 10.1152/ajpcell.212.2008. [DOI] [PubMed] [Google Scholar]
- 45.Markofski MM, Dickinson JM, Drummond MJ, Fry CS, Fujita S, Gundermann DM, Glynn EL, Jennings K, Paddon-Jones D, Reidy PT, Sheffield-Moore M, Timmerman KL, Rasmussen BB, Volpi E. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp Gerontol 65: 1–7, 2015. doi: 10.1016/j.exger.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin J, Horwich AL, Hartl FU. Prevention of protein denaturation under heat stress by the chaperonin Hsp60. Science 258: 995–998, 1992. doi: 10.1126/science.1359644. [DOI] [PubMed] [Google Scholar]
- 47.Mayhew DL, Hornberger TA, Lincoln HC, Bamman MM. Eukaryotic initiation factor 2B epsilon induces cap-dependent translation and skeletal muscle hypertrophy. J Physiol 589: 3023–3037, 2011. doi: 10.1113/jphysiol.2010.202432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller BF, Baehr LM, Musci RV, Reid JJ, Peelor FF III, Hamilton KL, Bodine SC. Muscle-specific changes in protein synthesis with aging and reloading after disuse atrophy. J Cachexia Sarcopenia Muscle. In press. doi: 10.1002/jcsm.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller BF, Hamilton KL, Majeed ZR, Abshire SM, Confides AL, Hayek AM, Hunt ER, Shipman P, Peelor FF III, Butterfield TA, Dupont-Versteegden EE. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J Physiol 596: 83–103, 2018. doi: 10.1113/JP275089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyabara EH, Nascimento TL, Rodrigues DC, Moriscot AS, Davila WF, AitMou Y, deTombe PP, Mestril R. Overexpression of inducible 70-kDa heat shock protein in mouse improves structural and functional recovery of skeletal muscles from atrophy. Pflugers Arch 463: 733–741, 2012. doi: 10.1007/s00424-012-1087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moro T, Brightwell CR, Deer RR, Graber TG, Galvan E, Fry CS, Volpi E, Rasmussen BB. Muscle protein anabolic resistance to essential amino acids does not occur in healthy older adults before or after resistance exercise training. J Nutr 148: 900–909, 2018. doi: 10.1093/jn/nxy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morton JP, MacLaren DP, Cable NT, Bongers T, Griffiths RD, Campbell IT, Evans L, Kayani A, McArdle A, Drust B. Time course and differential responses of the major heat shock protein families in human skeletal muscle following acute nondamaging treadmill exercise. J Appl Physiol (1985) 101: 176–182, 2006. doi: 10.1152/japplphysiol.00046.2006. [DOI] [PubMed] [Google Scholar]
- 53.Mosoni L, Malmezat T, Valluy MC, Houlier ML, Attaix D, Mirand PP. Lower recovery of muscle protein lost during starvation in old rats despite a stimulation of protein synthesis. Am J Physiol Endocrinol Metab 277: E608–E616, 1999. doi: 10.1152/ajpendo.1999.277.4.E608. [DOI] [PubMed] [Google Scholar]
- 54.Murlasits Z, Cutlip RG, Geronilla KB, Rao KM, Wonderlin WF, Alway SE. Resistance training increases heat shock protein levels in skeletal muscle of young and old rats. Exp Gerontol 41: 398–406, 2006. doi: 10.1016/j.exger.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Neufer PD, Benjamin IJ. Differential expression of B-crystallin and Hsp27 in skeletal muscle during continuous contractile activity. Relationship to myogenic regulatory factors. J Biol Chem 271: 24089–24095, 1996. doi: 10.1074/jbc.271.39.24089. [DOI] [PubMed] [Google Scholar]
- 56.Ogasawara R, Nakazato K, Sato K, Boppart MD, Fujita S. Resistance exercise increases active MMP and β1-integrin protein expression in skeletal muscle. Physiol Rep 2: e12212, 2014. doi: 10.14814/phy2.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oishi Y, Ishihara A, Talmadge RJ, Ohira Y, Taniguchi K, Matsumoto H, Roy RR, Edgerton VR. Expression of heat shock protein 72 in atrophied rat skeletal muscles. Acta Physiol Scand 172: 123–130, 2001. doi: 10.1046/j.1365-201X.2001.00847.x. [DOI] [PubMed] [Google Scholar]
- 58.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286: E321–E328, 2004. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 59.Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol (1985) 97: 243–248, 2004. doi: 10.1152/japplphysiol.01383.2003. [DOI] [PubMed] [Google Scholar]
- 60.Peretti D, Bastide A, Radford H, Verity N, Molloy C, Martin MG, Moreno JA, Steinert JR, Smith T, Dinsdale D, Willis AE, Mallucci GR. RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature 518: 236–239, 2015. doi: 10.1038/nature14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 62.Rantanen T, Guralnik JM, Sakari-Rantala R, Leveille S, Simonsick EM, Ling S, Fried LP. Disability, physical activity, and muscle strength in older women: the Women’s Health and Aging Study. Arch Phys Med Rehabil 80: 130–135, 1999. doi: 10.1016/S0003-9993(99)90109-0. [DOI] [PubMed] [Google Scholar]
- 63.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 20: 768–769, 2006. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr 54, Suppl 3: S40–S47, 2000. doi: 10.1038/sj.ejcn.1601024. [DOI] [PubMed] [Google Scholar]
- 65.Samelman TR. Heat shock protein expression is increased in cardiac and skeletal muscles of Fischer 344 rats after endurance training. Exp Physiol 85: 97–102, 2000. doi: 10.1111/j.1469-445X.2000.01894.x. [DOI] [PubMed] [Google Scholar]
- 66.Senf SM, Dodd SL, Judge AR. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am J Physiol Cell Physiol 298: C38–C45, 2010. doi: 10.1152/ajpcell.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Senf SM, Dodd SL, McClung JM, Judge AR. Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J 22: 3836–3845, 2008. doi: 10.1096/fj.08-110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smart F, Aschrafi A, Atkins A, Owens GC, Pilotte J, Cunningham BA, Vanderklish PW. Two isoforms of the cold-inducible mRNA-binding protein RBM3 localize to dendrites and promote translation. J Neurochem 101: 1367–1379, 2007. doi: 10.1111/j.1471-4159.2007.04521.x. [DOI] [PubMed] [Google Scholar]
- 69.Thomson DM, Gordon SE. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. J Physiol 574: 291–305, 2006. doi: 10.1113/jphysiol.2006.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tuckow AP, Vary TC, Kimball SR, Jefferson LS. Ectopic expression of eIF2Bepsilon in rat skeletal muscle rescues the sepsis-induced reduction in guanine nucleotide exchange activity and protein synthesis. Am J Physiol Endocrinol Metab 299: E241–E248, 2010. doi: 10.1152/ajpendo.00151.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Pelt DW, Confides AL, Judge AR, Vanderklish PW, Dupont-Versteegden EE. Cold shock protein RBM3 attenuates atrophy and induces hypertrophy in skeletal muscle. J Muscle Res Cell Motil 39: 35–40, 2018. doi: 10.1007/s10974-018-9496-x. [DOI] [PubMed] [Google Scholar]
- 72.Verhees KJ, Schols AM, Kelders MC, Op den Kamp CM, van der Velden JL, Langen RC. Glycogen synthase kinase-3β is required for the induction of skeletal muscle atrophy. Am J Physiol Cell Physiol 301: C995–C1007, 2011. doi: 10.1152/ajpcell.00520.2010. [DOI] [PubMed] [Google Scholar]
- 73.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286: 1206–1212, 2001. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vyas DR, Spangenburg EE, Abraha TW, Childs TE, Booth FW. GSK-3beta negatively regulates skeletal myotube hypertrophy. Am J Physiol Cell Physiol 283: C545–C551, 2002. doi: 10.1152/ajpcell.00049.2002. [DOI] [PubMed] [Google Scholar]
- 75.Waters-Banker C, Dupont-Versteegden EE, Kitzman PH, Butterfield TA. Investigating the mechanisms of massage efficacy: the role of mechanical immunomodulation. J Athl Train 49: 266–273, 2014. doi: 10.4085/1062-6050-49.2.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.West DWD, Marcotte GR, Chason CM, Juo N, Baehr LM, Bodine SC, Baar K. Normal Ribosomal Biogenesis but Shortened Protein Synthetic Response to Acute Eccentric Resistance Exercise in Old Skeletal Muscle. Front Physiol 9: 1915, 2019. doi: 10.3389/fphys.2018.01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.White JR, Confides AL, Moore-Reed S, Hoch JM, Dupont-Versteegden EE. Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways. Exp Gerontol 64: 17–32, 2015. doi: 10.1016/j.exger.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev 47: 123–132, 2018. doi: 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wood LK, Kayupov E, Gumucio JP, Mendias CL, Claflin DR, Brooks SV. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J Appl Physiol (1985) 117: 363–369, 2014. doi: 10.1152/japplphysiol.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang HJ, Ju F, Guo XX, Ma SP, Wang L, Cheng BF, Zhuang RJ, Zhang BB, Shi X, Feng ZW, Wang M. RNA-binding protein RBM3 prevents NO-induced apoptosis in human neuroblastoma cells by modulating p38 signaling and miR-143. Sci Rep 7: 41738, 2017. doi: 10.1038/srep41738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu X, Bührer C, Wellmann S. Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cell Mol Life Sci 73: 3839–3859, 2016. doi: 10.1007/s00018-016-2253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zou K, Meador BM, Johnson B, Huntsman HD, Mahmassani Z, Valero MC, Huey KA, Boppart MD. The α7β1-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol (1985) 111: 1134–1141, 2011. doi: 10.1152/japplphysiol.00081.2011. [DOI] [PubMed] [Google Scholar]