Abstract
The purpose of this study was to examine the effects of lifelong aerobic exercise on single-muscle fiber performance in trained women (LLE; n = 7, 72 ± 2 yr) by comparing them to old healthy nonexercisers (OH; n = 10, 75 ± 1 yr) and young exercisers (YE; n = 10, 25 ± 1 yr). On average, LLE had exercised ~5 days/wk for ~7 h/wk over the past 48 ± 2 yr. Each subject had a vastus lateralis muscle biopsy to examine myosin heavy chain (MHC) I and IIa single-muscle fiber size and function (strength, speed, power). MHC I fiber size was similar across all three cohorts (YE = 5,178 ± 157, LLE = 4,983 ± 184, OH = 4,902 ± 159 µm2). MHC IIa fiber size decreased (P < 0.05) 36% with aging (YE = 4,719 ± 164 vs. OH = 3,031 ± 153 µm2), with LLE showing a similar 31% reduction (3,253 ± 189 µm2). LLE had 17% more powerful (P < 0.05) MHC I fibers and offset the 18% decline in MHC IIa fiber power observed with aging (P < 0.05). The LLE contractile power was driven by greater strength (+11%, P = 0.056) in MHC I fibers and elevated contractile speed (+12%, P < 0.05) in MHC IIa fibers. These data indicate that lifelong exercise did not benefit MHC I or IIa muscle fiber size. However, LLE had contractile function adaptations that enhanced MHC I fiber power and preserved MHC IIa fiber power through different contractile mechanisms (strength vs. speed). The single-muscle fiber contractile properties observed with lifelong aerobic exercise are unique and provide new insights into aging skeletal muscle plasticity in women at the myocellular level.
NEW & NOTEWORTHY This is the first investigation to examine the effects of lifelong exercise on single-muscle fiber physiology in women. Nearly 50 yr of moderate to vigorous aerobic exercise training resulted in enhanced slow-twitch fiber power primarily by increasing force production, whereas fast-twitch fiber power was preserved primarily by increasing contractile speed. These unique muscle fiber power profiles helped offset the effects of fast-twitch fiber atrophy and highlight the benefits of lifelong aerobic exercise for myocellular health.
Keywords: aging, contractile function, lifelong exercise, Masters athletes, single muscle fiber
INTRODUCTION
The number of lifelong exercising women is on the rise, as the “exercise boom” generation is now reaching their eighth decade of life. Influenced by Title IX legislation in 1972 and women trailblazers including Roberta Gibbs, Kathrine Switzer, and Joan Benoit Samuelson, cultural shifts have led to a rise in the number of women who adopt exercise as a lifelong habit. In support of this cultural shift, women now outnumber men participating in running competitions (4). Along with the rising number of lifelong exercising women, more impressive performances are being produced in both track and field and swimming events (2, 30, 50). However, little research has been conducted on this emerging cohort of women who have engaged in recreational and more performance-based exercise regimens over their life span to gain insight into their physiological traits, particularly at the myocellular level.
Aging results in an overall reduction of physiological function, with habitual exercise showing positive effects to slow various aspects of the aging process (50). We recently reported that lifelong aerobic exercisers (>70 yr of age) had a substantial benefit in cardiovascular fitness [maximal oxygen consumption (V̇o2max)] and skeletal muscle metabolic fitness (capillary density and aerobic enzymes) (20). The present report presents a comprehensive single-muscle fiber size and contractile function (strength, speed, and power) profile from the same group of women who have engaged in aerobic exercise for nearly 50 yr. This is particularly important given that aging results in a loss of muscle mass and function, which is more pronounced in the fast-twitch [myosin heavy chain (MHC) IIa] muscle fibers (5, 31). As MHC IIa muscle fibers are four- to sixfold more powerful than slow-twitch (MHC I) muscle fibers, the age-related loss of MHC IIa fiber function is critical for whole muscle performance (8, 38, 43, 58). To date, the effects of various short-term exercise paradigms to improve the function and health profile of the fast-twitch muscle fibers in older individuals (>70 yr) have been mixed (24, 25, 43, 45, 46, 56, 60). The limited data from lifelong exercisers and Masters athletes (aging athletes who may not necessarily have exercised throughout their life) tend to support the idea that there is some benefit for fast-twitch muscle fibers, although these studies are generally on men <70 yr old (14, 15, 29, 42, 61, 68). The working hypothesis was that lifelong aerobic exercise would be an effective countermeasure (at least partially) for fast-twitch fiber health and enhance slow-twitch fiber properties because of recruitment during exercise throughout the life span. To our knowledge, this is the first study to provide insight into single-muscle fiber contractile function in lifelong exercising women.
METHODS
Subjects
Lifelong exercisers (LLE; n = 7), age-matched old healthy nonexercisers (OH; n = 10), and young exercisers (YE; n = 10) were included in this investigation (Table 1). Subjects were recruited from the greater Muncie, IN area with newspaper advertisements, mailed flyers, and personal interactions. Before participation, all subjects underwent an extensive physical examination that included medical history, blood samples for general health markers, resting and exercise electrocardiogram, and blood pressure measurement. Subjects were free from acute or chronic illness (cardiac, pulmonary, liver, or kidney abnormalities, cancer, uncontrolled hypertension, insulin-dependent or non-insulin-dependent diabetes, or other known metabolic disorders) and free from orthopedic limitations (including any artificial joints) and did not smoke or participate in other forms of tobacco use. Participants performed maximal graded exercise tests to assess V̇o2max (20), as well as measurements of whole muscle size and function. The study was approved by the Institutional Review Board of Ball State University. All study procedures, risks, and benefits were explained to the subjects before they gave written informed consent to participate.
Table 1.
Subject characteristics
| Young Exercisers (n = 10) | Lifelong Exercisers (n = 7) | Old Healthy Nonexercisers (n = 10) | |
|---|---|---|---|
| Age, yr | 25 ± 1 | 72 ± 2 | 75 ± 1 |
| Height, cm | 167 ± 2 | 164 ± 2 | 157 ± 2 |
| Weight, kg | 60 ± 2 | 61 ± 4 | 65 ± 1 |
| BMI, kg/m2 | 21 ± 1 | 23 ± 1 | 27 ± 1* |
| Body fat, % | 23 ± 1* | 30 ± 2† | 41 ± 2 |
| V̇o2max, mL·kg−1·min−1 | 44 ± 2* | 26 ± 2† | 18 ± 1 |
| Quadriceps CSA, cm2 | 59 ± 2* | 42 ± 2 | 40 ± 1 |
| Quadriceps strength, Nm | 138 ± 10† | 105 ± 9 | 82 ± 4 |
| Quadriceps 1RM, kg | 80 ± 6* | 54 ± 4 | 42 ± 2 |
| Quadriceps power, W | 404 ± 38* | 221 ± 20 | 146 ± 10 |
| Steps per day | 11,518 ± 1,404* | 7,463 ± 683 | 6,801 ± 823 |
Values are means ± SE. BMI, body mass index; CSA, cross-sectional area; 1RM, 1-repetition maximum; V̇o2max, maximal oxygen consumption.
P < 0.05 vs. other groups;
P < 0.05 vs. OH.
The exercise history of LLE subjects was reviewed for frequency, duration, intensity, and athletic achievements (20). Subjects of the LLE group were aerobic exercisers (primarily runners and cyclists) who had a history of participating in structured exercise on average 5 ± 1 days/wk for 7 ± 1 h/wk for 48 ± 2 yr (Table 2). Included in the LLE group were several individuals who continue to compete in local, regional, and national races, including one subject who cycled ~4,000 mi the year before testing and a national-level Masters track and field athlete. Although OH control subjects were not involved in any structured exercise training, participation in leisure activities (e.g., golfing, leisurely walking, and community service) was not grounds for exclusion. YE consisted of active individuals who exercised 4–6 days/wk for ~7 h/wk.
Table 2.
Exercise training histories
| Young Exercisers (n = 10) | Lifelong Exercisers (n = 7) | Old Healthy Nonexercisers (n = 10) | |
|---|---|---|---|
| Total training years | 5 ± 1 | 48 ± 2 | — |
| Lifetime average | |||
| Frequency, days/wk | — | 4.6 ± 0.3 | — |
| Duration, h/wk | — | 6.6 ± 0.6 | — |
| Intensity* | — | 1.9 ± 0.1 | — |
| Current decade | |||
| Frequency, days/wk | 5.4 ± 0.5 | 4.7 ± 0.4 | — |
| Duration, h/wk | 7.3 ± 1.1 | 6.8 ± 1.0 | — |
| Intensity* | 2.6 ± 0.1 | 2.1 ± 0.2 | — |
Values are means ± SE. Lifetime average reflects current decade exercise habits for young exercisers.
Levels of self-reported training intensity were 1 (light), 2 (moderate), and 3 (hard). In the case that a subject reported >1 training intensity, values were weighted and averaged (e.g., 80% of training at a 2 and 20% of training at a 3 resulted in an overall intensity of 2.2). More detailed exercise training histories are presented by us elsewhere (20).
Whole Muscle Function and Size
To assess quadriceps muscle strength and power among YE, LLE, and OH, isometric and dynamic contractile function tests were implemented in all three groups. Assessment of muscle function was distributed across three visits separated by at least 48 h. Subjects were familiarized with all testing procedures during the first visit. Magnetic resonance imaging (MRI) was used to analyze quadriceps cross-sectional area (CSA) in all three cohorts.
Isometric strength.
Maximal isometric force of the quadriceps was determined at 90° of knee extension on the custom-built ridged chair with a purpose-built leg cuff attached to a load cell (Omegadyne, Sundbury, OH) interfaced to computer software (LabVIEW) to record force (12, 27). Subjects were restrained in the chair to isolate the quadriceps contributions to the measurement. A total of three maximal repetitions, separated by rest periods, were completed for the left and right legs. The highest observed value was considered maximal isometric strength.
1RM.
Maximal isotonic strength [1-repetition maximum (1RM)] of the quadriceps (bilateral) was determined on a standard leg extension device (model 4107; Cybex Eagle, Medway, MA) outfitted with a custom range of motion position sensor that provided audible confirmation of a successful repetition (43, 64). After a 10-min warm-up on a cycle ergometer (Monark Ergomedic 828 E; Vansbro, Sweden; 25–50 W), subjects completed two sets of five repetitions at a low load (~40% 1RM). This was followed by single attempts to lift an incrementally heavier weight starting at a load estimated to be 70–80% 1RM. Attempts were separated by a 2-min rest period, and the approach was designed to determine 1RM in two to four attempts. The heaviest weight successfully lifted was considered the 1RM.
Power.
Bilateral quadriceps power was measured on a standard leg extension device (model 4107; Cybex Eagle, Medway, MA) outfitted with a strain gauge in a half-bridge configuration, a bridge sensor (Omega Engineering, Stamford, CT) to determine torque produced at the fulcrum of the device, and a potentiometer (Vishay Americas, Ontario, CA) to record angular displacement, all interfaced to LabVIEW software to determine power output. Power was determined at 40% of each subject’s 1RM, which is shown to elicit near-maximal power output (27, 52, 55). Three repetitions were performed at a maximal level, and the highest observed value was considered peak power.
MRI.
After 1 h of supine rest to control for the influence of posture-related fluid shifts on muscle size (9), MRI of the quadriceps was obtained by using a nonmetallic foot restraint to control joint angle (muscle length) and scan angle and to minimize leg compression, as we have previously described (63–65). All scans were completed in the morning, and no exercise or strenuous activity was allowed on the day of scanning. Imaging was completed in a 1.5-T scanner (Siemens, Munich, Germany) with serial interleaved images 8 mm thick (TR: 2,000 ms; TE: 8 ms; 512 × 512 matrix; field of view: 480 × 480 mm).
Quadriceps CSA.
MRI images were transferred electronically from the scanner to a personal computer (iMac) at the Human Performance Laboratory and analyzed with NIH image software (ImageJ, version 1.49). Quadriceps CSA (cm2) was determined via manual planimetry, and a detailed description of these measurements has been presented in our studies of aging and bed rest (63, 64). The quadriceps of the right leg of each subject was used for determination of CSA, which was assessed by the same investigator.
Muscle Biopsy
Since physical activity, diet, and time of day can impact various aspects of skeletal muscle physiology, standardized conditions were implemented before the muscle biopsy. Subjects were asked to refrain from structured exercise and physical activity outside of normal activities of daily living for 72 h before the muscle biopsy. Subjects consumed their normal evening meal and were instructed not to ingest any additional food or caloric beverage before arrival at the laboratory the following morning (~6:30 AM). Once in the laboratory, subjects rested quietly in the supine position for 30 min before undergoing a resting muscle biopsy of the vastus lateralis (6, 64). Muscle samples were processed and sectioned into longitudinal bundles. A portion of the muscle to be used for single-muscle fiber physiology was placed in cold skinning solution and stored at −20°C for later analysis. A portion of the muscle to be used for fiber type was immediately stored in 0.5 mL of RNAlater (Ambion, Austin, TX) at 4°C for a 24-h incubation period and then transferred to −20°C until analysis.
Skinning, Relaxing, and Activating Solutions
The skinning solution contained (in mM) 125.0 K propionate, 2.0 EGTA, 4.0 ATP, 1.0 MgCl2, and 20.0 imidazole (pH 7.0), with 50% (vol/vol) glycerol. The compositions of relaxing and activating solutions were calculated with an interactive computer program described by Fabiato and Fabiato (17). Solutions were adjusted for temperature, pH, and ionic strength by using stability constants in calculations (19). Each solution contained (in mM) 7.0 EGTA, 20.0 imidazole, 14.5 creatine phosphate, 1.0 free Mg2+, 4.0 free MgATP, KCl, and KOH to produce an ionic strength of 180 mM and a pH of 7.0. The relaxing and activating solutions had a free Ca2+ concentration ([Ca2+]) of pCa 9.0 and pCa 4.5, respectively (pCa = −log[Ca2+]).
Single-Muscle Fiber Experimental Procedure
On the day of experimentation, a 2.5- to 3.0-mm muscle fiber segment was randomly isolated from a muscle bundle and transferred to an experimental chamber filled with relaxing solution. The ends of the fiber were securely fastened between a force transducer (model 400A; Cambridge Technology, Watertown, MA) and a direct-current torque motor (model 308B; Cambridge Technology) as described by Moss (39). The force transducer and torque motor were calibrated before each experiment. The instrumentation was arranged so a muscle fiber could be rapidly transferred back and forth between experimental chambers filled with relaxing or activating solution. The apparatus was mounted on a microscope to view the fiber (×800) during an experiment. With an eyepiece micrometer, sarcomere length along the isolated muscle fiber was adjusted to 2.5 µm (60). All single-fiber experiments were performed at 15°C.
Unamplified force and length signals were sent to a digital oscilloscope (310; Nicolet, Madison, WI), enabling monitoring of muscle fiber performance throughout data collection. Analog force and position signals were amplified (Dual Differential Amplifier, 300-DIF2; Positron Development, Inglewood, CA), converted to digital signals (National Instruments, Austin, TX), and transferred to a computer (Micron Electronics, Nampa, ID) for analysis using customized software. Servomotor arm and isotonic force clamps were controlled with a computer-interfaced force-position controller (Force Controller, 300-FC1; Positron Development, Inglewood, CA).
For each single-muscle fiber experiment, a fiber with a compliance [calculated as fiber length (FL) divided by the y-intercept, which was generated from the unloaded shortening velocity evaluation] > 10% and/or a decrease in peak force of >10% was discarded and not used for analysis. The within-fiber test/retest variabilities of a single muscle fiber in our laboratory for the measurements of size, force-velocity relationships, force, and contractile velocity were <1%. The coefficients of variation for the force transducer, motor arm, and camera during the ~4-yr time frame of this investigation were <1%. After the completion of single-muscle fiber physiology experiments, each fiber was solubilized in 80 µL of 1% SDS sample buffer and stored at −20°C until being assayed for MHC fiber type.
Single-Muscle Fiber Physiology Experiments
Individual muscle fibers were analyzed for size (CSA), force (Po), specific tension (Po/CSA), maximal unloaded shortening velocity (Vo), force-velocity relationships and power, and fiber type. Experimental procedures were identical to those previously used in our human studies (55, 60). All size and contractile measurements were conducted by the same investigator and within a 4-wk period from when the biopsies were obtained to ensure tissue viability and data reliability.
Single-muscle fiber size.
A video camera (CCD-IRIS, DXC-107A; Sony, Japan) connected to the microscope and computer interface allowed viewing and storage of single-muscle fiber digital images. Fiber diameter was determined at three points along the segment length from an image captured with NIH public domain software (Scion Image, release Beta 4.0.2, for Windows) with the fiber briefly suspended in air (<5 s). For the fiber size-dependent variables (i.e., specific tension and normalized power), CSA was determined with the assumption that the fiber forms a cylindrical shape while suspended in air (36).
Single-muscle fiber Po.
Force and position transducer outputs were amplified and sent to a microcomputer via a Lab-PC+ 16-bit acquisition board (National Instruments, Austin, TX). Resting force was monitored, and then the fiber was maximally activated in activation solution. Po was determined in each fiber by computer subtraction of the baseline force from the peak force in the pCa 4.5 solution. Maximal force was then normalized to CSA to determine specific tension.
Single-muscle fiber Vo.
Vo was measured by the slack test technique as described by Edman (16). While fully activated, a shortening step was imposed and the fiber was held at the shortened length until force redevelopment was detected. Computer analysis determined the duration of unloaded shortening, or time between onset of slack and redevelopment of force. Four different activation and length steps [150, 200, 250, and 300 µm; ≤15% of fiber length (FL)] were used for each fiber, with the slack distance plotted as a function of the duration of Vo. The vertical intercept (y-intercept) was determined from where the resulting time vs. distance relationship intersected the y-axis. Vo (FL/s) was calculated by dividing the slope of the fitted line by the fiber segment length (data were normalized to a sarcomere length of 2.5 µm). Slope of this line defines fiber unloaded shortening velocity (Vo).
Single-muscle fiber power.
Submaximal isotonic load clamps were performed on each fiber for determination of force-velocity parameters and power. Each fiber segment was fully activated in activation solution and subjected to a series of three isotonic load steps. This procedure was performed at various loads so that each fiber underwent a total of 15–18 isotonic contractions.
For the resultant force-velocity relationships, load was expressed as P/Po (P = force during load clamping, Po = peak isometric force developed before submaximal load clamps). Force and shortening velocity data points were derived from the isotonic contractions and fit by the hyperbolic Hill equation (28). Only individual experiments in which R2 ≥ 0.98 were included for analysis.
Fiber peak power was calculated from the fitted force-velocity parameters [Po and maximal shortening velocity (Vmax)]. Absolute power (µN·FL/s) was defined as the product of force (µN) and shortening velocity (FL/s). Normalized power (watts per liter, W/L) was normalized to fiber volume and defined as the product of specific tension and shortening velocity.
Fiber Type Analysis
After the single-fiber contractile measurements, the MHC isoform profile was analyzed for each fiber segment by SDS-PAGE. Fiber type analyses were supplemented by extracting additional fibers (~360 fibers/subject) from RNAlater for MHC determination (YE = 8, LLE = 7, OH = 9). Briefly, samples were run overnight at 4°C on a Hoefer SE 600 gel electrophoresis unit (San Francisco, CA) utilizing a 3.5% (wt/vol) acrylamide stacking gel with a 5.0% separating gel (69). After electrophoresis, gels were silver stained as described by Giulian et al. (18). MHC isoforms (I, I/IIa, IIa, IIa/IIx, IIx, I/IIa/IIx) of each single muscle fiber were identified according to migration rate, as previously described (69).
Statistical Analysis
Statistical analyses were performed with Statistical Analysis Software (SAS; version 9.3; Cary, NC), and statistical tests yielding P values ≤ 0.05 were considered statistically significant. A two-way nested ANOVA was used to compare single-muscle fiber variables of interest (CSA, Po, Po/CSA, Vo, Vmax, absolute and normalized power) from MHC I and IIa muscle fibers among YE, LLE, and OH participants, with Tukey’s post hoc test for multiple comparisons. For each single-muscle fiber parameter within a subject, a mean value for each fiber type was determined. The mean value was then used to represent all fibers of that fiber type for that individual and weighted depending on the number of fibers studied for each person, to calculate a group (YE, LLE, and OH) mean that is presented in results. Because of the low number of hybrid (i.e., fibers expressing multiple MHC isoforms) and pure MHC IIx muscle fibers, analyses were restricted to MHC I and IIa fibers. A one-way ANOVA was used to compare muscle fiber type distribution among the groups, and a Tukey’s post hoc test was performed when significance was noted. All data are presented as means ± SE.
RESULTS
Single-Muscle Fiber Physiological Experiments
In total, 643 single muscle fibers (~24 per subject) were studied as a part of this investigation (Table 3). Additionally, a total of 31 fibers (YE = 12, LLE = 5, OH = 14) did not complete the experiment and were discarded. An additional 34 fibers (YE = 17, LLE = 9, OH = 8) were unable to be fiber typed with SDS-PAGE as a result of low or missing protein in the microcentrifuge tube. Because of the low number of hybrids (~14%, ~3 per subject) and MHC IIx (<1%, 4 total fibers), analysis was limited to MHC I and IIa fibers.
Table 3.
Number and percentage of single-muscle fiber physiology experiments studied by MHC classification
| Young Exercisers |
Lifelong Exercisers |
Old Healthy Nonexercisers |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| MHC isoform | ||||||
| I | 114 | 49 | 83 | 49 | 110 | 46 |
| I/IIa | 13 | 6 | 5 | 3 | 4 | 2 |
| IIa | 87 | 38 | 65 | 38 | 99 | 41 |
| IIa/IIx | 16 | 7 | 16 | 9 | 27 | 11 |
| IIx | 1 | <1 | 2 | 1 | 1 | <1 |
| Total fibers | 231 | 171 | 241 | |||
MHC, myosin heavy chain.
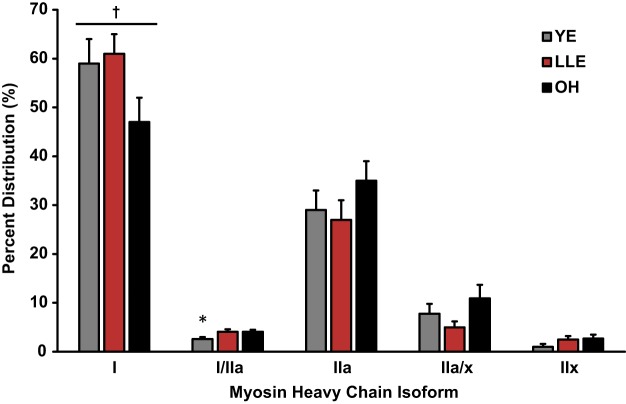
Myosin Heavy Chain Isoform Distribution
MHC fiber distribution is shown in Fig. 1. MHC I fiber distribution trended to be different among the groups (P = 0.079), whereas MHC I/IIa fiber distribution was greater in LLE and OH compared with YE (P < 0.05). No other differences in fiber type distribution were observed.
Fig. 1.
Myosin heavy chain fiber type distribution by SDS-PAGE. Fiber type analysis was completed with ~360 fibers/subject [young exercisers (YE) = 8, lifelong exercisers (LLE) = 7, old healthy nonexercisers (OH) = 9]. Values are means ± SE. *P < 0.05 vs. other groups; †P = 0.079 among groups.
Single-Muscle Fiber Size
Single muscle fiber size (CSA) data for MHC I and IIa fibers are presented in Table 4. MHC I fiber size was similar among the three cohorts. MHC IIa fiber size decreased 36% with aging (P < 0.05), with LLE showing a similar 31% reduction.
Table 4.
Single-muscle fiber size and contractile function in YE, LLE, and OH
| CSA, µm2 | Po, mN | Vo, FL/s | Vmax, FL/s | |
|---|---|---|---|---|
| MHC I fibers | ||||
| YE | 5,178 ± 157 | 0.62 ± 0.02† | 1.10 ± 0.02 | 0.97 ± 0.02 |
| LLE | 4,983 ± 184 | 0.69 ± 0.02 | 1.11 ± 0.02 | 1.01 ± 0.03 |
| OH | 4,902 ± 159 | 0.63 ± 0.02 | 1.09 ± 0.02 | 0.94 ± 0.02 |
| MHC IIa fibers | ||||
| YE | 4,719 ± 164* | 0.75 ± 0.03* | 3.29 ± 0.08 | 3.17 ± 0.08 |
| LLE | 3,253 ± 189 | 0.61 ± 0.03 | 3.71 ± 0.09* | 3.64 ± 0.09* |
| OH | 3,031 ± 153 | 0.56 ± 0.02 | 3.33 ± 0.07 | 3.23 ± 0.07 |
Values are means ± SE. CSA, cross-sectional area; FL, fiber length; LLE, lifelong exercisers; MHC, myosin heavy chain; OH, old healthy nonexercisers; Po, peak force; Vmax, maximal shortening velocity; Vo, unloaded shortening velocity; YE, young exercisers.
P < 0.05 vs. other groups;
P = 0.056 vs. LLE.
Single-Muscle Fiber Power
MHC I and IIa fiber power curves are presented in Figs. 2 and 3, respectively. MHC I fiber peak power was not altered with aging (YE vs. OH), whereas LLE were 17% more powerful (P < 0.05) than YE. MHC IIa fiber peak power was reduced 18% with aging (P < 0.05). In contrast, LLE had similar MHC IIa fiber peak power compared with YE.
Fig. 2.
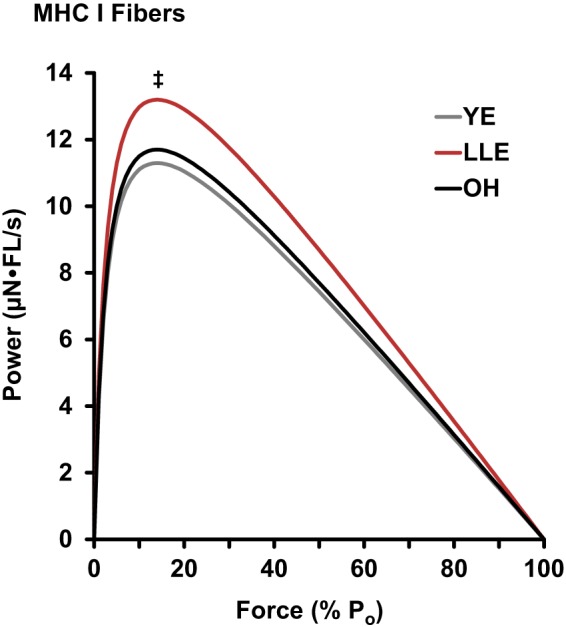
Mean power curves [µN·fiber length (FL)/s] of myosin heavy chain (MHC) I fibers for young exercisers (YE), lifelong exercisers (LLE), and old healthy nonexercisers (OH). Statistical analysis was conducted on the values for peak power (Po). Significant difference in these single values for specific groups: ‡P < 0.05 vs. YE.
Fig. 3.
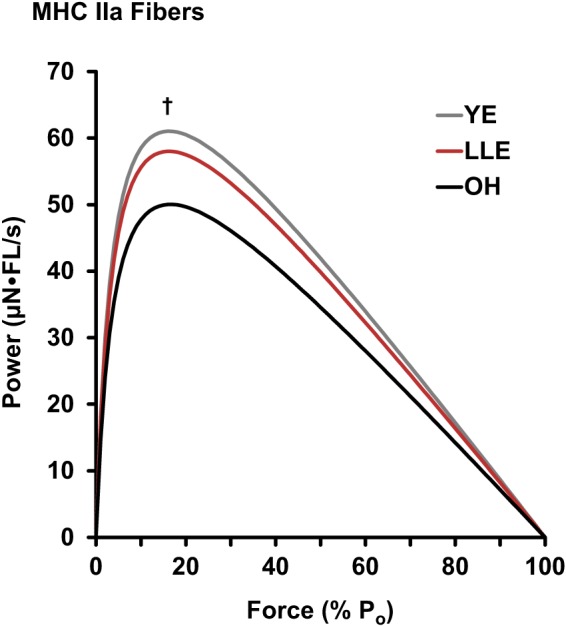
Mean power curves [µN·fiber length (FL)/s] of myosin heavy chain (MHC) IIa fibers for young exercisers (YE), lifelong exercisers (LLE), and old healthy nonexercisers (OH). Statistical analysis was conducted on the values for peak power (Po). Significant difference in these single values for specific groups: †P < 0.05 vs. OH.
Single-Muscle Fiber Force
Peak force (Po) data for MHC I and IIa fibers are presented in Table 4. MHC I fiber peak force was not altered with aging (YE vs. OH), whereas LLE produced 11% more force than YE (P = 0.056). MHC IIa fiber peak force was 25% lower (P < 0.05) with aging (YE vs. OH), with LLE showing a similar 19% reduction compared with YE (P < 0.05).
Single-Muscle Fiber Velocity
Unloaded shortening velocity (Vo) and velocity as calculated from the force-velocity test (Vmax) for MHC I and IIa fibers are presented in Table 4. No differences were observed in MHC I fibers among the groups. In MHC IIa fibers, LLE were ~12% faster than YE and OH (P < 0.05).
Single-Muscle Fiber Quality
Single-muscle fiber quality was assessed by normalizing force (specific tension) and power (normalized power) to cell size. These muscle quality measurements for MHC I and IIa fibers are presented in Table 5. In MHC I fibers, specific tension resulted in a hierarchical pattern (LLE > OH > YE; P < 0.05). Similarly, normalized power in LLE was greater than in YE (P < 0.05) and OH (P = 0.057), and that in OH was greater than in YE (P = 0.055). In MHC IIa fibers, specific tension and normalized power increased (P < 0.05) with aging (YE vs. OH), with LLE showing similar increases compared with YE (P < 0.05).
Table 5.
Single-muscle fiber quality in MHC I and IIa fibers of YE, LLE, and OH
| MHC I Fibers |
MHC IIa Fibers |
|||
|---|---|---|---|---|
| Specific Tension, kN/m2 | Normalized Power, W/L | Specific Tension, kN/m2 | Normalized Power, W/L | |
| YE | 121 ± 2a | 2.2 ± 0.1c,d | 162 ± 3a | 13.5 ± 0.5a |
| LLE | 140 ± 3b | 2.7 ± 0.1e | 193 ± 3 | 18.5 ± 0.6 |
| OH | 131 ± 2 | 2.4 ± 0.1 | 191 ± 3 | 17.3 ± 0.5 |
Values are means ± SE. LLE, lifelong exercisers; MHC, myosin heavy chain; OH, old healthy nonexercisers; YE, young exercisers.
P < 0.05 vs. other groups;
P < 0.05 vs. OH;
P < 0.05 vs. LLE;
P = 0.055 vs. OH;
P = 0.057 vs. OH.
DISCUSSION
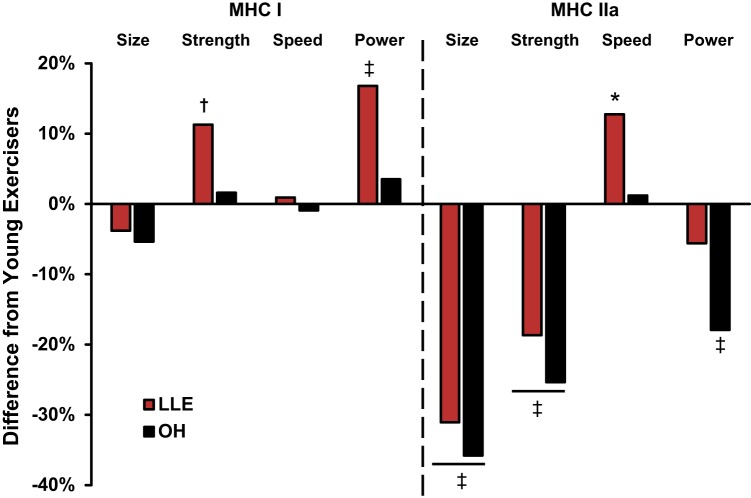
The women who adopted exercising consistently as a lifestyle as a result of cultural events including the exercise boom of the 1970s and Title IX legislation are now >70 yr old. This provided a unique opportunity to assess the effects of aging and lifelong exercise by comparing single-muscle fiber contractile properties of lifelong exercisers with young exercisers and age-matched nonexercisers. To our knowledge, this is the first investigation to assess single-muscle fiber contractile properties of lifelong aerobic exercising women. The main findings from this investigation were that 1) lifelong exercise did not provide any apparent benefit for MHC I or IIa fiber size, 2) LLE had enhanced MHC I fiber power and preserved MHC IIa fiber power, which typically declines with aging (49, 55), and 3) the myocellular power adaptations of the LLE cohort were driven by strength in the MHC I fibers and speed in the MHC IIa fibers (Fig. 4). The single-muscle fiber contractile properties observed with lifelong aerobic exercise are unique and provide new insights into aging skeletal muscle plasticity in women at the myocellular level.
Fig. 4.
Percent difference of single-muscle fiber function of lifelong exercisers (LLE) and old healthy nonexercisers (OH) compared with young exercisers (YE). MHC, myosin heavy chain. *P < 0.05 vs. other groups; ‡P < 0.05 vs. YE; †P = 0.056 vs. YE.
From Lexell’s pioneering work in the 1980s to the present study, it is well established that aging primarily targets the MHC IIa fibers, resulting in decreased size, strength, and power (11, 31, 40, 49, 55). This age-related loss of MHC IIa fiber power has significant implications for whole muscle function, as MHC IIa fibers are best suited for explosive tasks and produce four to six times more power than MHC I fibers (55, 68). The present study shows that lifelong aerobic exercise appears to preserve MHC IIa fiber power, as the lifelong aerobic exercisers were similar to young exercisers and 16% (8 µN·FL/s) more powerful than age-matched nonexercisers. Previous research investigating high-intensity training, such as resistance exercise, has been shown to increase MHC IIa fiber power by 20–60% in young and old individuals (44, 56, 60, 66). Likewise, aerobic exercise has also been shown to increase MHC IIa fiber power after a taper program (reduced training load) in swimmers (53) and runners (32, 57). Furthermore, 12 wk of moderate to vigorous cycle training in older men has also resulted in increased MHC IIa fiber power output (25). Together, these data support the idea that various exercise paradigms improve MHC IIa fiber power, and the present study extends these data to indicate that 50 yr of consistent aerobic exercise is beneficial for MHC IIa fiber power and prevents the typical decline that is observed with aging.
The preservation of MHC IIa fiber power in LLE appears to be driven by increased contractile velocity, as MHC IIa fibers of the LLE cohort were similar in size and force production to those of the OH cohort. This combination of increasing velocity and decreasing size/force appears to be a remodeling mechanism as a result of lifelong aerobic exercise. Interestingly, the top performers in the LLE cohort [~4,000 mi/yr cyclist and national level runner; both exhibited the highest skeletal muscle metabolic fitness of the LLE cohort (20)] had the fastest (Vo = 3.95 and 4.15 FL/s) and smallest (CSA = 2,460 and 2,700 µm) MHC IIa fibers of the lifelong exercisers. Furthermore, their normalized power (21.1 and 21.0 W/L) values were also the highest of the LLE cohort, suggesting that exercising at a high level across the life span may decrease MHC IIa fiber size, while increasing velocity to ultimately preserve power. A higher contractile velocity is not totally surprising, since it can be modified with various exercise regimens (41, 53, 60, 68), but in the context that it offset the decrease in fiber size and force with age to preserve fiber power is a novel finding. Although the mechanism of this myocellular remodeling is not well understood, alterations in the specific proteins (i.e., content, abundance, posttranslational modifications), calcium kinetics, and packing density of the cell are plausible hypotheses (7, 10, 48). Overall, the myocellular remodeling likely reflects the balance among fiber size, contractile performance, and metabolic demands that has resulted from ~50 yr of consistent aerobic exercise.
Lifelong exercise was also beneficial for MHC I fiber power, as the LLE cohort was 17% more powerful than the YE cohort. Along with resistance training (44, 56, 60, 66), aerobic exercise in relatively untrained individuals can increase power of MHC I fibers from the gastrocnemius through marathon training (57) and the vastus lateralis through cycle training (24, 25). Interestingly, collegiate runners that are in a base training phase have MHC I fibers that are ~130% more powerful than recreational runners (26). However, when these collegiate runners incorporate more high-intensity training as the season progresses, the MHC I fiber power drops by nearly 40%, essentially eliminating the differences observed in the base training phase (23). Thus training background and intensity can play a big role in single-muscle fiber dynamics. Middle-aged elite Masters runners have 13% lower MHC I fiber power compared with sedentary controls, mirroring the collegiate runner’s paradigm during heavy training (68). On the basis of the available literature, we interpret the improved MHC I fiber power with ~50 yr of lifelong aerobic exercise to be more reflective of the type of training that would generally be classified as base training among younger endurance athletes, which makes sense given the age and training patterns of these individuals.
Contrary to the MHC IIa fibers, the higher MHC I fiber power observed in the LLE cohort was primarily due to enhanced force production as opposed to velocity. This MHC I myocellular profile appears to be unique to lifelong exercise, as the previous training programs mentioned above that increased MHC I fiber power are a result of velocity (24, 26, 60) and/or hypertrophy of the fibers (24–26, 44, 56, 60, 66). As the LLE cohort had greater force than and similar size as YE and OH, the ~50 yr of aerobic exercise resulted in improved specific tension (i.e., muscle fiber quality). Enhancements in MHC I fiber specific tension have been previously reported with endurance training in young runners as these fibers became smaller with training (23, 57). Specific tension appears to be a function of packing density of contractile proteins and/or passive stiffness (37). Therefore, as fiber size was similar among the groups in the present study, lifelong aerobic exercise may enhance the amount of proteins or alter the protein’s function (i.e., posttranslational modifications) to cause these functional adaptations, likely augmenting power in these fatigue-resistant fibers.
Although some of the functional properties of the MHC I and IIa muscle fibers were positively impacted by lifelong aerobic exercise, there did not appear to be any benefit for fiber size. In our cohorts, both YE and OH had typical fiber size patterns that would be generally expected (55), as the MHC I and IIa fibers from YE were similar in size (within 10%) whereas the MHC IIa fibers from OH were 38% smaller than their MHC I fibers. The LLE women mirrored OH, as their MHC IIa fibers were 35% smaller than their MHC I fibers and nearly identical in size to OH. Although the MHC IIa fibers were smaller among LLE and OH, these fibers had greater function per unit size (i.e., specific tension and normalized power). This increased muscle fiber quality with aging has been observed previously by our laboratory (21, 43) and others (48, 49) and now can be extended to include older lifelong exercisers.
We hypothesized that the MHC IIa fibers, which atrophy to a greater extent with aging (5, 31), would have some benefit from the lifelong aerobic exercise due to general use/recruitment during exercise (3). This idea is generally supported by previous work in middle-aged to older (>45 to <70 yr of age) Masters athletes (14, 29, 61, 67). Aerobic exercise has been shown to be anabolic (protein synthesis and myogenic gene networks) after acute exercise (22, 34, 70). Furthermore, aerobic exercise training ranging from 12 wk to 12 mo has proven effective to induce fast-twitch muscle fiber hypertrophy in young and old individuals (13, 24, 25). When aerobic exercise is combined with resistance exercise, it enhances hypertrophy during short-term training (5 wk) and prevents atrophy during long-term (60 days) bed rest (33, 54). However, these anabolic benefits of aerobic training did not appear to translate to an enhanced fiber size in the lifelong exercisers, which has been similarly shown in lifelong (~70 yr of age) women cyclists (n = 2) (42), women track athletes (~80 yr of age) (47), ~70-yr-old men who began cycle training in their thirties (35), and lifelong (~70 yr of age) endurance-trained men (1). We speculate that the MHC IIa fibers were optimized for aerobic exercise, as MHC IIa fiber size may have contributed to enhanced metabolic efficiency as previously shown in lifelong exercisers (15) and warrants further research.
Although we observed enhanced functional adaptations in both fiber types with lifelong aerobic exercise compared with OH, they did not appear to translate to whole muscle size and function (Table 1). For whole muscle size, this appears to be mostly explained by the similar fiber size profile of the LLE and OH cohorts. The similar whole muscle function between the older cohorts may be partially explained by the fiber type profiles, as the lifelong exercisers trended to have a greater distribution of the relatively less powerful, albeit fatigue-resistant, MHC I fibers (61% and 47% in LLE and OH, respectively). Elite Masters runners (~50 yr) also exhibited no differences in whole muscle size or strength with 20–25 yr of run training compared with age-matched nonexercisers (62). Although the middle-aged Masters runners had less powerful MHC IIa fibers (−27%) than nonrunners, they had a large abundance of slow-twitch fibers (~75%) compared with the nonexercisers (~50%), which likely explains the similar whole muscle strength findings (67). This phenomenon of altered fiber type distribution influencing whole muscle performance was identified over 40 years ago (51). Our team expanded on this idea to include contractile properties, showing that a large slow-twitch to fast-twitch fiber transition during 90-day bed rest resulted in composite myofiber power profiles that were masked at the whole muscle level (59). Taken together, these data show that alterations within muscle fibers are not always reflected at the whole muscle level, which appears to be the case in the present investigation.
The exercise boom generation ushered in an increased participation in exercise habits among everyday people that turned into a lifestyle. We had the unique opportunity to examine the potential health benefits at the myocellular level from women, now in their eighth decade of life, who had regularly exercised for nearly 50 yr. The key finding from this investigation was that lifelong aerobic exercise did not provide any apparent benefit for MHC I or IIa fiber size. However, LLE enhanced slow-twitch (MHC I) muscle fiber power and preserved fast-twitch (MHC IIa) muscle fiber power. The underlying mechanisms for these lifelong aerobic exercise adaptations varied between the MHC I fibers (strength) and MHC IIa fibers (speed). The single-muscle fiber contractile properties observed with lifelong aerobic exercise are unique and provide new insights into aging skeletal muscle plasticity in women at the myocellular level. We acknowledge the limitation that these lifelong exercisers participated in various forms of aerobic exercise across their life and we were thus unable to make mode-specific comparisons. However, these subjects were representative of the exercise boom generation given that individuals likely altered their exercise habits for various reasons (e.g., injuries or change in interests). Future research should consider other modes of exercise (i.e., resistance exercise) and the potential wide range of benefits across multiple physiological systems with lifelong exercise.
GRANTS
This research was supported by NIH Grant R01 AG-038576 and the Ball State University Academic Excellence Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
U.R., T.A.T., and S.T. conceived and designed research; K.J.G., K.M., U.R., G.J.G., G.B., B.G., T.A.T., and S.T. performed experiments; K.J.G., K.M., U.R., G.J.G., G.B., W.H.F., T.A.T., and S.T. analyzed data; K.J.G. and S.T. interpreted results of experiments; K.J.G. and S.T. prepared figures; K.J.G. and S.T. drafted manuscript; K.J.G., K.M., U.R., G.J.G., G.B., W.H.F., B.G., T.A.T., and S.T. edited and revised manuscript; K.J.G., K.M., U.R., G.J.G., G.B., W.H.F., B.G., T.A.T., and S.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the many Human Performance Laboratory graduate students and support staff who were involved with various aspects of data collection and interaction with the volunteers. We are grateful to all the volunteers who graciously gave time, energy, and support to this project.
REFERENCES
- 1.Aagaard P, Magnusson PS, Larsson B, Kjaer M, Krustrup P. Mechanical muscle function, morphology, and fiber type in lifelong trained elderly. Med Sci Sports Exerc 39: 1989–1996, 2007. doi: 10.1249/mss.0b013e31814fb402. [DOI] [PubMed] [Google Scholar]
- 2.Akkari A, Machin D, Tanaka H. Greater progression of athletic performance in older Masters athletes. Age Ageing 44: 683–686, 2015. doi: 10.1093/ageing/afv023. [DOI] [PubMed] [Google Scholar]
- 3.Altenburg TM, Degens H, van Mechelen W, Sargeant AJ, de Haan A. Recruitment of single muscle fibers during submaximal cycling exercise. J Appl Physiol (1985) 103: 1752–1756, 2007. doi: 10.1152/japplphysiol.00496.2007. [DOI] [PubMed] [Google Scholar]
- 4.Andersen JJ. The State of Running 2019 (Online). Fort Collins, CO: RunRepeat https://runrepeat.com/state-of-running?fbclid=IwAR3x_Z4MeyKxCaLBwOTBL8uSqcAnz64s5H_Lh8aGHbsm72GxRz_G4Su1zcU [18 Nov 2019]. [Google Scholar]
- 5.Aniansson A, Hedberg M, Henning GB, Grimby G. Muscle morphology, enzymatic activity, and muscle strength in elderly men: a follow-up study. Muscle Nerve 9: 585–591, 1986. doi: 10.1002/mus.880090702. [DOI] [PubMed] [Google Scholar]
- 6.Bergström J. Muscle electrolytes in man. Scand J Clin Lab Invest 14: 1–110, 1962. [Google Scholar]
- 7.Bottinelli R, Betto R, Schiaffino S, Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. J Physiol 478: 341–349, 1994. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottinelli R, Pellegrino MA, Canepari M, Rossi R, Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J Electromyogr Kinesiol 9: 87–95, 1999. doi: 10.1016/S1050-6411(98)00040-6. [DOI] [PubMed] [Google Scholar]
- 9.Brill PA, Macera CA, Davis DR, Blair SN, Gordon N. Muscular strength and physical function. Med Sci Sports Exerc 32: 412–416, 2000. doi: 10.1097/00005768-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Brocca L, McPhee JS, Longa E, Canepari M, Seynnes O, De Vito G, Pellegrino MA, Narici M, Bottinelli R. Structure and function of human muscle fibres and muscle proteome in physically active older men. J Physiol 595: 4823–4844, 2017. doi: 10.1113/JP274148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan DM, Bedrin NG, Subramanian M, Berking J, Ades PA, Toth MJ, Miller MS. Age-related structural alterations in human skeletal muscle fibers and mitochondria are sex specific: relationship to single-fiber function. J Appl Physiol (1985) 116: 1582–1592, 2014. doi: 10.1152/japplphysiol.01362.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol (1985) 105: 1907–1915, 2008. doi: 10.1152/japplphysiol.00059.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol (1985) 72: 1780–1786, 1992. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 14.Coggan AR, Spina RJ, Rogers MA, King DS, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic characteristics of skeletal muscle in master athletes. J Appl Physiol (1985) 68: 1896–1901, 1990. doi: 10.1152/jappl.1990.68.5.1896. [DOI] [PubMed] [Google Scholar]
- 15.Dubé JJ, Broskey NT, Despines AA, Stefanovic-Racic M, Toledo FG, Goodpaster BH, Amati F. Muscle characteristics and substrate energetics in lifelong endurance athletes. Med Sci Sports Exerc 48: 472–480, 2016. doi: 10.1249/MSS.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol 291: 143–159, 1979. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 75: 463–505, 1979. [PubMed] [Google Scholar]
- 18.Giulian GG, Moss RL, Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem 129: 277–287, 1983. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- 19.Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol 80: 279–297, 1982. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gries KJ, Raue U, Perkins RK, Lavin KM, Overstreet BS, D’Acquisto LJ, Graham B, Finch WH, Kaminsky LA, Trappe TA, Trappe S. Cardiovascular and skeletal muscle health with lifelong exercise. J Appl Physiol (1985) 125: 1636–1645, 2018. doi: 10.1152/japplphysiol.00174.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosicki GJ, Standley RA, Murach KA, Raue U, Minchev K, Coen PM, Newman AB, Cummings S, Harris T, Kritchevsky S, Goodpaster BH, Trappe S; Health ABC Study . Improved single muscle fiber quality in the oldest-old. J Appl Physiol (1985) 121: 878–884, 2016. doi: 10.1152/japplphysiol.00479.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harber MP, Crane JD, Dickinson JM, Jemiolo B, Raue U, Trappe TA, Trappe SW. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Regul Integr Comp Physiol 296: R708–R714, 2009. doi: 10.1152/ajpregu.90906.2008. [DOI] [PubMed] [Google Scholar]
- 23.Harber MP, Gallagher PM, Creer AR, Minchev KM, Trappe SW. Single muscle fiber contractile properties during a competitive season in male runners. Am J Physiol Regul Integr Comp Physiol 287: R1124–R1131, 2004. doi: 10.1152/ajpregu.00686.2003. [DOI] [PubMed] [Google Scholar]
- 24.Harber MP, Konopka AR, Douglass MD, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol 297: R1452–R1459, 2009. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol (1985) 113: 1495–1504, 2012. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harber M, Trappe S. Single muscle fiber contractile properties of young competitive distance runners. J Appl Physiol (1985) 105: 629–636, 2008. doi: 10.1152/japplphysiol.00995.2007. [DOI] [PubMed] [Google Scholar]
- 27.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol (1985) 103: 2068–2076, 2007. doi: 10.1152/japplphysiol.00670.2007. [DOI] [PubMed] [Google Scholar]
- 28.Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci 126: 136–195, 1938. doi: 10.1098/rspb.1938.0050. [DOI] [PubMed] [Google Scholar]
- 29.Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, Schnohr P, Saltin B. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand 140: 41–54, 1990. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- 30.Kundert AM, Di Gangi S, Nikolaidis PT, Knechtle B. Jumping and throwing performance in the World Masters’ Athletic Championships 1975–2016. Res Sports Med 27: 374–411, 2019. doi: 10.1080/15438627.2018.1528975. [DOI] [PubMed] [Google Scholar]
- 31.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 32.Luden N, Hayes E, Minchev K, Louis E, Raue U, Conley T, Trappe S. Skeletal muscle plasticity with marathon training in novice runners. Scand J Med Sci Sports 22: 662–670, 2012. doi: 10.1111/j.1600-0838.2011.01305.x. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg TR, Fernandez-Gonzalo R, Gustafsson T, Tesch PA. Aerobic exercise does not compromise muscle hypertrophy response to short-term resistance training. J Appl Physiol (1985) 114: 81–89, 2013. doi: 10.1152/japplphysiol.01013.2012. [DOI] [PubMed] [Google Scholar]
- 34.Lundberg TR, Fernandez-Gonzalo R, Tesch PA, Rullman E, Gustafsson T. Aerobic exercise augments muscle transcriptome profile of resistance exercise. Am J Physiol Regul Integr Comp Physiol 310: R1279–R1287, 2016. doi: 10.1152/ajpregu.00035.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKendry J, Joanisse S, Baig S, Liu B, Parise G, Greig CA, Breen L. Superior aerobic capacity and indices of skeletal muscle morphology in chronically trained master endurance athletes compared with untrained older adults. J Gerontol A Biol Sci Med Sci 2019: glz142, 2019. doi: 10.1093/gerona/glz142. [DOI] [PubMed] [Google Scholar]
- 36.Metzger JM, Moss RL. Shortening velocity in skinned single muscle fibers. Influence of filament lattice spacing. Biophys J 52: 127–131, 1987. doi: 10.1016/S0006-3495(87)83197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller MS, Bedrin NG, Ades PA, Palmer BM, Toth MJ. Molecular determinants of force production in human skeletal muscle fibers: effects of myosin isoform expression and cross-sectional area. Am J Physiol Cell Physiol 308: C473–C484, 2015. doi: 10.1152/ajpcell.00158.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME 2nd, Ades PA, Maughan DW, Palmer BM, Toth MJ. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J Appl Physiol (1985) 115: 1004–1014, 2013. doi: 10.1152/japplphysiol.00563.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moss RL. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol 292: 177–192, 1979. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murgia M, Toniolo L, Nagaraj N, Ciciliot S, Vindigni V, Schiaffino S, Reggiani C, Mann M. Single muscle fiber proteomics reveals fiber-type-specific features of human muscle aging. Cell Rep 19: 2396–2409, 2017. doi: 10.1016/j.celrep.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 41.Pansarasa O, Rinaldi C, Parente V, Miotti D, Capodaglio P, Bottinelli R. Resistance training of long duration modulates force and unloaded shortening velocity of single muscle fibres of young women. J Electromyogr Kinesiol 19: e290–e300, 2009. doi: 10.1016/j.jelekin.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Pollock RD, O’Brien KA, Daniels LJ, Nielsen KB, Rowlerson A, Duggal NA, Lazarus NR, Lord JM, Philp A, Harridge SD. Properties of the vastus lateralis muscle in relation to age and physiological function in master cyclists aged 55–79 years. Aging Cell 17: e12735, 2018. doi: 10.1111/acel.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol (1985) 106: 1611–1617, 2009. doi: 10.1152/japplphysiol.91587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shoepe TC, Stelzer JE, Garner DP, Widrick JJ. Functional adaptability of muscle fibers to long-term resistance exercise. Med Sci Sports Exerc 35: 944–951, 2003. doi: 10.1249/01.MSS.0000069756.17841.9E. [DOI] [PubMed] [Google Scholar]
- 45.Singh MA, Ding W, Manfredi TJ, Solares GS, O’Neill EF, Clements KM, Ryan ND, Kehayias JJ, Fielding RA, Evans WJ. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol Endocrinol Metab 277: E135–E143, 1999. doi: 10.1152/ajpendo.1999.277.1.E135. [DOI] [PubMed] [Google Scholar]
- 46.Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295: R273–R280, 2008. doi: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonjak V, Jacob K, Morais JA, Rivera-Zengotita M, Spendiff S, Spake C, Taivassalo T, Chevalier S, Hepple RT. Fidelity of muscle fibre reinnervation modulates ageing muscle impact in elderly women. J Physiol 597: 5009–5023, 2019. doi: 10.1113/JP278261. [DOI] [PubMed] [Google Scholar]
- 48.Straight CR, Ades PA, Toth MJ, Miller MS. Age-related reduction in single muscle fiber calcium sensitivity is associated with decreased muscle power in men and women. Exp Gerontol 102: 84–92, 2018. doi: 10.1016/j.exger.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundberg CW, Hunter SK, Trappe SW, Smith CS, Fitts RH. Effects of elevated H+ and Pi on the contractile mechanics of skeletal muscle fibres from young and old men: implications for muscle fatigue in humans. J Physiol 596: 3993–4015, 2018. doi: 10.1113/JP276018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka H. Aging of competitive athletes. Gerontology 63: 488–494, 2017. doi: 10.1159/000477722. [DOI] [PubMed] [Google Scholar]
- 51.Thorstensson A, Grimby G, Karlsson J. Force-velocity relations and fiber composition in human knee extensor muscles. J Appl Physiol 40: 12–16, 1976. doi: 10.1152/jappl.1976.40.1.12. [DOI] [PubMed] [Google Scholar]
- 52.Tihanyi J, Apor P, Fekete G. Force-velocity-power characteristics and fiber composition in human knee extensor muscles. Eur J Appl Physiol Occup Physiol 48: 331–343, 1982. doi: 10.1007/BF00430223. [DOI] [PubMed] [Google Scholar]
- 53.Trappe S, Costill D, Thomas R. Effect of swim taper on whole muscle and single muscle fiber contractile properties. Med Sci Sports Exerc 32: 48–56, 2000. [PubMed] [Google Scholar]
- 54.Trappe S, Creer A, Slivka D, Minchev K, Trappe T. Single muscle fiber function with concurrent exercise or nutrition countermeasures during 60 days of bed rest in women. J Appl Physiol (1985) 103: 1242–1250, 2007. doi: 10.1152/japplphysiol.00560.2007. [DOI] [PubMed] [Google Scholar]
- 55.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol 281: C398–C406, 2001. doi: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- 57.Trappe S, Harber M, Creer A, Gallagher P, Slivka D, Minchev K, Whitsett D. Single muscle fiber adaptations with marathon training. J Appl Physiol (1985) 101: 721–727, 2006. doi: 10.1152/japplphysiol.01595.2005. [DOI] [PubMed] [Google Scholar]
- 58.Trappe S, Luden N, Minchev K, Raue U, Jemiolo B, Trappe TA. Skeletal muscle signature of a champion sprint runner. J Appl Physiol (1985) 118: 1460–1466, 2015. doi: 10.1152/japplphysiol.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol 557: 501–513, 2004. doi: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol (1985) 89: 143–152, 2000. doi: 10.1152/jappl.2000.89.1.143. [DOI] [PubMed] [Google Scholar]
- 61.Trappe SW, Costill DL, Fink WJ, Pearson DR. Skeletal muscle characteristics among distance runners: a 20-yr follow-up study. J Appl Physiol (1985) 78: 823–829, 1995. doi: 10.1152/jappl.1995.78.3.823. [DOI] [PubMed] [Google Scholar]
- 62.Trappe SW, Costill DL, Goodpaster BH, Pearson DR. Calf muscle strength in former elite distance runners. Scand J Med Sci Sports 6: 205–210, 1996. doi: 10.1111/j.1600-0838.1996.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 63.Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf) 191: 147–159, 2007. doi: 10.1111/j.1748-1716.2007.01728.x. [DOI] [PubMed] [Google Scholar]
- 64.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300: R655–R662, 2011. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol (1985) 90: 2070–2074, 2001. doi: 10.1152/jappl.2001.90.6.2070. [DOI] [PubMed] [Google Scholar]
- 66.Widrick JJ, Stelzer JE, Shoepe TC, Garner DP. Functional properties of human muscle fibers after short-term resistance exercise training. Am J Physiol Regul Integr Comp Physiol 283: R408–R416, 2002. doi: 10.1152/ajpregu.00120.2002. [DOI] [PubMed] [Google Scholar]
- 67.Widrick JJ, Trappe SW, Blaser CA, Costill DL, Fitts RH. Isometric force and maximal shortening velocity of single muscle fibers from elite master runners. Am J Physiol Cell Physiol 271: C666–C675, 1996. doi: 10.1152/ajpcell.1996.271.2.C666. [DOI] [PubMed] [Google Scholar]
- 68.Widrick JJ, Trappe SW, Costill DL, Fitts RH. Force-velocity and force-power properties of single muscle fibers from elite master runners and sedentary men. Am J Physiol Cell Physiol 271: C676–C683, 1996. doi: 10.1152/ajpcell.1996.271.2.C676. [DOI] [PubMed] [Google Scholar]
- 69.Williamson DL, Godard MP, Porter DA, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain coexpression in single muscle fibers from older men. J Appl Physiol (1985) 88: 627–633, 2000. doi: 10.1152/jappl.2000.88.2.627. [DOI] [PubMed] [Google Scholar]
- 70.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 98: 1745–1752, 2005. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]