Abstract
Arterial oxygen tension and oxyhemoglobin saturation () decrease in parallel during hypoxia. Distinguishing between changes in oxygen tension and oxygen content as the relevant physiological stimulus for cardiorespiratory alterations remains challenging. To overcome this, we recruited nine individuals with hemoglobinopathy manifesting as high-affinity hemoglobin [HAH; partial pressure at 50% (P50) = 16 ± 0.4 mmHg] causing greater at a given oxygen partial pressure compared with control subjects (n = 12, P50 = 26 ± 0.4 mmHg). We assessed ventilatory and cardiovascular responses to acute isocapnic hypoxia, iso-oxic hypercapnia, and 20 min of isocapnic hypoxia (arterial Po2 = 50 mmHg). Blood gas alterations were achieved with dynamic end-tidal forcing. When expressed as a function of the logarithm of oxygen partial pressure, ventilatory sensitivity to hypoxia was not different between groups. However, there was a significant difference when expressed as a function of . Conversely, the rise in heart rate was blunted in HAH subjects when expressed as a function of partial pressure but similar when expressed as a function of . Ventilatory sensitivity to hypercapnia was not different between groups. During sustained isocapnic hypoxia, the rise in minute ventilation was similar between groups; however, heart rate was significantly greater in the controls during 3 to 9 min of exposure. Our results support the notion that oxygen tension, not content, alters cellular Po2 in the chemosensors and drives the hypoxic ventilatory response. Our study suggests that in addition to oxygen partial pressure, oxygen content may also influence the heart rate response to hypoxia.
NEW & NOTEWORTHY We dissociated the effects of oxygen content and pressure of cardiorespiratory regulation studying individuals with high-affinity hemoglobin (HAH). During hypoxia, the ventilatory response, expressed as a function of oxygen tension, was similar between HAH variants and controls; however, the rise in heart rate was blunted in the variants. Our work supports the notion that the hypoxic ventilatory response is regulated by oxygen tension, whereas cardiovascular regulation may be influenced by arterial oxygen content and tension.
Keywords: heart rate, high-affinity hemoglobin, ventilation
INTRODUCTION
Exposure to hypoxia is a longstanding method to stress physiological responses to study pulmonary and cardiovascular regulation. Due to the sigmoidal shape of the oxygen-hemoglobin dissociation curve, inspiring a mildly hypoxic gas results in decreases in with only modest changes in oxyhemoglobin saturations () and thus arterial oxygen content (). As a result, mild hypoxia is often not physiologically significant, in awake humans, because it often will not reach the threshold for a detectable response. Decreasing the inspired oxygen concentration further results in decreases in both and , which alter cellular environments and elicit a robust physiological response. However, because of the parallel decrease in and in significant hypoxia, dissociating whether the upstream stimulus driving the ventilatory response is altered oxygen tension or content remains equivocal. For example, while it is generally agreed that governs the hypoxic ventilatory response, there are cases from comparative biology suggesting has an independent impact (6). Several methods have been used to address this biological challenge, including isovolumetric hemodilution or inhalation of carbon monoxide (CO). Isovolumetric hemodilution (removal of red blood cells while maintaining blood volume) can decrease hemoglobin (Hb) concentrations and thus . However, without the addition of a hypoxic inspirate, the remaining Hb is fully saturated, and thus cardiovascular changes due to partial pressure status do not occur (4). Hemodilution can also be time consuming and the hypoxic ventilatory response can change considerably within 2 h (27). Inhaling CO, which preferentially binds to Hb and decreases without changes in , itself alters carotid chemosensor activity (14, 24), which render interpretation of ventilatory responses more complex.
A solution to the limitations in previous work is to use individuals with rare hemaglobinopathies causing increased Hb oxygen affinity that manifests as different where Hb is 50% saturated (P50). In individuals with normal Hb, the P50 is ~27 mmHg (normal range 24–30 mmHg), whereas in individuals with altered oxygen affinity variant Hb, the oxygen dissociation curve (ODC) can be “left-shifted” (higher affinity, P50 < 24 mmHg) or “right-shifted” (lower affinity, P50 > 30 mmHg). Therefore, using individuals with variant Hb as “experiments of nature” offers an approach to dissociate the effects of and . Previously, others have used this approach by studying individuals with high-affinity Hb (P50 = 17 mmHg; HAH) and concluded that , rather than , is the primary hypoxic ventilatory stimulus (9). The authors also demonstrated that the heart rate response to hypoxia was blunted in those with HAH, but the hypercapnic ventilatory response was similar. However, the previous study was limited by small sample size (n = 2 with variant Hb and no statistical analysis), the subjects were relatively young (12–18 yr), the testing was short (4–5 min), and minimal cardiovascular measures were taken.
Thus the purpose of this study was to dissociate the effects of and on the control of breathing and cardiopulmonary response to acute hypoxia using individuals with rare HAH. We sought to comprehensively test the ventilatory sensitivity to hypoxia and hypercapnia along with the cardiopulmonary response to sustained acute hypoxia. We hypothesized that those participants with HAH would have similar hypoxic ventilatory response to control subjects, indicating that oxygen tension is the primary stimulus. Moreover, we hypothesized that the heart rate response would be mediated by oxygen content, and thus the HAH would have blunted response to various severities of hypoxia.
METHODS
Ethical approval.
All study procedures were approved by the Mayo Clinic Institutional Review Board (16–007719), and the study was in accordance with the Declaration of Helsinki except registration in a database. Subjects provided written informed consent before beginning the study.
Participants.
Subjects (n = 9; 6 women:3 men) with rare high-affinity Hb were recruited from a Mayo Clinic database. The Hb variants were either Hb-Malmö (n = 8) or Hb-San Diego (n = 1). The eight subjects with Hb-Malmö were all related. Both Hb-Malmö and San Diego are β-substitutions: Malmö (Hsn97Gln) (1, 5) and San Diego (Val109Met) (19). Both variants maintain a normal Bohr effect but are reported to have decreased cooperativity (1, 19). Individuals with normal Hb (n = 12) were continually recruited to match the Hb variants. One Hb variant and one matched control subject were current smokers (<1 pack per day).
Experimental design.
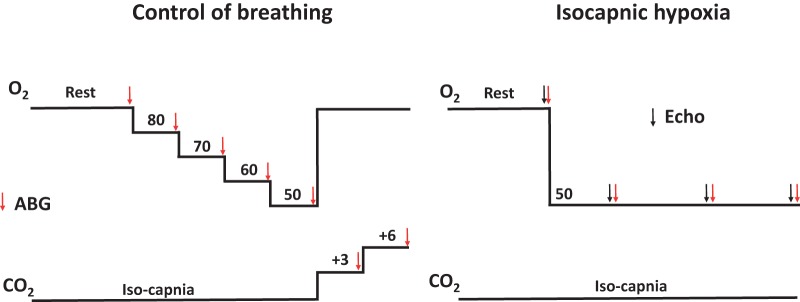
Upon arrival to the laboratory, we obtained a venous blood sample from each subject to assess the ODC and measure P50 to verify if their Hb was variant or control. Thereafter, each subject was instrumented with an arterial catheter and fitted with a face mask. Subjects then rested for 10 min in the supine positions while breathing room air on the mask. Subjects’ ventilatory sensitivity to oxygen and carbon dioxide was then determined. The subjects were allowed to recover breathing room air (off the face mask) for 30 min before performing the final test consisting of 20 min of isocapnic hypoxia. An experimental schematic is presented in Fig. 1. For all testing, we utilized a dynamic end-tidal forcing system that adjusted the inspirate on a breath-by-breath basis to bring end-tidal gases to their desired level (36, 37). The system allows for the independent control of end-tidal oxygen and carbon dioxide levels irrespective of ventilation. Transthoracic echocardiogram images were obtained before and at time points during the 20-min isocapnic hypoxia exposure.
Fig. 1.
Schematic of the testing. Numerical values for O2 and CO2 are end-tidal values targeted during testing. For the “Control of breathing” aspect of testing, each change in end-tidal gases was sustained for 3 min each. ABG, arterial blood gas sample; Echo, transthoracic echocardiography measurement.
Data collection.
Heart rate was continuously measured via ECG in lead II configuration. Respiratory gas flow was measured with a pneumotachograph calibrated with a 3-liter syringe. Volumes were obtained via numerical integration of flow. End-tidal oxygen and carbon dioxide were sampled from a port in the mouthpiece that was connected to a calibrated mass spectrometer (MGA-1100; Perkin-Elmer, Kansas City, MO). Oxyhemoglobin saturation was measured on a distal phalanx with pulse oximetry (Cardiocap/5; Datex-Ohmeda, Louisville, CO). Signals were analogue-to-digital converted (PowerLab; AD Instruments, Colorado Springs, CO) and recorded simultaneously (200 Hz) using data acquisition software (LabChart v8.1).
Arterial blood samples and pressure.
After administration of local anesthesia (2% lidocaine), an indwelling arterial catheter was placed in either the radial or brachial artery using sterile techniques. The catheter was connected to commercially available blood sampling kit that kept patency with pressurized saline. The kit allowed repeated arterial blood sampling and continuous pressure measurements. Arterial blood samples were analyzed immediately with a commercial blood gas analyzer (ABL90 COOX; Radiometer, Copenhagen, Denmark). Offline, blood gases were corrected for blood temperature (30) as measured by a rapid response thermistor (IT-18; Physiemp Instruments, Clinton, NJ) placed in the catheter hub. Arterial blood pressure (Cardiocap/5) was referenced to the level of the heart.
Acute hypoxic and hypercapnic ventilatory response.
To determine the subject’s ventilatory sensitivity to oxygen and carbon dioxide, we manipulated end-tidal gases in discrete stages. First, we obtained 10 min of resting data while the subjects were quiet and supine (wearing face mask). Thereafter, we determined subjects eucapnic acute hypoxic ventilatory response by exposing them to four, 3-min step reductions in end-tidal Po2 (; 80, 70, 60, and 50 mmHg) while maintaining isocapnia at resting levels. Conventionally, when assessing the acute hypoxic ventilatory response, the target s are ~65, 57, and 47 mmHg, which correspond to set decrements in oxyhemoglobin saturation (34). However, due to the varying relationship between Po2 and in the Hb variants, we elected to use equal decrements in . Subsequently, we determined the iso-oxic hypercapnic ventilatory response by increasing 3 and 6 mmHg above eucapnic levels for 3 min each while was held constant at baseline levels (~95 mmHg). An arterial blood sample was obtained during the last 15 s of each 3-min stage for the acute hypoxic ventilatory response and hypercapnic tests.
Sustained isocapnic hypoxia.
After 30 min of recovery, we exposed the subjects to 20 min of isocapnic hypoxia while transthoracic echocardiogram images were obtained. Briefly, subjects first rested on the facemask breathing room air while baseline echocardiography measures were optimized and taken. This period of quiet rest lasted 5–20 min with the subjects connected to the face mask for at least the final 5 min to determine baseline levels. Thereafter, subjects were exposed to 20 min of isocapnic hypoxia. The target was 50 mmHg, and was maintained at resting levels measured during baseline (~40 mmHg). Arterial blood samples and echocardiography measurements were obtained at baseline and at the 5-, 12-, and 20-min marks of hypoxia. Variables measured continuously (e.g., heart rate, ventilation, etc.) were averaged in 1-min epochs for 5 min during rest and the entire 20 min of exposure.
Echocardiography.
Before and throughout the isocapnic hypoxia test at 5, 12, and 20 min, two-dimensional echocardiographic examinations (CX50 or IE33 and S5-1 transducer; Philips Healthcare, Eindhoven, The Netherlands) were performed by an experienced cardiac sonographer in accordance with American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines (17). Images were acquired from standard parasternal and apical windows, and all system settings were adjusted to ensure optimal signal-to-noise ratio and endocardial delineation and held constant throughout the experimental testing sessions for each individual. A minimum of three cardiac cycles were captured, and all echocardiographic data were analyzed offline on a commercially available workstation (Q-LAB 10.8.5; Philips Healthcare). Stroke volume was calculated as the product of left ventricular outflow tract cross-sectional area and pulsed-wave Doppler-derived blood velocity-time integral, measured immediately proximal to the aortic valve during systole, and cardiac output was calculated by multiplication with heart rate.
Analysis.
To determine the partial pressure at 50% saturation, heparinized venous blood samples were analyzed for hemoglobin oxygen saturation and partial pressure of oxygen by dual wavelength spectrophotometry and a Clark electrode, respectively (Hemox Analyzer; TCS Medical Products, Huntington Valley, PA). After deoxygenation with nitrogen, the compressed air reoxygenation curve was measured at pH 7.6 and 37°C using a laboratory developed protocol (40). During the acute hypoxic ventilatory response and hypercapnic ventilatory response tests, the final 30 s of each stages was averaged. Thereafter, linear regressions for V̇e versus and V̇e versus log were calculated for each subject using each stage from the acute hypoxic ventilatory response (rest and 4 hypoxic stages). Similarly, linear regression for V̇e versus and V̇e versus hydrogen ion concentration was employed to the acute hypercapnic ventilatory response (rest and 2 hypercapnic stages). The slope and intercept for each subject were pooled and used to generate average curves and perform statistical comparisons. For the 20 min of isocapnic hypoxic exposure, data were averaged in 1-min intervals starting 5 min before and continuing for 20 min of isocapnic hypoxia. Systemic vascular resistance was calculated as the quotient of mean arterial pressure (via arterial catheter) and cardiac output (via echocardiography).
Statistics.
Descriptive variables and resting arterial blood gases were compared using unpaired Student’s t tests. Ventilatory sensitivity to oxygen and carbon dioxide was compared by determining the slope and intercept for each subject and comparing those means using an unpaired Student’s t test. Variables during isocapnic hypoxia were compared between groups using a 2-way (Hb type [control, high-affinity]) × time (1–20 min) repeated measures ANOVA. When significant F ratios were detected, a Tukey post hoc test was used to determine where the significance lay. Significance was set at P < 0.05, and data are presented as means ± SE.
RESULTS
Subjects.
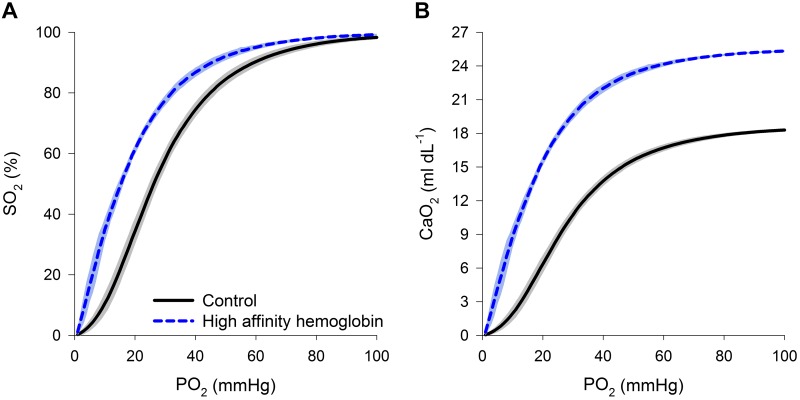
There were no differences in the subjects’ age, sex, mass, or body mass index, however the control subjects were significantly taller (Table 1). As expected, those with HAH had a significantly lower P50 (Fig. 2A), and thus was greater despite no differences in , , or pH (Table 1). We (31) and others (3) have previously demonstrated that P50 is negatively correlated with Hb concentration, and the current participants confirm this with the HAH variants demonstrating marked polycythemia and elevated (Fig. 2B). Other hemoglobinopathies can result in a disagreement between and pulse oximeter-derived values (12). We found the summation of the oxyhemoglobin saturation and minimal fraction of methemoglobin (<1%) and carboxyhemoglobin (1–2%) resulted in a nearly identical to pulse oximetry-derived saturation in all subjects.
Table 1.
Demographics and resting arterial blood samples and pressure
| Control (n = 12) | High-Affinity Hb (n = 9) | |
|---|---|---|
| Sex | 6M:6F | 3M:6F |
| Age, yr | 41 ± 3 | 43 ± 4 |
| Mass, kg | 82 ± 4 | 91 ± 10 |
| Height, cm | 174 ± 3 | 164 ± 3* |
| BMI, kg m2 | 27.0 ± 1.0 | 33.8 ± 3.7 |
| P50, mmHg | 26 ± 0.4 | 16 ± 0.4* |
| , mmHg | 89 ± 2 | 87 ± 2 |
| , mmHg | 40 ± 1 | 39 ± 1 |
| pH | 7.41 ± 0.003 | 7.41 ± 0.004 |
| cBase, mmol/L | 0.5 ± 0.5 | −0.01 ± 0.5 |
| , mmol/L | 25 ± 0.5 | 25 ± 0.5 |
| Lac, mmol/L | 0.6 ± 0.1 | 0.6 ± 0.0 |
| Hb, g/dL | 13.7 ± 0.3 | 19.0 ± 0.6* |
| , %)/ | 96 ± 0.1 | 97 ± 0.2* |
| , ml dL−1 | 17.8 ± 0.4 | 25.0 ± 0.9* |
| MAP, mmHg | 109 ± 4 | 107 ± 3 |
| Heart rate, beats/min | 60 ± 4 | 77 ± 4* |
| ‡Stroke volume, ml | 74 ± 6 | 71 ± 7 |
| ‡Cardiac output, L/min | 4.2 ± 0.2 | 5.5 ± 1.5* |
Values are means ± SE. BMI, body mass index; MAP, mean arterial pressure; P50, pressure at which 50% of hemoglobin is saturated with oxygen; , arterial oxygen tension; , arterial carbon dioxide tension; cBase, base excess concentration; Lac, arterial lactate concentration; Hb, hemoglobin concentration; , arterial oxyhemoglobin saturation; , arterial oxygen content.
P < 0.05, significantly different from control.
Cardiac output and stroke volume were derived from echocardiography in n = 5 high affinity hemoglobin and n = 11 controls.
Fig. 2.
A and B: composite oxyhemoglobin dissociation curves (A) and arterial oxygen content curves (B) for the control subjects and those with high-affinity hemoglobin. Arterial oxygen content was calculated with corresponding oxyhemoglobin dissociation curves and resting hemoglobin concentration. The line represents the mean, and the shaded area represents 1 SD. So2, oxyhemoglobin saturation; Po2, oxygen tension; , arterial oxygen content.
Acute hypoxic ventilatory response.
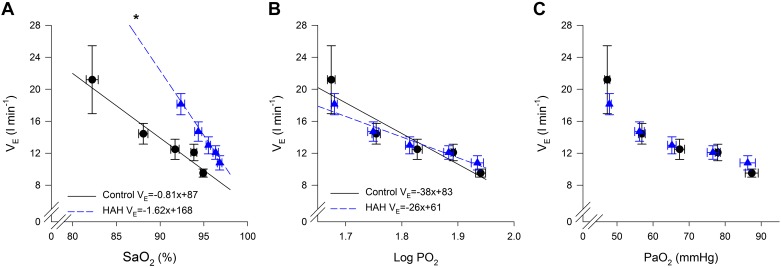
The relationships between oxygen and the ventilatory response are shown in Fig. 3, A–C. When the ventilatory response to hypoxia is expressed as a function of , there are marked differences between the groups (Fig. 3A). Specifically, for a given decrement in saturation, the increase in V̇e is significantly greater in that with HAH (−1.62 ± 0.27 vs. −0.81 ± 0.25 L·min−1·%−1). The intercept was also significantly greater for the HAH group (168 ± 26 vs. 87 ± 23 L/min). There was no difference between groups for the average R2 between and V̇e (0.82 ± 0.05 vs. 0.88 ± 0.03 for controls and HAH, respectively). When the ventilatory response is expressed as function of log however, there are no significant differences between the groups (Fig. 3B, the average slope, intercept, and R2 was −38 ± 14 L·min−1·mmHg−1, 83 ± 26 L/min, and 0.80 ± 0.04 for controls and −26 ± 0.3 L·min−1·mmHg−1, 61 ± 7 L/min, and 0.83 ± 0.05 for HAH). When is expressed as logarithmic function, there is no difference in the linear relationship between log and V̇e between the groups (Fig. 3B). Similarly, when absolute is used, both groups demonstrate the expected hyperbolic relationship (Fig. 3C).
Fig. 3.
Ventilatory response to altered levels of oxygen. A–C: oxyhemoglobin saturation (), oxygen tension (Po2), and arterial oxygen tension (). V̇E, ventilation. HAH, high-affinity hemoglobin. *P < 0.05, significantly different slope than controls.
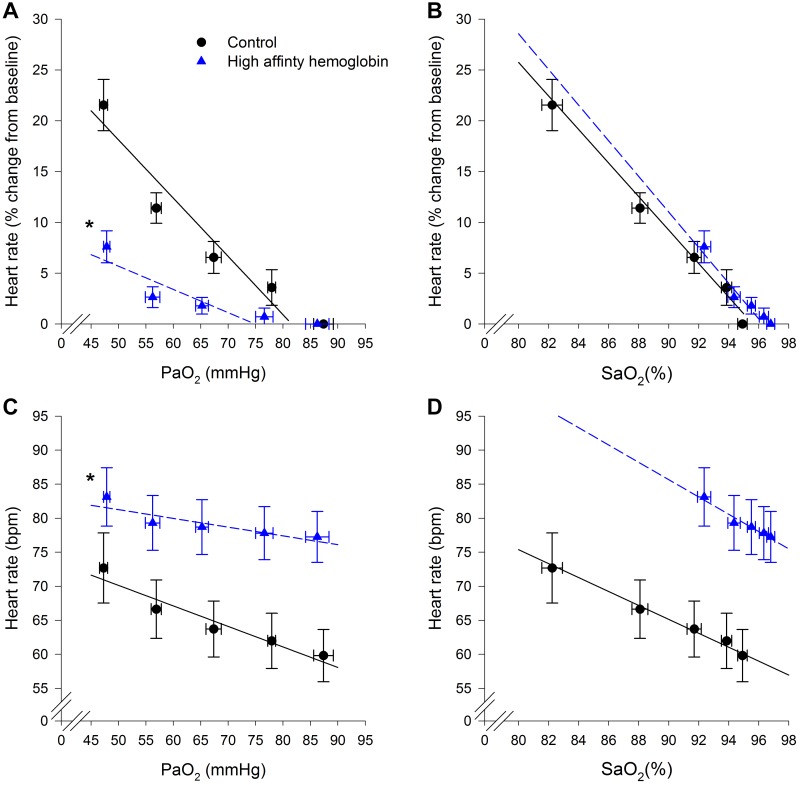
Conversely, heart rate (HR) responses showed the opposite effect compared with V̇e during the acute hypoxia (Fig. 4). When the change in HR (Fig. 4A, relative, or Fig. 4C, absolute) was plotted as a function of , our data show as was lowered the degree of increase in HR was significantly less in the HAH group. For Fig. 4A, the average slope, intercept, and R2 were −0.57 ± 0.07 beats·min−1·mmHg−1, 47 ± 5 beats/min, and 0.80 ± 0.07 for controls and −0.21 ± 0.05 beats·min−1·mmHg−1, 16 ± 4 beats/min, and 0.65 ± 0.08 for HAH. For Fig. 4C, the average slope, intercept and R2 were −0.31 ± 0.04 beats·min−1·mmHg−1, 85 ± 6 beats/min, and 0.80 ± 0.04 for controls and −0.13 ± 0.03 beats·min−1·mmHg−1, 88 ± 5 beats/min, and 0.63 ± 0.09 for HAH. Moreover, some HAH subjects had only a weak relationship between the increase in HR and . However, when the change in HR was expressed as function of , there were no differences in the slopes between groups (Fig. 4, B and D, respectively). For Fig. 4B, the average slope, intercept, and R2 were −1.64 ± 0.24 beats·min−1·%−1, 158 ± 21 beats/min, and 0.87 ± 0.05 for controls and −1.66 ± 0.39 beats·min−1·%−1, 161 ± 37 L/min and 0.81 ± 0.08 for HAH. For Fig. 4D, the average slope, intercept, and R2 were −1.02 ± 0.15 beats·min−1·%−1, 157 ± 15 beats/min, and 0.88 ± 0.04 for controls and −1.24 ± 0.27 beats·min−1·%−1, 197 ± 28 L/min, and 0.79 ± 0.08 for HAH.
Fig. 4.
Changes in heart rate during altered oxygen levels. A and C: arterial oxygen tension (). B and D: oxyhemoglobin saturation (). *P < 0.05, significantly different slope compared with the control subjects.
Acute hypercapnic ventilatory response.
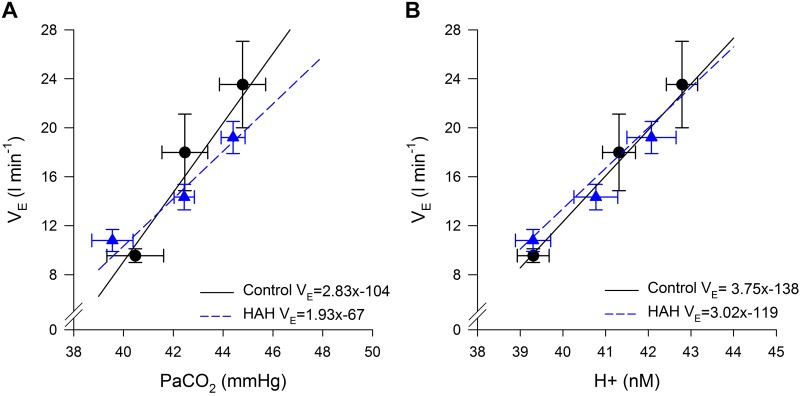
Figure 5 depicts acute hypercapnic ventilatory response. Both groups demonstrated a linear relationship between V̇e versus and V̇e versus arterial hydrogen ion concentration with no significant differences between the groups. The average R2 for the relationship between V̇e and (0.90 ± 0.04 vs. 0.93 ± 0.03 for controls and HAH, respectively) or V̇e and hydrogen ion concentration (0.93 ± 0.02 vs. 0.94 ± 0.03 for controls and HAH, respectively) were not different between groups in either case.
Fig. 5.
Ventilatory response to altered levels of carbon dioxide. A: average slope and intercept was 2.83 ± 0.46 L·min−1·mmHg−1 and −104 ± 19 L/min for controls and 1.94 ± 0.31 L·min−1·mmHg−1and −67 ± 13 L/min for high-affinity hemoglobin (HAH). B: average slope and intercept was 3.75 ± 0.63 L·min−1·mmHg−1and −138 ± 25 L/min for controls and 3.30 ± 0. L·min−1·mmHg−1 and −119 ± 23 L/min for HAH. V̇e, ventilation; , arterial carbon dioxide tension; H+, hydrogen ion concentration.
Sustained isocapnic hypoxia.
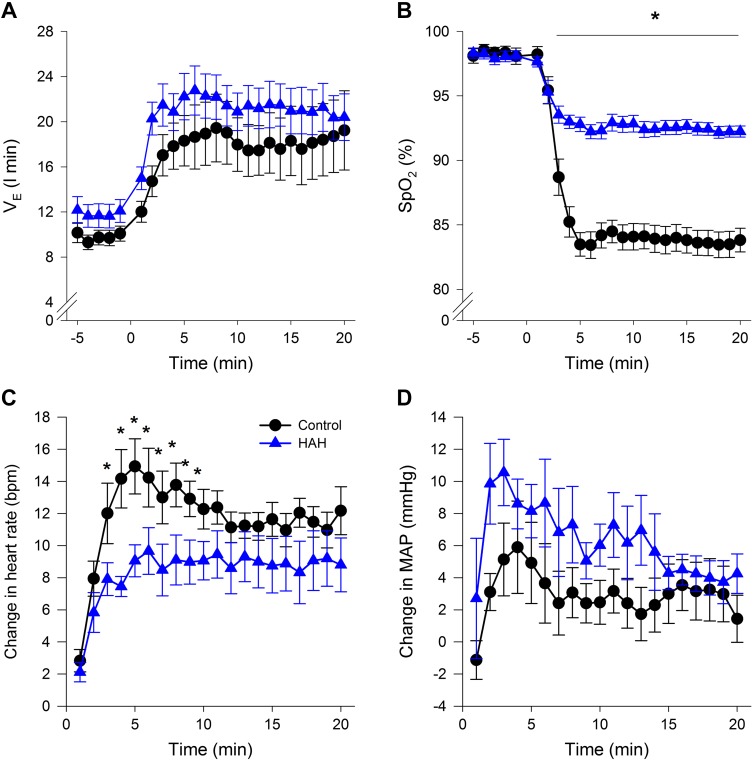
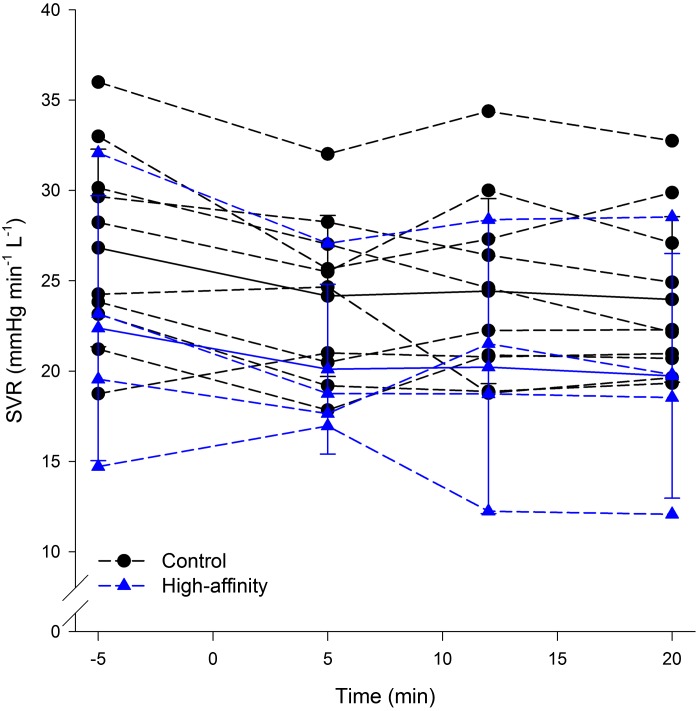
Figure 6 depicts the stimulus and response to 20 min of isocapnic hypoxia. Our intended stimulus of sustained arterial hypoxia and isocapnia was identical between groups (Table 2). There were no differences in end-tidal oxygen or carbon dioxide tensions between groups (P > 0.05). As expected, levels are ~1–2 mmHg lower than values, with no differences in between groups (Table 2). Arterial Pco2 was unchanged compared with rest and not different between groups (Table 2). Despite the identical , as expected the HAH variants maintained a significantly higher during hypoxia due to the shape of the ODC (Fig. 6B and Table 2). There were no differences in V̇e (Fig. 6A) or mean arterial blood pressure (Fig. 6D) at baseline or throughout hypoxia. However, similar to the acute hypoxic ventilatory tests, the rise in HR was significantly blunted in the HAH subjects (Fig. 6C). Systemic vascular resistance is shown in Fig. 7 with both groups showing a decrease during hypoxic exposure.
Fig. 6.
A–D: ventilation (A), oxyhemoglobin saturation (B), heart rate (C), and arterial blood pressure (AD) during the 20 min of hypoxic exposure. Variables were continuously derived variables binned into 1-min epochs and expressed as a change from baseline. V̇e, ventilation; , oxyhemoglobin saturation measured with pulse oximetry; MAP, mean arterial pressure. *P < 0.05, significantly different from HAH subjects.
Table 2.
Arterial blood variables during throughout the 20-min hypoxia exposure
| Variables and Time, mins | Control | High-Affinity Hb |
|---|---|---|
| , mmHg | ||
| Baseline | 90 ± 2 | 85 ± 3 |
| 5 | 49 ± 1 | 47 ± 1 |
| 12 | 48 ± 1 | 48 ± 1 |
| 20 | 48 ± 1 | 48 ± 1 |
| , mmHg | ||
| Baseline | 40 ± 1 | 39 ± 1 |
| 5 | 40 ± 1 | 39 ± 0 |
| 12 | 40 ± 1 | 39 ± 0 |
| 20 | 40 ± 1 | 39 ± 0 |
| pH | ||
| Baseline | 7.40 ± 0.004 | 7.41 ± 0.005 |
| 5 | 7.41 ± 0.004 | 7.41 ± 0.006 |
| 12 | 7.41 ± 0.004 | 7.41 ± 0.006 |
| 20 | 7.40 ± 0.005 | 7.41 ± 0.004 |
| Lac, mmol/L | ||
| Baseline | 0.5 ± 0.1 | 0.6 ± 0.0 |
| 5 | 0.5 ± 0.1 | 0.6 ± 0.0 |
| 12 | 0.5 ± 0.1 | 0.6 ± 0.0 |
| 20 | 0.5 ± 0.0 | 0.6 ± 0.0 |
| , % | ||
| Baseline | 95 ± 0 | 97 ± 0* |
| 5 | 83 ± 1 | 92 ± 1* |
| 12 | 83 ± 1 | 92 ± 1* |
| 20 | 82 ± 1 | 92 ± 1* |
| , ml/dL | ||
| Baseline | 17.7 ± 0.4 | 24.9 ± 0.9* |
| 5 | 15.4 ± 0.4 | 23.6 ± 0.9* |
| 12 | 15.2 ± 0.5 | 23.7 ± 0.9* |
| 20 | 15.2 ± 0.5 | 23.6 ± 0.9* |
Values are means ± SE. , arterial oxygen tension; , arterial carbon dioxide tension; Lac, arterial lactate concentration; , arterial oxyhemoglobin saturation; , arterial oxygen content.
P < 0.05, significantly different from control.
Fig. 7.
Systemic vascular resistance (SVR) derived from echocardiography and measured before and at the 5-, 12-, and 20-min mark of hypoxic exposure.
DISCUSSION
Major findings.
The findings from our study are twofold. First, our data support the notion that the hypoxic ventilatory response is regulated by changes in rather than . Unique and novel in our study was the ability to test this hypothesis in healthy humans without many confounding experimental factors. Second, compensatory HR increases during acute hypoxia may have additional regulating mechanisms other than . Our findings are based on the observation that a similar change in resulted in a smaller increase in heart rate in those with HAH. Overall, our study supports the notion that arterial oxygen tension alters the cellular Po2 in the carotid chemosensors and this is the primary stimulus contributing to acute increases in ventilation during hypoxic exposure. Conversely, the heart rate response to hypoxia appears to be less respective of and modulated by arterial oxygen content.
Acute hypoxic and hypercapnic ventilatory response.
We found that the acute ventilatory response to progressive decrements in was similar in our HAH and control subjects. Specifically, linear increase in V̇e was not different when was expressed as logarithmic function (Fig. 3B). A logarithmic function has been previously shown to be useful when comparing groups with different ODCs (33, 35). The ventilatory response was also not different when expressed in absolute units; however, the response was nonlinear as expected (Fig. 4C) (34). This occurred despite significant differences in and provides further evidence that the hypoxic ventilatory response is governed by changes in that alter cellular Po2 (25).
Much of the research aimed at dissociating the effect of versus relies on comparative literature, and for a more comprehensive review on peripheral chemosensors, the interested readers are directed elsewhere (13). A notable example in humans where and are dissociated is in previous work with HAH subjects that showed similar results (9). In their study, Hebbel et al. (9) studied two young individuals with an Hb mutation that results in a similar P50 as our subjects (Hb Andrew-Minneapolis; P50 ~17 mmHg) and their siblings with typical Hb (P50 = 27 mmHg). Their findings also support that the acute hypoxic ventilatory response was similar between groups when expressed as a function of but different when expressed as a change in .
Further examples from the comparative literature also support these findings. Rats with chemically altered P50 have the same acute hypoxic ventilatory response when expressed in terms of a change in yet divergent in terms of (2). Similarly, the acute hypoxic ventilatory response and P50 have been shown to be independent of each other in chemically mutated rats and guinea pigs (26) and the ODC does not impact V̇e in toads (38). Finally, in goats, it has been demonstrated that O2 tension was the driver of peripheral chemosensor activity (28). Notably however, the lack of a ventilatory response to decreases in is not a universal finding, as others found that decreases in alone caused similar increase in V̇e despite no change in (6). As such, there is still debate in the comparative literature regarding the exact role of in the acute hypoxic ventilatory response (18). Difficulties with interpreting the above findings are the vastly different species and experimental technical limitations. Furthermore, some animals (e.g., Barheaded goose) have evolved to have increased ventilatory sensitivity when compared with related species (29). Additionally, there may be key differences in the role that each peripheral chemosensor (aortic and carotid bodies) has in the acute hypoxic ventilatory response. While it has been convincingly shown that the carotid body responds to changes in , less is known about the aortic body (23). It has been suggested that the aortic body is sensitive to changes in , and this is further discussed in Heart rate.
We found that the iso-oxic hypercapnic ventilatory response was similar between our groups. Specifically, both the controls and HAH demonstrated a linear increase in V̇e during our tests regardless of whether the stimulus was plotted as or H+ concentration (Fig. 5). Our findings suggest that higher Hb oxygen binding affinity does not influence the chemosensitivity to CO2. The finding that HAH does not impact ventilatory sensitivity to CO2 is similar to others who used similar resting measures (9). Another group also demonstrated that subjects with different HAH mutations with greater affinity (P50 = 13 mmHg) have that is within normal limits at rest (39–42 mmHg), was maintained during mild exercise, and was reduced as exercise intensity approached maximal levels (41); all of which would be considered a typical response. Interestingly, on the opposite spectrum of Hb affinity, mutant mice with low-affinity Hb (Hb Presbyterian) have a blunted hypercapnic ventilatory response (10, 22). There is also a case report of resting and exercise blood gases in a single subject with Hb Presbyterian (32). Although the single subject had blood gases at rest that were unremarkable, upon beginning moderate exercise, the subject became hypercapnic ( = 49.1 mmHg), acidotic (pH = 7.23), and mildly hypoxemic ( = 81.8 mmHg), suggesting a blunted ventilatory response (32). Although the specific mechanism blunting CO2 sensitivity has not been identified, it could relate other regulatory mechanisms sensing CO2 production over tension. Although a V̇co2 sensor has yet to be fully elucidated, a coupling between V̇co2 and V̇e at rest (21) and during exercise (20) has been demonstrated in sheep.
Heart rate.
The increase in HR was significantly greater in the controls when expressed as function of , but not as , during both the acute hypoxic ventilatory response testing (Fig. 4) and 20 min of isocapnic hypoxia (Fig. 6). Although the intercepts are different between groups when heart rate is expressed in absolute units (Fig. 4, C and D), it is clear that that the slopes are different for and similar for . This would suggest there are tissues that are sensitive to or the associated cellular effects and may be involved in HR regulation. Evidence for this come from studies finding that while the carotid chemosensors only respond to oxygen tensions, the aortic chemosensors also respond to (7, 15, 16, 23). In line with our findings, others have also demonstrated that those with HAH have a blunted increase in heart rate in response to acute (9) and chronic (8) hypoxia. It is possible that the attenuated increase in HR in subjects with HAH is due to the decreased sensory stimulus from the aortic chemosensors. Interestingly, we found that estimated mixed venous Po2 () was also correlated with the changes in HR. To demonstrate this, we calculated based on measured cardiac output, , Hb, and estimated resting VO2 (31). As detailed in depth elsewhere (31), those with HAH will have a lower resting and we estimated that it would only decrease ~1.5 mmHg when was reduced to 50 mmHg, whereas in the controls would decrease over 4mmHg when going from rest to a of 50 mmHg. While the reduction in is less in those with HAH, similar to the versus HR relationship, the slopes of the relationship between and HR was not different between groups. Specifically, both controls and HAH subjects demonstrated a similarly strong linear relationship (R2 = 0.98 and 0.96 for HAH and controls, respectively). As the slopes are similar between groups, we view this as further evidence that an additional mechanism is regulating changes in HR during hypoxia. However, as and are intertwined, we are unable to determine which is the mechanistic cause or if they are resulting in (or the result of) relative cellular hypoxia.
We caution, however, there are other physiological theories that could explain the changes in HR. First, there was a 17 beats/min difference in resting heart rate between the groups (60 ± 4 vs. 77 ± 4 for the controls and HAH, respectively). The greater vagal tone in controls subjects at rest could allow for a greater increase in heart rate during the acute hypoxic exposure. Although on average the HAH subjects had a greater resting HR, there was a considerable range (56–85 beats/min) that overlapped with controls. The controls were also trending toward (P = 0.06) having a greater maximal aerobic capacity (determined separately from the current study). We found the expected significant relationship between percent predicted maximal aerobic capacity and resting heart rate for each group separately or pooled. As such, the greater resting HR in the HAH is most likely due to their lower maximal aerobic capacity. Second, the higher hematocrits in the HAH subjects may have resulted in greater myocardial work and thus a blunted heart rate response. Third, in response to systemic hypoxia, the systemic circulation will vasodilate to maintain oxygen delivery to peripheral tissues (11). However, there were no differences in systemic vascular resistance (Fig. 7) or mean arterial pressure (Fig. 6D) between groups, suggesting that the degree of peripheral vasodilation was similar. Furthermore, if there was less sensory input from the aortic chemosensors, why would the ventilatory response be similar? Potentially ventilatory control is governed primarily by feedback from the carotid chemosensor whereas cardiovascular regulation also has inputs from the aortic chemosensors.
For both the ventilatory and heart rate response to hypoxia, it is important to reaffirm that the physiological response is governed not by changes in Po2 or So2// per se but rather cellular changes in response to hypoxia (13, 23, 25, 34). The specific mechanism behind the cellular changes is beyond the scope of this paper, and the interested reader is directed elsewhere (23). Briefly, due to the highly vascularized nature of the carotid chemosensors, is very similar to the intracellular Po2. As such, when is reduced so too is cellular Po2, and this results in a signal that alters the chemosensors activity and produces a physiological response. However, in humans, directly measuring the cellular milieu and specific receptor response (e.g., carotid chemosensor and associated neural impulse) is not readily possible. As such, we are only able to measure and present the upstream variables (e.g., ) that subsequently modulate the response.
Methodological considerations.
Investigating cardiorespiratory responses to hypoxia in awake humans has technical limitations. For example, direct recordings of the carotid sinus or vagus nerve were not performed. Therefore, we were unable to directly assess neural discharge from the carotid and aortic chemoreceptors, respectively. The ventilatory response to hypoxia can also be influenced by behavior responses. However, all subjects were given similar explanations and testing was completed with a similar environment and investigators. Therefore, while behavior responses would certainly introduce variability into our measures, we do not believe they have a systematic effect. Eight of the nine subjects with HAH were related, and there is a strong familial effect in the ventilatory response to hypoxia (39). However, three of the control subjects were also related to those eight with Hb Malmö and their responses were similar to the other controls rather than their relatives with HAH. As such, any familial effect on the response to hypoxia is less compared with the effect of variant Hb. Other hemoglobinopathies can result in a disagreement between and pulse oximeter-derived values (12). However, we found the summation of with the minimal fraction of arterial methemoglobin (<1%) and carboxyhemoglobin (1–2%) resulted in a nearly identical to pulse oximetry-derived saturation in all subjects regardless of Hb. Although our HAH subject had normal and elevated , it is possible that their cellular environment was relatively hypoxic due to diminished oxygen unloading ability. Indirect evidence for this comes from our previous work where we calculated that mixed venous Po2 would be lower in HAH subjects at rest (31). Despite potentially having a lower mixed venous Po2, we believe the compensatory polycythemia in our HAH subjects prevents cellular hypoxia, and this is reflected in a pH and lactate that was not different from controls (Tables 1 and 2).
Conclusions.
Using individuals with rare HAH we were able to dissociate the effects of and on the integrative response to hypoxia. We demonstrate that the increase in V̇E during acute hypoxic exposure is regulated by changes in rather than . Conversely, the cardiovascular response appears to be influenced by , but another factor is also regulating the response. At present we are unable to determine if the other factor is , , or if these are merely indicative of what is impacting the response. Overall, our findings reinforce the notion that the ventilatory response to acute hypoxia is governed by the carotid chemosensor responding to changes in arterial oxygen pressure and subsequently intracellular Po2. Future work should attempt to identify the additional mechanism responsible for the cardiovascular response to hypoxia.
GRANTS
The work was funded by National Heart, Lung, and Blood Institute (NHLBI) Grant R-35-HL-139854. P. B. Dominelli was supported by a post-doctoral fellowship from the Natural Science and Engineering Council of Canada. S. E. Baker was supported by NHLBI Grant F32-HL-131151. C. C. Wiggins was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant T32-DK-007352-39. G. E. Foster is a Michael Smith Foundation for Health Research Scholar. G. M. Stewart was supported by American Heart Association Grant 19POST34450022 and a Mayo Clinic Career Development Award in Cardiovascular Disease Research Honoring Dr. Earl H. Wood.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.B.D., S.E.B., C.C.W., G.M.S., P.S., J.R.S., T.K.R., T.B.C., J.D.H., J.L.O., G.E.F., and M.J.J. conceived and designed research; P.B.D., S.E.B., C.C.W., G.M.S., P.S., J.R.S., S.K.R., T.B.C., and M.J.J. performed experiments; P.B.D., S.E.B., C.C.W., G.M.S., P.S., J.R.S., T.K.R., T.B.C., J.D.H., J.L.O., G.E.F., and M.J.J. analyzed data; P.B.D., S.E.B., C.C.W., G.M.S., P.S., J.R.S., T.K.R., T.B.C., J.D.H., J.L.O., G.E.F., and M.J.J. interpreted results of experiments; P.B.D., S.E.B., C.C.W., T.K.R., G.E.F., and M.J.J. prepared figures; P.B.D., S.E.B., C.C.W., G.M.S., P.S., J.R.S., S.K.R., T.K.R., T.B.C., J.D.H., J.L.O., G.E.F., and M.J.J. drafted manuscript; P.B.D., S.E.B., C.C.W., G.M.S., P.S., J.R.S., S.K.R., T.K.R., T.B.C., J.D.H., J.L.O., G.E.F., and M.J.J. edited and revised manuscript; P.B.D., S.E.B., C.C.W., G.M.S., P.S., J.R.S., S.K.R., T.K.R., T.B.C., J.D.H., J.L.O., G.E.F., and M.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We are thankful for the enthusiastic participation of our research subjects.
REFERENCES
- 1.Berglund S. The oxygen dissociation curve of whole blood containing haemoglobin Malmö. Scand J Haematol 9: 377–386, 1972. doi: 10.1111/j.1600-0609.1972.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 2.Birchard GF, Tenney SM. The hypoxic ventilatory response of rats with increased blood oxygen affinity. Respir Physiol 66: 225–233, 1986. doi: 10.1016/0034-5687(86)90075-7. [DOI] [PubMed] [Google Scholar]
- 3.Birchard GF, Tenney SM. Relationship between blood-oxygen affinity and blood volume. Respir Physiol 83: 365–373, 1991. doi: 10.1016/0034-5687(91)90055-N. [DOI] [PubMed] [Google Scholar]
- 4.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 24: 107–116, 2009. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fairbanks VF, Maldonado JE, Charache S, Boyer SH 4th. Familial erythrocytosis due to electrophoretically undetectable hemoglobin with impaired oxygen dissociation (hemoglobin Malmö, alpha 2 beta 2 97 gln). Mayo Clin Proc 46: 721–727, 1971. [PubMed] [Google Scholar]
- 6.Garland RJ, Kinkead R, Milson WK. The ventilatory response of rodents to changes in arterial oxygen content. Respir Physiol 96: 199–211, 1994. doi: 10.1016/0034-5687(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 7.Hatcher JD, Chiu LK, Jennings DB. Anemia as a stimulus to aortic and carotid chemoreceptors in the cat. J Appl Physiol 44: 696–702, 1978. doi: 10.1152/jappl.1978.44.5.696. [DOI] [PubMed] [Google Scholar]
- 8.Hebbel RP, Eaton JW, Kronenberg RS, Zanjani ED, Moore LG, Berger EM. Human llamas: adaptation to altitude in subjects with high hemoglobin oxygen affinity. J Clin Invest 62: 593–600, 1978. doi: 10.1172/JCI109165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebbel RP, Kronenberg RS, Eaton JW. Hypoxic ventilatory response in subjects with normal and high oxygen affinity hemoglobins. J Clin Invest 60: 1211–1215, 1977. doi: 10.1172/JCI108874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izumizaki M, Tamaki M, Suzuki Y, Iwase M, Shirasawa T, Kimura H, Homma I. The affinity of hemoglobin for oxygen affects ventilatory responses in mutant mice with Presbyterian hemoglobinopathy. Am J Physiol Regul Integr Comp Physiol 285: R747–R753, 2003. doi: 10.1152/ajpregu.00104.2003. [DOI] [PubMed] [Google Scholar]
- 11.Joyner MJ, Casey DP. Muscle blood flow, hypoxia, and hypoperfusion. J Appl Physiol (1985) 116: 852–857, 2014. doi: 10.1152/japplphysiol.00620.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh R, Miyake T, Arai T. Unexpectedly low pulse oximeter readings in a boy with unstable hemoglobin Köln. Anesthesiology 80: 472–473, 1994. doi: 10.1097/00000542-199402000-00029. [DOI] [PubMed] [Google Scholar]
- 13.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2: 141–219, 2012. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahiri S, Iturriaga R, Mokashi A, Ray DK, Chugh D. CO reveals dual mechanisms of O2 chemoreception in the cat carotid body. Respir Physiol 94: 227–240, 1993. doi: 10.1016/0034-5687(93)90050-K. [DOI] [PubMed] [Google Scholar]
- 15.Lahiri S, Mulligan E, Nishino T, Mokashi A, Davies RO. Relative responses of aortic body and carotid body chemoreceptors to carboxyhemoglobinemia. J Appl Physiol 50: 580–586, 1981. doi: 10.1152/jappl.1981.50.3.580. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri S, Nishino T, Mokashi A, Mulligan E. Relative responses of aortic body and carotid body chemoreceptors to hypotension. J Appl Physiol 48: 781–788, 1980. doi: 10.1152/jappl.1980.48.5.781. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28: 1–39.e14, 2015. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Milsom WK, Wang T. Is the hypoxic ventilatory response driven by blood oxygen concentration? J Exp Biol 220: 956–958, 2017. doi: 10.1242/jeb.151316. [DOI] [PubMed] [Google Scholar]
- 19.Nute PE, Stamatoyannopoulos G, Hermodson MA, Roth D. Hemoglobinopathic erythrocytosis due to a new electrophoretically silent variant, hemoglobin San Diego (beta109 (G11)val--met). J Clin Invest 53: 320–328, 1974. doi: 10.1172/JCI107553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillipson EA, Bowes G, Townsend ER, Duffin J, Cooper JD. Role of metabolic CO2 production in ventilatory response to steady-state exercise. J Clin Invest 68: 768–774, 1981. doi: 10.1172/JCI110313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillipson EA, Duffin J, Cooper JD. Critical dependence of respiratory rhythmicity on metabolic CO2 load J Appl Physiol 50: 45–54, 1981. doi: 10.1152/jappl.1981.50.1.45. [DOI] [PubMed] [Google Scholar]
- 22.Pokorski M, Izumizaki M, Shirasawa T. Chemosensory ventilatory responses in the mutant mice with Presbyterian hemoglobinopathy. Respir Physiol Neurobiol 187: 18–25, 2013. doi: 10.1016/j.resp.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Prabhakar NR. O2 and CO2 detection by the carotid and aortic bodies. In: Chemosensory Transduction, edited by Zufall F, Munger. Amsterdam, The Netherlands: Elsevier Academic, 2016, chapt 18, p. 321–338. [Google Scholar]
- 24.Prabhakar NR, Dinerman JL, Agani FH, Snyder SH. Carbon monoxide: a role in carotid body chemoreception. Proc Natl Acad Sci USA 92: 1994–1997, 1995. doi: 10.1073/pnas.92.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prabhakar NR, Peng YJ. Peripheral chemoreceptors in health and disease. J Appl Physiol (1985) 96: 359–366, 2004. doi: 10.1152/japplphysiol.00809.2003. [DOI] [PubMed] [Google Scholar]
- 26.Rivera-Ch M, León-Velarde F, Huicho L, Monge-C C. Ventilatory response to severe acute hypoxia in guinea pigs and rats with low hemoglobin-oxygen affinity induced by phytic acid. Comp Biochem Physiol A Physiol 112: 411–416, 1995. doi: 10.1016/0300-9629(95)02008-X. [DOI] [PubMed] [Google Scholar]
- 27.Sahn SA, Zwillich CW, Dick N, McCullough RE, Lakshminarayan S, Weil JV. Variability of ventilatory responses to hypoxia and hypercapnia. J Appl Physiol 43: 1019–1025, 1977. doi: 10.1152/jappl.1977.43.6.1019. [DOI] [PubMed] [Google Scholar]
- 28.Santiago TV, Edelman NH, Fishman AP. The effect of anemia on the ventilatory response to transient and steady-state hypoxia. J Clin Invest 55: 410–418, 1975. doi: 10.1172/JCI107945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott GR, Hawkes LA, Frappell PB, Butler PJ, Bishop CM, Milsom WK. How bar-headed geese fly over the Himalayas. Physiology (Bethesda) 30: 107–115, 2015. doi: 10.1152/physiol.00050.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Severinghaus JW. Oxyhemoglobin dissociation curve correction for temperature and pH variation in human blood. J Appl Physiol 12: 485–486, 1958. doi: 10.1152/jappl.1958.12.3.485. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd JR, Dominelli PB, Roy TK, Secomb TW, Hoyer JD, Oliveira JL, Joyner MJ. Modelling the relationships between haemoglobin oxygen affinity and the oxygen cascade in humans. J Physiol 597: 4193–4202, 2019. doi: 10.1113/JP277591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirasawa T, Izumizaki M, Suzuki Y, Ishihara A, Shimizu T, Tamaki M, Huang F, Koizumi K, Iwase M, Sakai H, Tsuchida E, Ueshima K, Inoue H, Koseki H, Senda T, Kuriyama T, Homma I. Oxygen affinity of hemoglobin regulates O2 consumption, metabolism, and physical activity. J Biol Chem 278: 5035–5043, 2003. doi: 10.1074/jbc.M211110200. [DOI] [PubMed] [Google Scholar]
- 33.Teppema LJ, Boulet LM, Hackett HK, Dominelli PB, Cheyne WS, Dominelli GS, Swenson ER, Foster GE. Influence of methazolamide on the human control of breathing: A comparison to acetazolamide. Exp Physiol. In press. doi: 10.1113/EP088058. [DOI] [PubMed] [Google Scholar]
- 34.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 90: 675–754, 2010. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- 35.Teppema LJ, van Dorp ELA, Dahan A. Arterial [H+] and the ventilatory response to hypoxia in humans: influence of acetazolamide-induced metabolic acidosis. Am J Physiol Lung Cell Mol Physiol 298: L89–L95, 2010. doi: 10.1152/ajplung.00255.2009. [DOI] [PubMed] [Google Scholar]
- 36.Tymko MM, Ainslie PN, MacLeod DB, Willie CK, Foster GE. End tidal-to-arterial CO2 and O2 gas gradients at low- and high-altitude during dynamic end-tidal forcing. Am J Physiol Regul Integr Comp Physiol 308: R895–R906, 2015. doi: 10.1152/ajpregu.00425.2014. [DOI] [PubMed] [Google Scholar]
- 37.Tymko MM, Hoiland RL, Kuca T, Boulet LM, Tremblay JC, Pinske BK, Williams AM, Foster GE. Measuring the human ventilatory and cerebral blood flow response to CO2: a technical consideration for the end-tidal-to-arterial gas gradient. J Appl Physiol (1985) 120: 282–296, 2016. doi: 10.1152/japplphysiol.00787.2015. [DOI] [PubMed] [Google Scholar]
- 38.Wang T, Branco LG, Glass ML. Ventilatory responses to hypoxia in the toad Bufo paracnemis before and after a decrease in haemoglobin oxygen-carrying capacity. J Exp Biol 186: 1–8, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Weil JV. Variation in human ventilatory control-genetic influence on the hypoxic ventilatory response. Respir Physiol Neurobiol 135: 239–246, 2003. doi: 10.1016/S1569-9048(03)00048-X. [DOI] [PubMed] [Google Scholar]
- 40.Winslow RM, Swenberg ML, Berger RL, Shrager RI, Luzzana M, Samaja M, Rossi-Bernardi L. Oxygen equilibrium curve of normal human blood and its evaluation by Adair’s equation. J Biol Chem 252: 2331–2337, 1977. [PubMed] [Google Scholar]
- 41.Wranne B, Berlin G, Jorfeldt L, Lund N. Tissue oxygenation and muscular substrate turnover in two subjects with high hemoglobin oxygen affinity. J Clin Invest 72: 1376–1384, 1983. doi: 10.1172/JCI111094. [DOI] [PMC free article] [PubMed] [Google Scholar]