Abstract
Patients with type 2 diabetes mellitus (T2DM) have a greater risk of developing life-threatening cardiac arrhythmias. Because the underlying mechanisms and potential influence of diabetic autonomic neuropathy are not well understood, we aimed to assess the relevance of a dysregulation in cardiac autonomic tone. Ventricular arrhythmia susceptibility was increased in Langendorff-perfused hearts isolated from mice with T2DM (db/db). Membrane properties and synaptic transmission were similar at cardiac postganglionic parasympathetic neurons from diabetic and control mice; however, a greater asynchronous neurotransmitter release was present at sympathetic postganglionic neurons from the stellate ganglia of db/db mice. Western blot analysis showed a reduction of tyrosine hydroxylase (TH) from the ventricles of db/db mice, which was confirmed with confocal imaging as a heterogeneous loss of TH-immunoreactivity from the left ventricular wall but not the apex. In vivo stimulation of cardiac parasympathetic (vagus) or cardiac sympathetic (stellate ganglion) nerves induced similar changes in heart rate in control and db/db mice, and the kinetics of pacing-induced Ca2+ transients (recorded from isolated cardiomyocytes) were similar in control and db/db cells. Antagonism of cardiac muscarinic receptors did not affect the frequency or severity of arrhythmias in db/db mice, but sympathetic blockade with propranolol completely inhibited arrhythmogenicity. Collectively, these findings suggest that the increased ventricular arrhythmia susceptibility of type 2 diabetic mouse hearts is due to dysregulation of the sympathetic ventricular control.
NEW & NOTEWORTHY Patients with type 2 diabetes mellitus have greater risk of suffering from sudden cardiac death. We found that the increased ventricular arrhythmia susceptibility in type 2 diabetic mouse hearts is due to cardiac sympathetic dysfunction. Sympathetic dysregulation is indicated by an increased asynchronous release at stellate ganglia, a heterogeneous loss of tyrosine hydroxylase from the ventricular wall but not apex, and inhibition of ventricular arrhythmias in db/db mice after β-sympathetic blockade.
Keywords: autonomic nervous system, diabetic neuropathy, sudden cardiac death, ventricular arrhythmias
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a pandemic of the 21st century (26, 63) as the number of patients will double within the next three decades (18), resulting in 592 million patients with T2DM by the year 2035 (27). The Framingham study has long confirmed the link between diabetes and cardiovascular disease, which is still the leading cause of mortality in T2DM (32). Patients with T2DM have a greater risk of dying from sudden cardiac death (4, 41), but the underlying mechanisms leading to fatal cardiac arrhythmias have not been fully elucidated.
Cardiac autonomic neuropathy is the most common overlooked complication in diabetes and has a prevalence of over 40% in patients between the age of 40 and 70 yr (36). It is associated with QTc time prolongation and imbalanced cardiac sympathetic/parasympathetic control (58) and may result in silent myocardial infarction, intraoperative cardiovascular instability, ventricular arrhythmias, and sudden cardiac death (71, 80). Cardiac arrhythmias can arise from abnormalities in de- or repolarization (38, 67) induced by a number of identified cellular mechanisms (13, 68), including alterations in neural pathways (16, 24, 47, 66). In T2DM, changes in the structure and function of the peripheral nerves regulating cardiac function contribute to the increased cardiovascular mortality (25, 46, 55, 73, 81).
Leptin receptor-deficient mice (db/db) provide a unique animal model of T2DM, which is similar in pathogenesis to T2DM in humans (11, 35, 75). Db/db mouse hearts have recently been shown to be sensitive to stimulus evoked arrhythmias from myocardial Ca2+ dyshomeostasis or sinoatrial node dysfunction (56). A depression in ganglionic nicotinic signaling at peripheral ganglia (superior cervical ganglion) has been observed in db/db mice (9), and interruption of ganglionic neurotransmission at intrinsic cardiac ganglia of healthy mice is proarrhythmic (31).
To determine whether the increased arrhythmia burden in T2DM is attributable to a dysregulation of the cardiac autonomic tone, we studied the arrhythmogenic potential of db/db mouse hearts in combination with an analysis of the structure and function of the cardiac sympathetic and parasympathetic innervation. We found that db/db mice are more susceptible to pacing-induced arrhythmias, which can be inhibited by β-sympathetic receptor blockade, have an increased asynchronous neurotransmitter release at stellate ganglia, and show a heterogeneous loss of tyrosine hydroxylase (TH) expression from the ventricular wall but not the apex, whereas cardiac parasympathetic neurotransmission remains unchanged.
MATERIALS AND METHODS
Studies were performed using 10- to 16-wk-old mice homozygous (db/db) or heterozygous (db/het; control) for the diabetes spontaneous mutation (Leprdb; BKS.Cg-Dock7m+/+ Leprdb/J, cat. no. 642, Jackson, Bar Harbor, ME). The study protocol was approved by the local authorities of the State of Hamburg, the Animal Care and Use Committees of the University of California, Los Angeles, and the University of Hamburg and conforms to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (2011).
Langendorff studies.
After cervical dislocation, hearts of male mice were quickly removed from the thoracic cavity, then submerged and dissected in ice-cold modified Krebs-Henseleit solution, consisting of (in mM) 119 NaCl, 25 NaHCO3, 4.6 KCL, 1.2 KH2PO4, 1.1 MgSO4, 2.5 CaCl2, 8.3 glucose, and 2 Na-pyruvate (pH 7.4, 95% O2-5% CO2). The aorta was quickly attached to the perfusion system (Hugo Sachs Elektronik/Harvard Aparatus, Germany) and the heart was retrogradely perfused at constant pressure (80 mmHg). Data were recorded using a digital data acquisition system and corresponding software (Powerlab 8/30 & Labchart, ADInstruments, Dunedin, New Zealand). A 2-Fr octapolar electrophysiology catheter (CIB’ER Mouse, NuMed, Hopkinton, NY) recorded intracardiac electrograms via an ECG amplifier (F104, ADInstruments, Dunedin, New Zealand) after being inserted into the right atrium and right ventricle. Programmed stimulation was applied using a designated digital stimulus generator (STG4002, Multi Channel Systems, Reutlingen, Germany) at twice the atrial or ventricular pacing threshold to determine standard electrophysiological parameters (60). Sinus node recovery time was calculated after fixed-rate atrial pacing and defined as the maximum return cycle length after 10 s of fixed-rate pacing at S1S1 cycle length 120, 100, and 80 ms. The atrioventricular His-Purkinje conduction properties were evaluated by fixed-rate atrial pacing. The Wenckebach point was defined as longest S1S1 cycle length (8 stimuli; S1S1: 100 ms; 2-ms stepwise reduction) with loss of 1:1 atrioventricular-nodal conduction. The atrioventricular nodal refractory periods were determined as longest S1S2 (12 stimuli S1S1: 120, 110, and 100 ms; one short coupled extra stimulus with a 2-ms stepwise S1S2 reduction) with loss of atrioventricular-nodal conduction. The atrial and ventricular refractory periods were defined as longest S1S2 (12 stimuli S1S1: 120, 110, and 100 ms; one short coupled extrastimulus with a 2-ms stepwise S1S2 reduction) with absent atrial response. Programmed extra- and burst stimulation protocols were performed to evaluate arrhythmogenesis in line with the Lambeth Conventions (47, 52). For programmed extrastimulation, three different baseline pacing cycle lengths (S1S1: 120, 100, and 80 ms) have been used and were followed by 2 and 3 extra beats (60–20 ms with 2-ms stepwise reduction). For burst pacing, a 5-s-long stimulus (S1S1: 50–10 ms) with 10-ms stepwise reduction was used. Ventricular tachycardia was defined as ≥4 consecutive premature ventricular complexes and classified by an established scoring system (17, 21). Besides baseline experiments, atropine (muscarinic blockade, 1×10−6 M; Sigma-Aldrich, St. Louis, MO) and atropine with propranolol (β-receptor blockade, 1×10−6 M; mibe, Sandersdorf-Brehna, Germany) were added to the perfusion solution before the experiment was started. Additionally, myocardial wave propagation velocity and dispersion in conduction direction were determined by epicardial activation mapping (23). The 32-electrode array (EcoFlexMEA36, Multi Channel Systems, Reutlingen, Germany) was positioned at both ventricles during epicardial pacing and recorded unipolar electrograms (ME128-FAI-MPA-System, Multi Channel Systems, Reutlingen, Germany) with a sampling rate of 25 kHz. Data were bandpass filtered (50 Hz), digitized with 12 bit and a signal range of 20 mV.
Microelectrode recording.
Intrinsic cardiac or stellate ganglia were isolated from adult male mice under deep isoflurane (5%) anesthesia. The thorax was removed and placed in cold physiological salt solution containing the following: (in mM) 121 NaCl, 5.9 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 25 NaHCO3, 2 CaCl2, and 8 d-glucose (pH 7.4), maintained by 95% O2-5% CO2 aeration. Excised ganglia were pinned to the SylGard floor of a glass bottom petri dish. The dish was transferred to the stage of a Zeiss AxioExaminer microscope equipped with differential interference contrast (DIC) optics, a Zeiss Axiocam camera, and ×5 air and ×40 water-immersion objectives.
Ganglion preparations were superfused continuously (6–7 mL/min) with physiological salt solution (32–35°C). Individual neurons were impaled using microelectrodes filled with either 2M KCl (60–120 MΩ) or 2M KCl + 2% neurobiotin (80–160 MΩ; Vector Laboratories). Membrane voltage was recorded using a Multiclamp 700B amplifier coupled with a Digidata 1550B data acquisition system and pCLAMP 10 software (Molecular Devices, Sunnyvale, CA). Current injected through the recording electrode was used to characterize membrane physiology. Depolarizing current steps (0.1–0.5 nA amplitude, 500-ms or 1-s duration) were used to assess neuronal excitability. Cells were classified as either phasic [<2 action potentials (APs)], bursting (2–5 spikes in short burst), or tonic (>5 spikes continuously) based on the number of APs elicited by depolarizing current pulses. Hyperpolarizing current steps (0.1–0.5 nA, 500 ms) were used to determine cell input resistance. AP amplitude and duration were measured from spontaneous or nerve evoked APs. After-hyperpolarization amplitude and duration were measured from brief intracellular current injections (100–500 pA, 5 ms), spontaneous APs, or nerve-evoked APs.
For extracellular stimulation of preganglionic nerves, concentric bipolar stimulating electrodes (FHC; Bowdoin, ME) were placed on the thoracic sympathetic trunk (T2–T3) of the stellate ganglia or interganglionic nerves of intrinsic cardiac ganglia. Graded shocks (200–600 uA; 100 µs) (AMPI Master 8 and IsoFlex optical Isolation Unit) given to the preganglionic nerves generated synaptic potentials, as recorded with sharp microelectrodes, in ganglion neurons. Trains of stimuli, given presynaptically at 5, 10, or 20 Hz, assessed spike-following frequency in the postsynaptic neuron and the number and amplitude of posttetanic asynchronous excitatory postsynaptic potentials (aEPSPs). aEPSP number and amplitude were quantified using Mini Analysis software (Synaptosoft).
Tissue fixation and immunohistochemistry.
After intracellular recording experiments, ganglia were fixed overnight in 4% paraformaldehyde. Fixed tissue was rinsed in phosphate buffered saline (PBS) and stored in PBS + 0.02% sodium azide. The whole mounts were permeabilized in ‘block’ solution containing 2% horse serum, 0.1% Triton X-100, 0.02% sodium azide, and 0.01 M PBS, with pH 7.2. Ganglia were stained with antibodies directed to either TH or protein gene product 9.5 (PGP 9.5). Secondary staining with streptavidin conjugated ATTO-647N was used to visualize Neurobiotin-filled cells. Stained tissue was rinsed in PBS, mounted on glass slides with antifade media (Citifluor CFM-1+; Electron Microscopy Sciences), and coverslipped.
Tile-scans of whole ganglion neuroanatomy were acquired on a confocal microscope using a dry ×10 objective (Leica). Cells backfilled with Neurobiotin were tile-scanned using a ×40 oil-immersion objective (Leica).
In vivo nerve stimulation studies.
Male mice were anesthetized with isoflurane [2–3%, in oxygen (1 L/min)] and placed on a heated surgical pad. Body temperature was monitored rectally and maintained at 37°C. A midline cervical incision was made to facilitate cannulation of the trachea with a 20-gauge SURFLO IV catheter. Vagal nerve stimulation and stellate ganglion stimulation experiments were completed in separate animals (all male). In animals receiving stellate ganglion stimulation, the lungs were mechanically ventilated (130 breaths/min) using a positive pressure ventilator (SAR-830/P ventilator, IITC, Woodland Hills, CA). Surface ECGs (3 lead) were recorded with platinum subdermal needle electrodes placed in the limbs and connected to a Grass amplifier (P511). Blood samples were centrifuged (10 min, 4°C, 4,000 g) and stored at –80°C. Analysis of fasting blood glucose, cholesterol, triglycerides, and high-density lipoprotein was performed by a central laboratory using routine techniques.
For vagal stimulation experiments, the right cervical vagus was isolated and transected and the distal nerve was placed over bipolar platinum stimulating electrodes. Nerve and electrode were immersed in mineral oil to protect tissue. For stellate ganglion stimulation, after intubation animals were placed on the left side and the right forelimb was retracted above the head. An incision was made between second and third ribs, and tissue layers were transected to reveal the upper lobe of the right lung. Retractors were placed within the intercostal space to open an operating window of ~1 cm2. The upper lobe of the right lung was retracted exposing the cranial-medial pole of the right stellate ganglion. A concentric bipolar stimulating electrode (FHC) was advanced through the surgical window and placed on the surface of the stellate.
Stimulating electrodes were coupled to an optoelectrically isolated, constant current, stimulus isolation unit (PSIU6, Grass Instruments) driven by a digital square-wave generator (S88X, Astro-Med). Analog output from the rectal thermistor, ECG amplifier, and stimulator were connected to a Powerlab 8/35 A/D converter (ADInstruments). Digitized output was acquired with a personal computer (Apple) running Labchart software (ADInstruments). Heart rate was calculated from the ECG signal in the Labchart software.
After a 30-min equilibration period, nerve stimulation studies are begun to evaluate cardiac responsiveness to either stellate or vagal stimulation. Indices of cardiac responsiveness include changes in the ECG and heart rate. For both vagal and stellate stimulation, nerves were first stimulated at 10 Hz (5-s duration vagus, 10-s duration stellate; 2-ms pulse width) to establish the current intensity required to produce a 50% decrease in heart rate (vagus) or 10% increase in heart rate (stellate). Thereafter, multiple trials of stimulation at 5, 10, or 20 Hz were randomly tested with a 10-min recovery interval between stimuli.
Immunoblotting and microscopy.
Immunoblotting was performed as described previously (31) using the following primary antibodies: rabbit α TH (1:1,000; Merck Millipore), rabbit α substance P (1:1,000; Bioss), goat α vesicular acetylcholine transporter (VAChT) (1:500; Merck Millipore), and rat α PGP 9.5 (1:500; a kind gift from Dr. C. Meyer-Schwesinger) (57). Subsequently, membranes were incubated with goat anti-rabbit, donkey anti-goat, or goat anti-rat IgG conjugated to horseradish peroxidase (HRP, 1:10,000; Dianova). HRP-conjugated rabbit α GAPDH (1:2,000; Cell Signaling) was used to detect GAPDH as a loading control. Immunoreactive proteins were visualized with enhanced chemiluminescence and quantified using the Fusion Solo S gel documentation system and software (VWR International).
For TH immunostaining, hearts of db/db and control mice (all male) were Langendorff-perfused, fixed in neutral-buffered formalin (Sigma-Aldrich) for 24 h at room temperature, and embedded in 5% agarose. Hearts were sectioned with a vibratome (50 µm) from apex to the atria. Sections were bleached in Dent’s bleach (4:1:1 MeOH: H2O2: DMSO; Merck, Darmstadt, Germany) for 2 h at room temperature and subsequently rehydrated to PBS in a series of descending MeOH concentrations in PBS (100, 75, 50, and 25%; 10 min each). Quenching of autofluorescence was performed in 0.25% Sudan black (Roth) in 70% ethanol for 2 h at room temperature. Sections were washed briefly in 50% ethanol and subsequently blocked overnight at 4°C in 5% bovine serum albumin (BSA)/PBS. Rabbit anti-TH (1:1,000; EMD Millipore) was incubated for 36 h at 4°C. After 6 washes in PBS for 30 min each, secondary antibody (donkey anti-rabbit 488, 1:500; Life Technologies) was incubated for 24 h. After being washed, sections were embedded in DAPI-Fluoromount G (Southern Biotech). Sections were digitalized using an Axio Scan.Z1 (kindly provided by Prof. Schumacher, University Clinic Hamburg-Eppendorf, Hamburg, Germany).
Whole mount apex staining.
To visualize sympathetic innervation at the heart apex, hearts isolated from control and db/db mice (all male) were fixed in 4% paraformaldehyde overnight and cleared using a passive clarity technique (65, 78). Cleared hearts were stained with antibodies against TH. Apexes were mounted on glass slides and coverslipped for confocal (Zeiss LSM780) imaging with a ×10 objective (numerical aperture = 0.45). Apical nerve fibers immunoreactive for TH were hand traced in two dimensions using three-dimensional image stacks and a digitizing tablet (Wacom Cintiq) with ImageJ software (50). The area of traced fibers was calculated within three concentric rings (Zone 1 diameter = 750 μm, Zone 2 = 500 μm, Zone 3 = 250 μm; Fig. 6D) centered on the apex. The density of nerve fibers immunoreactive for TH is expressed as the percentage of traced fibers per unit area.
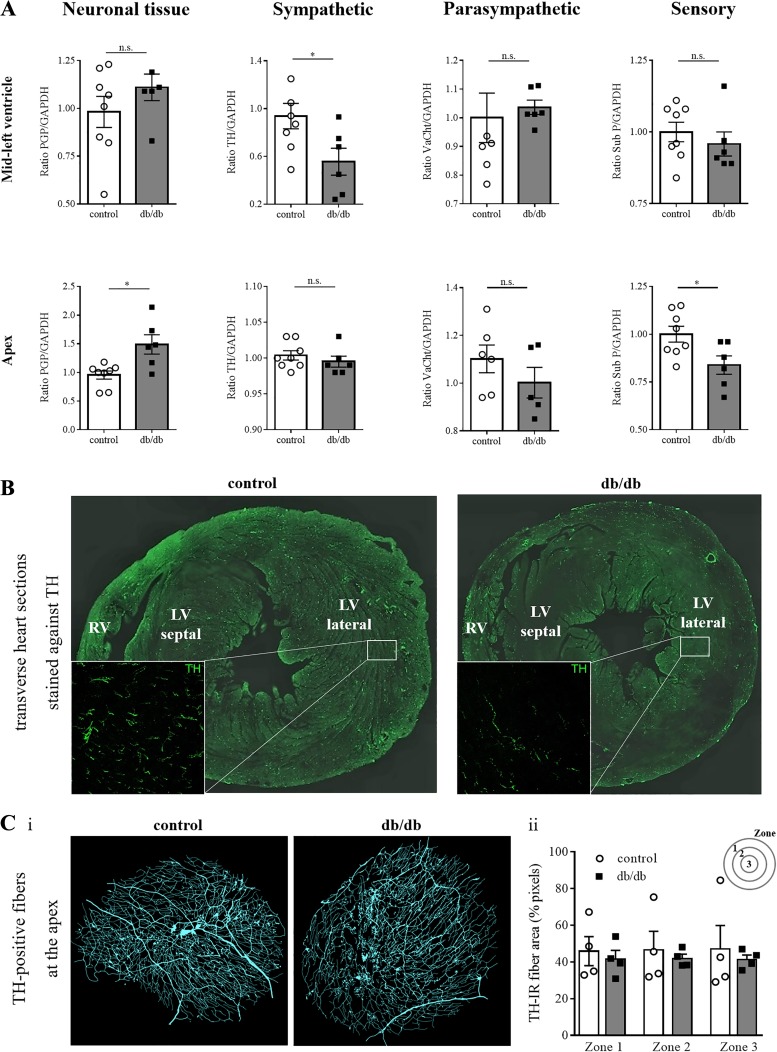
Fig. 6.
Characteristics of the cardiac autonomic innervation. A: Western blot analysis (control: n = 8, db/db: n = 6) after normalization with GAPDH confirms a heterogeneous expression of tyrosine hydroxylase (TH) in db/db mice in the midleft ventricle but not in the apex. Differences in the expression of a pan-neuronal marker (PGP 9.5) and a sensory marker (Substance P) were observed in the apex. B: transverse heart sections reveal abundant TH-positive fibers at the midleft ventricle in control hearts, whereas only sparse TH-positive fibers are present in db/db hearts, demonstrating a reduced sympathetic innervation. C: antibody staining against TH at the cardiac apex (n = 4 for control and db/db), the most peripheral area of the heart, revealed no differences in the amount of TH-positive fibers in a semiquantitative analysis. *P ≤ 0.05. LV, left ventricle; n.s., not significant; RV, right ventricle; TH-IR, TH-immunoreactivity; VAChT, vesicular acetylcholine transporter.
Ca2+ transient measurements in ventricular myocytes.
Cardiomyocytes were isolated from control (16 cells from 3 animals; cells per animal: 2 male/7 male/7 female) and db/db (19 cells from 4 animals; cells per animal 2 male/5 female/7 female/5 female) mouse hearts as previously described (19, 45). After retrograde perfusion with Ca2+-free buffer solution consisting of (in mM) 113 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4, 12 NaHCO3, 10 KHCO3, 30 taurine, 5.55 glucose, 10 2,3-butanedione monoxime, and 10 HEPES (pH 7.46) for 6.5 min, hearts were digested with 0.075 mg/mL Liberase (Roche Diagnostics, Mannheim, Germany) and dissolved in buffer solution containing 12.5 µM CaCl2 for 7–8 min. Ca2+ was introduced stepwise up to a concentration of 1 mM (22). Intracellular Ca2+ concentrations ([Ca2+]i) during contraction were evaluated using the IonOptix system (IonWizard; IonOptix; Milton, MA). Only rod-shaped cells without membrane blebs, hypercontractile zones, and spontaneous activity showing a stable contraction amplitude and rhythm at 1-Hz pacing frequency were measured (22). Cells were kept in modified Tyrode’s solution containing (in mM) 135 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4, 1.5 CaCl2, 20 glucose, and 10 HEPES (pH 7.46) and measured at 37°C with a perfusion rate of 1 mL/min. Cells were incubated with 1 µM Fura-2-AM (Invitrogen) for 15 min, which was followed by a 20-min washing period allowing for deesterification of Fura-2 for Ca2+ transient measurements. Fura-2-loaded cells were excited with 340- and 380-nm light using the IonOptix hyperswitch light source. Emitted 510-nm light was recorded with a photon multiplier tube, and the F340-to-F380 ratio was used as an index of [Ca2+]i after the signal was corrected for background. Sixteen cells of control mice (n = 3) and 19 cells of db/db mice (n = 4) were used. Measurements were made in normal glucose (5 mM) for control cells and high glucose (20 mM) for T2 diabetic cells.
Statistical analysis.
Statistical analysis was performed using Graphpad Prism (Graphpad, La Jolla, CA). For continuous variables Student’s t-test Mann-Whitney or Wilcoxon signed rank tests were used as appropriate. Two-way ANOVA was used to compare responses from nerve stimulation in control and db/db mice. Post hoc analysis was performed with Sidak’s multiple comparisons test. Differences in ventricular tachycardia occurrence were analyzed using Fisher’s exact test. Continuous variables are reported as means ± SE and categorical variables as absolute and relative numbers. Data were considered statistically significant with a P value < 0.05.
RESULTS
Increased blood glucose, lipid levels, and body weight in diabetic mice.
Compared with control mice, the leptin deficient (db/db) mice show hyperglycemia, hyperlipidemia, and an increase in body weight as well as a decrease in the heart-to-body weight ratio (Table 1) consistent with T2DM.
Table 1.
Elevated blood parameters and weight in db/db mice
| Control | db/db | P Value | |
|---|---|---|---|
| Glucose, mg/dL (196–476) |
175 ± 4 | >500 | <0.0001 |
| Cholesterol, mg/dL (0–89) |
97 ± 3 | 129 ± 14 | 0.052 |
| Trigylceride, mg/dL (52–170) |
123 ± 10 | 150 ± 16 | 0.197 |
| HDL, mg/dL (45–85) |
93 ± 2 | 128 ± 9 | 0.005 |
| Heart-to-body weight ratio (n = 12) |
0.80 ± 0.02 | 0.47 ± 0.01 | <0.0001 |
Values are means ± SE; n = 5 mice for both groups. In db/db mice, blood levels of glucose and lipids are elevated. Increased body weight in db/db mice leads to a lower heart-to-body weight ratio. Normal values are shown in parentheses. HDL, high-density lipoprotein.
Increased arrhythmia occurrence in diabetic mice.
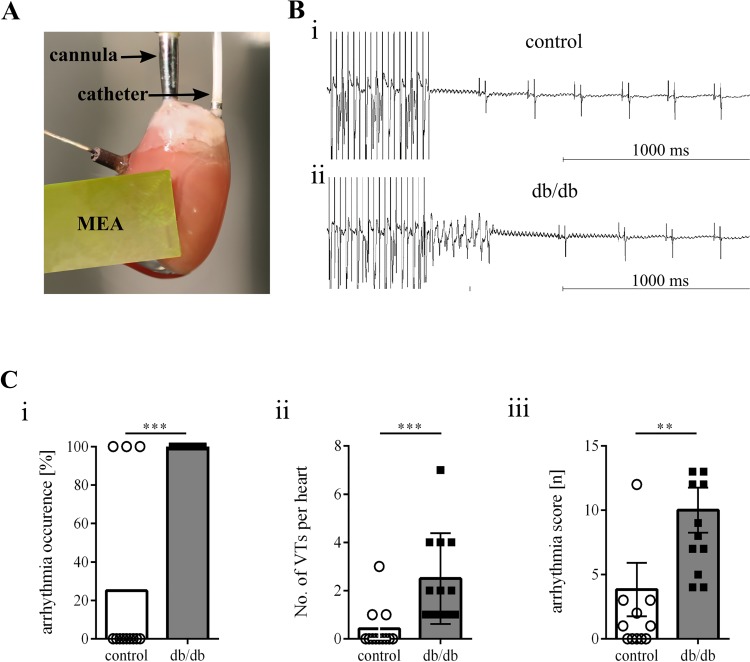
An ex vivo setup was used to determine arrhythmia susceptibility and baseline electrophysiological parameter in control and db/db mouse hearts. Ventricular tachycardias were easily inducible in db/db mice (Fig. 1). Of 12, a total of 12 db/db mice developed ventricular arrhythmias upon using programmed extrastimuli and burst pacing protocols. The calculated arrhythmia score reflects the severity of induced arrhythmias (Fig. 1C,iii) and was greater in db/db mice (3.8 ± 2.1 vs. 10.0 ± 1.8, P = 0.002). Baseline electrophysiological parameters, including heart rate as well as epicardial conduction properties, were similar in db/db and control mice (Table 2).
Fig. 1.
Increased arrhythmia occurrence in db/db mice. A: exemplary image of the ex vivo Langendorff setup, including the octapolar catheter positioned in the right atrium and right ventricle as well as the epicardial multielectrode array (MEA). B: exemplary tracing of arrhythmia induction by using burst stimulation. In the upper tracing, no ventricular arrhythmia occurred in control mice in comparison to the lower tracing, in which a self-terminating arrhythmia could be induced in db/db mice. C: arrhythmia occurrence (i), number of ventricular tachycardias per heart (ii), and the arrhythmia score (iii) upon programmed stimulation were increased in db/db mice. Control: n = 12 mice; db/db: n = 12 mice. **P ≤ 0.05; ***P ≤ 0.003.
Table 2.
Similar endocardial and epicardial electrophysiological parameter
| SNRT/SCL, % | WBP, ms | AVNRP100, ms | ARP100, ms | VRP100, ms | WPV, m/s | sCHI | |
|---|---|---|---|---|---|---|---|
| Control | 130 ± 4.6 | 82 ± 2.5 | 68 ± 1.7 | 28 ± 1.1 | 26 (76) ± 0.8 | 0.99 ± 0.1 | 1.65 ± 0.2 |
| db/db | 125 ± 4.6 | 79 ± 1.5 | 68 ± 2.3 | 27 ± 1.2 | 29 ± 1.2 | 0.88 ± 1.4 | 1.88 ± 0.1 |
Values are means ± SE; n = 12 mice for both groups. The percentage sinus node recovery time (SNRT)/sinus cycle length (SCL), Wenckebach point (WBP), atrioventricular nodal recovery period (AVNRP), and atrial refractory period (ARP) represent baseline electrophysiological parameter of the atria, whereas the ventricular refractory period (VRP) denotes ventricular electrophysiological parameter. The wave propagation velocity (WPV) and conduction heterogeneity index (sCHI) denote epicardial parameter. There were no differences in endocardial or epicardial electrophysiological parameters in control and db/db mice.
Preserved synaptic transmission at intrinsic cardiac neurons.
To investigate the contribution of autonomic neuropathy to the increased arrhythmia susceptibility of diabetic hearts, intracellular microelectrode recordings were made from intrinsic cardiac neurons (ICNs). Mouse ICNs consist of the postganglionic parasympathetic neurons, supplying cholinergic innervation to the heart. Some ICNs contain TH but are most likely not functionally sympathetic (30). A single ICN (backfilled with Neurobiotin) from a control cardiac ganglion is shown in Fig. 2A. The image is representative of all cells imaged (n = 3). Mouse ICNs appear to have only a soma and single axon with no dendrites. Because of the limited number of cells backfilled, no comparisons of basic cellular morphology were made between control and diabetic mice. Basic membrane properties, including resting membrane potential [−56 ± 1.7 mV control (12) vs. −54 ± 1.6 mV db/db (17), P = 0.49], input resistance (187 ± 28 MΩ control vs. 175 ± 24 MΩ db/db, P = 0.75), after-hyperpolarization amplitude (10.46 ± 1.0 mV control vs. 10.65 ± 0.68 mV db/db, P = 0.87), and after-hyperpolarization duration (115 ± 38 ms control vs. 158 ± 33 mV db/db, P = 0.41) were not different between the two groups of mice.
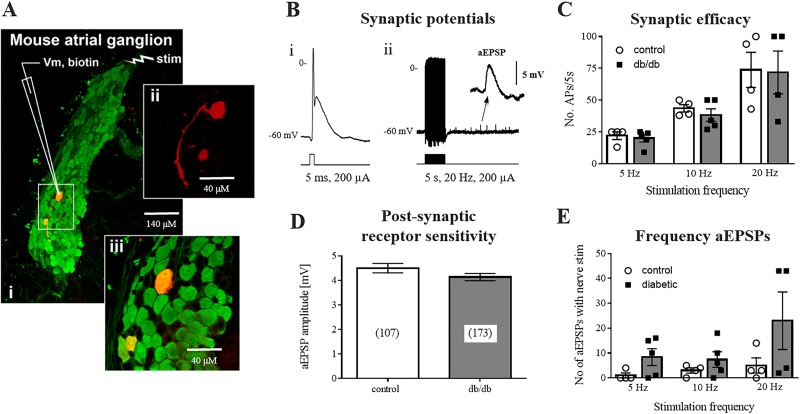
Fig. 2.
Preserved synaptic transmission at intrinsic cardiac neurons (ICNs). A: intracellular membrane potential was recorded from individual ICNs impaled with glass microelectrodes in isolated preparations of intrinsic cardiac ganglia. A single atrial ganglion (i) is shown [protein gene product 9.5 (PGP9.5), green], containing an individual neuron backfilled with Neurobiotin (red) (ii and iii). Scale bars = 140 μM (i) and 40 μM (ii and iii). B: examples of the suprathreshold postsynaptic potential recorded from a neuron during either a single (i) or train (ii) of presynaptic shocks. Note the occurrence of asynchronous excitatory postsynaptic potentials (aEPSPs; inset) following tetanic stimulation. C: synaptic efficacy, shown as the number of action potentials (APs) elicited by a train of presynaptic stimuli. D: summed amplitude of aEPSPs was not significantly different between control or db/db mice, indicating no loss of function of postganglionic nicotinic receptors. E: number of aEPSPs occurring at multiple frequencies of presynaptic stimulation were not different between control and db/db mice. Control: 12 cells from 5 animals; db/db: 17 cells from 7 animals.
Previous reports have suggested that ganglionic neurotransmission is depressed at peripheral autonomic ganglia of db/db mice (9, 48). Both single and repetitive stimulation of presynaptic inputs to ICNs were used to test synaptic efficacy and the postsynaptic receptor sensitivity to evoked neurotransmitter release (Fig. 2). Single stimuli always produced suprathreshold excitatory postsynaptic potentials (EPSPs), eliciting APs, in both control and db/db mice. Treatment with 500 μM hexamethonium completely blocked nerve evoked responses (n = 4 control ICNs, n = 4 db/db ICNs), demonstrating mediation by acetylcholine release and postsynaptic activation of ganglionic nicotinic receptors. Presynaptic stimulation at 5-, 10-, or 20-Hz frequencies (5-s duration) was used to test the ability of the postganglionic neuron to follow the presynaptic stimulus. The number of APs evoked by stimulus trains at these frequencies was not different between control and db/db mice [F(2,20) = 0.035, P = 0.97)], demonstrating similar synaptic efficacy for the two groups of mice.
aEPSPs and small transient EPSPs occurring stochastically after nerve stimulation were observed both during and after tetanic stimulation at 5, 10, or 20 Hz in control and db/db mice (Fig. 2). In both groups of mice, a greater number of events were observed at higher stimulation frequencies. The number of aEPSPs tended to be greater in db/db mice, but the difference was not significant [F(2,20) = 0.98, P = 0.39].
aEPSPs are thought to be mediated by asynchronous release of synaptic vesicles at the synapse and thus provide a definitive measurement of postsynaptic receptor sensitivity to released acetylcholine (64). aEPSP amplitudes were not different between control (4.5 ± 0.19 mV, n = 107) and db/db mice (4.1 ± 0.15 mV, n = 173; P = 0.13).
Greater asynchronous neurotransmitter release at stellate ganglia of diabetic mice.
Intracellular microelectrode recordings were made from stellate ganglia isolated from control and db/db mice to investigate ganglionic neurotransmission at cardiac sympathetic ganglia (Fig. 3). A single stellate ganglion neuron backfilled with Neurobiotin is shown in Fig. 3A. All backfilled stellate neurons (n = 4) showed a similar morphology consisting of a complex dendritic arbor emanating from the soma with a single axon that leaves the ganglion. Basic membrane properties of stellate ganglion neurons, including resting membrane potential (60 ± 1.5 vs. 61 ± 0.8 mV, P = 0.60) and input resistance (194 ± 47 vs. 185 ± 44 MΩ, P = 0.89), were not different between control and db/db mice.
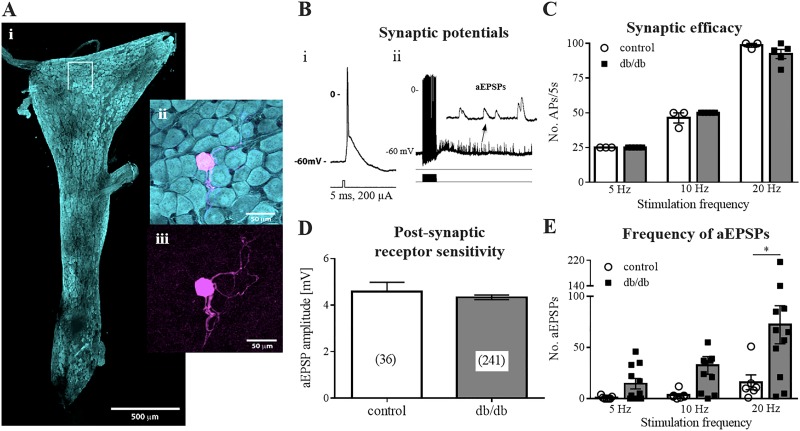
Fig. 3.
Increased asynchronous neurotransmitter release at stellate ganglion neurons. A: fixed whole mount preparation of a stellate ganglion (i) stained with antibody to protein gene product 9.5 (PGP9.5). ii: a single cell from within the boxed region in i, backfilled with Neurobiotin and labeled with streptavidin (magenta). iii: location of the backlabeled cell among PGP 9.5+ (cyan) and neurons. Scale bars = 500 μM (i) and 50 μM (ii and iii). B: exemplary intracellular potential recordings during single (i) and repetitive (ii) stimuli delivered at the thoracic sympathetic trunk. i: single stimuli evoked excitatory postsynaptic potentials (EPSPs), which were always suprathreshold for the generation of action potentials. ii: volleys of asynchronous EPSPs (aEPSPs) (inset) followed trains of stimuli. C: number of action potentials evoked during stimulus trains was equivalent in control and db/db mice. D: mean amplitude of aEPSPs was similar between control and diabetic mice. E: number of aEPSPs following 20-Hz tetanic stimulation was significantly greater in db/db mice. Control: 7 cells from 3 animals; db/db: 11 cells from 4 animals. *P ≤ 0.05.
As with the ICNs, single stimuli elicited a large suprathreshold EPSP, which was blocked by hexamethonium (500 μM) in all cells (n = 5 control, n = 4 db/db). Trains of stimuli at 5, 10, or 20 Hz (5-s duration) also evoked an equivalent number of APs in both the control and db/db mice (Fig. 3C). Again, as observed at ICNs, stimulus trains evoked aEPSPs. However, the number of aEPSPs observed at all stimulation frequencies was greater in db/db mice relative to controls (0.3 ± 0.3 vs. 25 ± 9.7 aEPSPs at 5 Hz; 5 ± 3.5 vs. 58 ± 15 aEPSPs at 10 Hz; 23 ± 10 vs. 120 ± 37 aEPSPs at 20 Hz; P = 0.0028). The amplitude of these events was not different between groups [4.6 ± 0.4 (n = 36) vs. 4.3 ± 0.10 mV (n = 241); P = 0.43], which had similar resting membrane potentials and similar input resistances.
Similar chronotropic responses to cardiac parasympathetic and cardiac sympathetic stimulation in vivo.
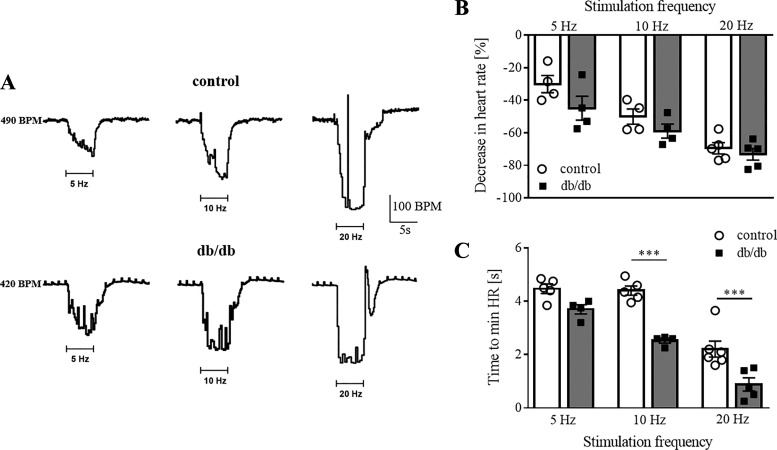
To determine cardiac responsiveness to endogenous neurotransmitter release, heart rate changes in response to preganglionic parasympathetic stimulation were assessed in isoflurane anesthetized, freely breathing mice. Following a 30-min equilibration period after completion of surgical procedures, sinus rhythm in db/db mice (n = 5) was lower than control mice (n = 5) (377 ± 13 vs. 419 ± 19 beats/min, P = 0.02). Stimulation of the right vagus nerve caused a direct decrease in heart rate in both control and db/db mice (Fig. 4). The time required for the development of the maximum negative chronotropic effect, measured from the start of vagal stimulation, was shorter in db/db mice at 10 Hz (4.40 ± 0.18 vs. 2.53 ± 0.09 s, P < 0.0001) and 20 Hz (2.21 ± 0.30 vs. 0.88 ± 0.25 s, P = 0.0006) (Fig. 4C). At all three stimulation frequencies tested, the relative decrease in heart rate was similar for control and db/db mice [Fig. 4B; F(2,20) = 0.6959, P = 0.51]. In both groups, increasing the stimulation frequency caused progressively greater cardiac decelerations, with the largest bradycardias observed during 20-Hz stimulation. The mean current intensity required to produce a maximum bradycardia at 10 Hz was similar between control (60 ± 6.8 μA, n = 6) and db/db (68 ± 10.2 μA, n = 5; P = 0.52) mice.
Fig. 4.
Stimulation of the vagus nerve evoked equivocal changes in heart rate (HR) in control and db/db mice. A: representative tracings of the HR response to vagal stimulation in anesthetized control and db/db mice. B: summary data showing the mean change in HR. There was no difference between control and db/db mice. C: time required for the maximum bradycardic response was less in db/db mice at 10 Hz and 20 Hz. Control: n = 5 animals; db/db: n = 5 animals. ***P ≤ 0.003; BPM, beats/min.
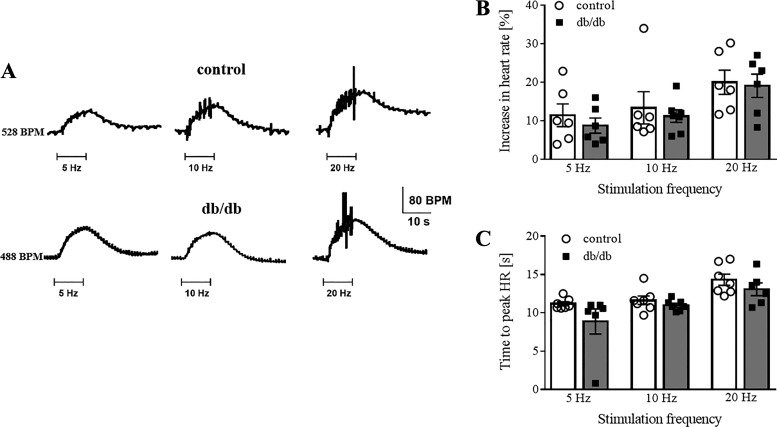
Heart rate changes in response to right stellate ganglion stimulation were also investigated in anesthetized control (n = 5) and db/db (n = 7) mice. Ten seconds of right stellate ganglion stimulation (at either 5, 10, or 20 Hz) caused a slowly developing increase in heart rate, which reached similar relative maximums in both control and db/db mice (Fig. 5A). The delay in the tachycardia development, from the start of stimulation to the maximum effect, did not differ between control and db/db mice [Fig. 5C; F(2,31) = 0.5608, P = 0.5759]. The relative increase in heart rate was greatest at 20-Hz stimulation for both groups but was not different between groups at any frequency tested [Fig. 5B; F(2,31) = 0.0497, P = 0.95]. The current intensity required to elicit a threshold response (10% baseline) was similar between control (233 ± 21 μA, n = 6) and db/db (225 ± 25 μA, n = 6; P = 0.80) mice.
Fig. 5.
Heart rate (HR) responses to stellate ganglion stimulation were also similar for control and db/db mice. A: representative increases in HR in response to stellate stimulation for a control and a db/db mouse. B: mean percent increases in HR for each stimulation frequency tested were similar between groups. C: time to peak tachycardia after the start of stimulation was similar between control and db/db mice. Control: n = 7 mice; db/db: n = 5 mice.
Reduced expression of tyrosine hydroxylase at db/db mouse ventricles.
To determine differences in cardiac neuronal protein expression, Western blot analysis of ventricular tissue using antibodies against PGP 9.5, TH, VAChT, and substance P were performed. TH, the rate-limiting enzyme of catecholamine biosynthesis, was reduced in the midleft ventricles of db/db mice. No differences were detectable in the expression of the pan-neuronal marker (PGP 9.5), VAChT (cholinergic), or substance P (sensory) in the midleft ventricle (Fig. 6A). To confirm, fewer TH-positive fibers were detectable in transverse vibratome sections of the midleft ventricle of db/db hearts (Fig. 6B). At the apex, a reduced expression of PGP 9.5 and substance P was observed, with no change in TH or VAChT (Fig. 6A). The preservation of TH-immunoreactive nerve fibers at the heart apex was confirmed in cleared, immunolabeled whole mount preparations of db/db and control mouse heart apexes (Fig. 6C).
Normal Ca2+ transients in diabetic adult mouse cardiomyocytes.
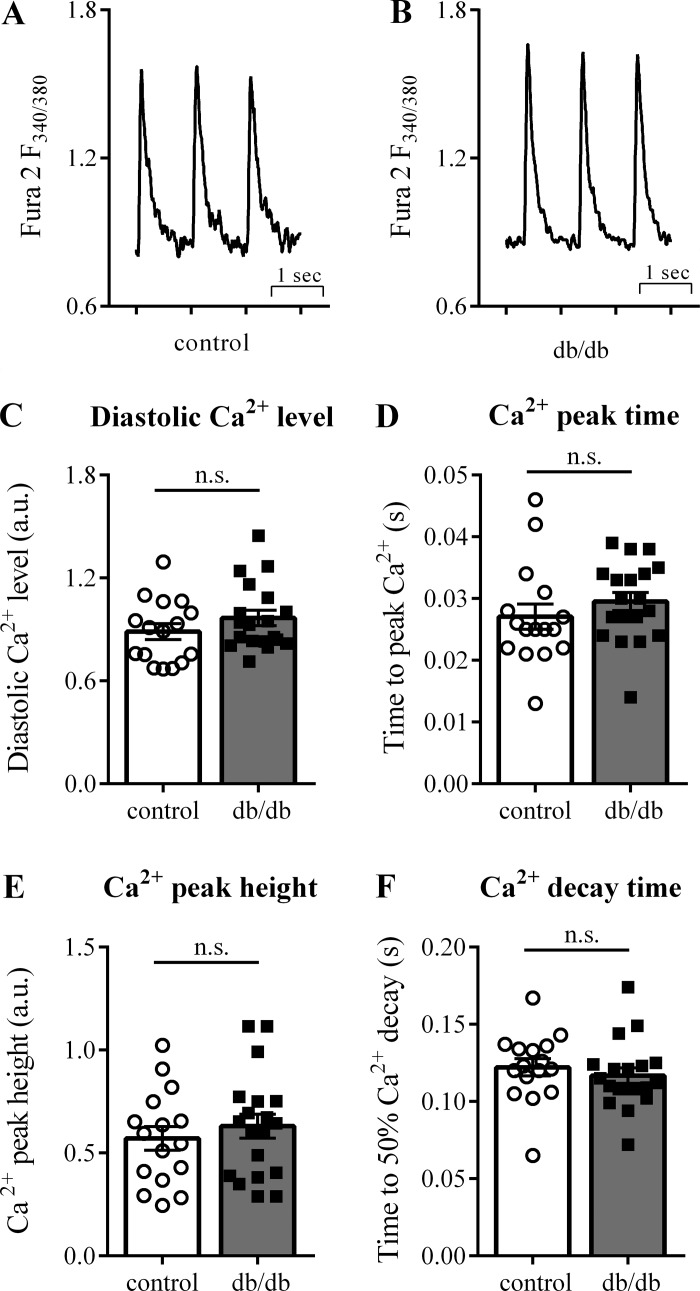
To investigate the influence of modified myocyte function on arrhythmogenesis, we analyzed [Ca2+]i kinetics in adult mouse cardiomyocytes. Global [Ca2+]i transients were recorded from either isolated control (5 mM glucose) or diabetic (20 mM glucose) cardiomyocytes (Fig. 7, A and B). Baseline diastolic [Ca2+]i [0.89 ± 0.05 arbitrary units (au) controls vs. 0.97 ± 0.04 au db/db, P = 0.23; Fig. 7C], [Ca2+]i peak time (27.1 ± 2.0 ms controls vs. 29.5 ± 1.5 ms db/db, P = 0.32; Fig. 7D), [Ca2+]i peak height (0.57 ± 0.06 au controls vs. 0.63 ± 0.06 au db/db, P = 0.47; Fig. 7E), and time to 50% [Ca2+]i decay (0.25 ± 0.02 s controls vs. 0.22 ± 0.01 s db/db, P = 0.09; Fig. 7F) were all similar in control and db/db mice. Using different glucose concentrations for control (20 mM glucose) and diabetic (5 mM glucose) cardiomyocytes revealed similar results (data not shown).
Fig. 7.
Cardiac myocyte [Ca2+]i transients are not altered in db/db mice. Exemplary tracings of [Ca2+]i transients from control (A) and db/db (B) mouse cardiomyocytes. The kinetics of the [Ca2+]i transients, including diastolic [Ca2+] (C), peak time (D), peak height (E), and decay time (F), were similar in control and db/db mice. Control: 16 cells from 3 animals (cells per animal: 2, 7, 7); db/db: 19 cells from 4 animals (cells per animal: 2, 5, 7, 5). a.u., arbitrary units; n.s., not significant.
Inhibition of β-sympathetic receptors prevents ventricular arrhythmias in diabetic mice.
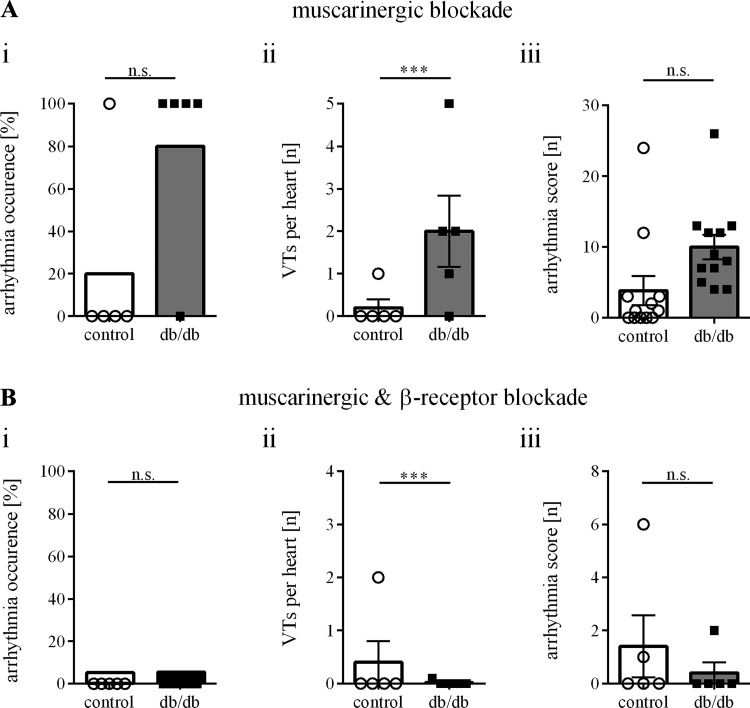
Additional Langendorff experiments were conducted to test the efficacy of autonomic blockade on arrhythmia inducibility. Inhibition of cardiac muscarinic receptors with atropine did not alter baseline electrophysiological measurements (data not shown) or arrhythmia susceptibility in paced hearts (Fig. 8A). Sinus node recovery time (SNRT) was longer in db/db mice after atropine [SNRT/sinus cycle length (SCL) = 141 ± 7.4 vs. 120 ± 2.1%, P = 0.026]. In the presence of both atropine and propranolol, the duration of basic cardiac electrophysiological parameters was prolonged in control [Wenckebach points (WBP) = 80 ± 2.6 vs. 100 ± 4.5 ms; atrioventricular nodal recovery period (AVNRP)100 = 68 ± 4.2 vs. 83 ± 3.7 ms; atrial refractory period (ARP)100 = 24 ± 1.2 vs. 41 ± 3.0 ms; ventricular refractory period (VRP)100 = 28 ± 2.3 vs. 40 ± 4.1 ms; P < 0.05 for all] and diabetic hearts (SNRT/SCL 141 ± 7.4 vs. 155 ± 14.5%; WBP = 83 ± 1.6 vs. 93 ± 5.2 ms; AVNRP100 = 73 ± 1.4 vs. 82 ± 7.5 ms; ARP100 = 26 ± 1.3 vs. 40 ± 5.0 ms; VRP100 = 25 ± 1.5 vs. 47 ± 4.2 ms; P < 0.05 for all). Although baseline cardiac electrophysiological parameters were similar between control and diabetic hearts, the combined treatment completely abrogated all stimulus-evoked arrhythmias in db/db mice (Fig. 8B).
Fig. 8.
Sympathetic blockade prevents ventricular tachycardias in db/db mice. A: muscarinic blockade (control: n = 5 mice, db/db: n = 5 mice) did not influence arrhythmia inducibility (i), the number of ventricular tachycardias per heart (ii) or the arrhythmia score (iii). B: sympathetic β-receptor-blockade, combined with muscarinic blockade (control: n = 5 mice, db/db: n = 5 mice), reduced the arrhythmia occurrence (i), the number of ventricular tachycardias per heart (ii), and the arrhythmia score (iii) upon ventricular stimulation in db/db mice. ***P ≤ 0.05.
DISCUSSION
The present study investigated the relevance of different components of the autonomic nervous system to the increased arrhythmia burden in db/db mice. The main findings are as follows: 1) hearts isolated from db/db mice were more susceptible to pacing-induced ventricular arrhythmias, 2) greater asynchronous neurotransmitter release was evident at preganglionic nerve terminals of sympathetic ganglia (stellate) from db/db mice, 3) heterogeneous loss in the expression of TH was observed in the mid-left ventricle but not the apex of db/db mice, and 4) inhibition of cardiac β-sympathetic receptors prevented stimulus-evoked arrhythmias in db/db mice. Collectively these results suggest that the increased susceptibility of type 2 diabetic mouse hearts to ventricular arrhythmias is due to sympathetic dysfunction in the absence of overt degeneration of postganglionic nerve fibers.
Cardiac arrhythmias are implicated as the underlying cause for sudden cardiac death in patients with T2DM (4, 40). The principle determinants of myocardial excitability are ion channel kinetics, Ca2+ handling, and waveform propagation, all of which can be modulated by the sympathetic, parasympathetic, and sensory nerves that interact together to form the multilevel neural circuits for autonomic control of the heart (3). In this study, we identified no differences in epicardial conduction velocity or cardiomyocyte Ca2+ homeostasis in T2 diabetic mice aged 10–16 wk. To confirm, in other mouse models of diabetes, e.g., in transgenic mice with cardiac specific overexpression of peroxisome proliferator activated receptor γ 1 (PPARγ1), no alterations in conduction velocity were documented despite reduced Cx43 expression (40). Also, in streptozotocin (STZ)-induced type 1 diabetic rats, Cx43 expression level was unchanged in the ventricles (39). However, reports of decreased conduction velocity measured in female db/db mice at an age of 22 wk (13) and in Zucker diabetic Fatty rats with T2DM (43) suggests that changes in myocardial waveform propagation may appear in older diabetic animals.
Deficits in cardiac myocyte Ca2+ homeostasis can be observed in db/db mice, including prolonged [Ca2+]i transient duration and reduced [Ca2+]i transient amplitude (7). Hyperglycemia with diabetes has also been shown to activate Ca2+/calmodulin-dependent protein kinase II (CaMKII), leading to activation of spontaneous sarcoplasmic reticulum Ca2+ release events (20), although Ca2+-sparks are less frequent in db/db mice (44). We found no differences in [Ca2+]i transients evoked by electrical stimulation of cardiomyocytes isolated from db/db mice at 12–16 wk of age. Multiple experimental factors likely underlie the discrepancies in cardiac myocyte Ca2+ homeostasis with type 2 diabetes. This includes both variation in methodology of intracellular Ca2+ measurements as well as differences in the metabolic and cardiac phenotypes of the models used. Baseline conditions between our study and that published by Belke et al. (7) were different. This group used 1 mM Ca2+ (1.5 mM in our setting), 0.3-Hz stimulation (1.0 Hz in our setting), room temperature (37°C in our setting), and Indo-1 instead of Fura-2 as calcium sensor. They observed a decrease in contractile function for 12-wk-old mice in one publication (7) but not in another (7). Pereira et al. (44) derived Ca2+-sparks from two-photon line scans in Rhod-2 loaded mouse hearts (15 wk of age). Both studies used the db/db mouse generated on a C57BL/6J background, whereas we used a db/db mouse on the BKS background. At 12–16 wk of age, our mice were hyperglycemic with cardiac insulin resistance. At this age, cardiac dysfunction starts to develop and diastolic dysfunction becomes obvious, but commonly no cardiomyopathy is present yet (29, 53, 70, 79). One study describes differences in calcium homeostasis and evidence of cardiomyopathy in BKS-db/db mice but at an age of 20 wk (61). Clearly, differences in the age and strain of the mice affect both the metabolic and cardiac phenotypes. The lack of a dysregulation of whole cell Ca2+-currents in our mice may be a reflection of the young age and absence of overt cardiomyopathy.
The increased arrhythmia susceptibility in both rat and mouse models of T2DM (13, 20) is postulated to be caused by disturbed sympathetic/parasympathetic interactions (42, 69). Ventricular electrophysiology is controlled by postganglionic sympathetic efferent axons (8, 15), as well as the postganglionic parasympathetic neurons of the intrinsic cardiac ganglia (10, 12, 31, 77). Inhibition of neurotransmission at intrinsic cardiac ganglia has also been shown to be proarrhythmic in otherwise healthy db/db hearts (31), and changes in intracardiac ganglion structure and activity are observed in the Akita mouse model of type 1 diabetes (5).
Tetanic stimulation of either sympathetic or parasympathetic nerves in vivo elicited similar changes in heart rate in both control and diabetic mice, indicating innervation of the sinoatrial node is unaffected in 12-wk-old db/db mice. In contrast to results following intraperitoneal injection of isoproterenol in db/db mice (56), we did not observe increased variability in R-R interval duration during sympathetic nerve stimulation (data not shown). This is likely related to the differences in the specific actions of nerve-released catecholamines versus global activation of sympathetic receptors following intraperitoneal injection. Decreased chronotropic responses to vagal stimulation are observed in mouse models of type 1 diabetes STZ-treated (37) or Akita mouse (5), again suggesting differences in etiology of diabetic cardiac autonomic neuropathy between type 1 and type 2 diabetic animal models. The similar responses on sinoatrial node automaticity observed in this study also demonstrate that evoked changes in heart rate do not accurately predict the state of the ventricular innervation in diabetes. It is also important to note that changes in heart rate recorded under anesthesia may not be a wholly accurate representation of differences in heart rate in the conscious state.
In the db/db mouse model, axonal degeneration, slowed sensory and motor nerve conduction velocity, impaired axonal transport, and decreased neurotransmitter levels are manifestations of diabetic neuropathy (9, 54, 62, 64). We found greater asynchronous neurotransmitter release presynaptically at the stellate ganglia in db/db mice, which was also observed at the pelvic ganglia of db/db mice (64). The increase in asynchronous neurotransmitter release may be associated with Ca2+ dyshomeostasis at preganglionic nerve endings (33, 64) or may be an effect of increased nerve activity and a resulting increase in neurotransmitter release probability. The effect of asynchronous neurotransmitter release on the ventricular myocardium remains to be determined, although one could hypothesize that the differential release of norepinephrine could result in a greater action potential duration dispersion.
Further supporting a role of sympathetic neuron dysfunction in arrhythmogenesis in type 2 diabetic mice is the heterogeneous loss of TH from db/db mouse hearts. We observed both a reduction in total ventricular TH expression and an absence of TH-immunoreactive nerve fibers from the ventricular wall of db/db mice, whereas the presence of TH+ fibers at the heart apex was unaffected. Significant proximal-distal heterogeneity in TH expression was previously observed in STZ-treated rats, which was partially restored by nerve growth factor infusion into the stellate ganglia (76). Consistently in patients with pure autonomic failure, a sympathetic dysfunction with TH reduction has been suggested to occur ahead of denervation (28). In diabetic patients, nuclear medicine imaging of the sympathetic nervous system revealed heterogeneous ventricular denervation (1, 34, 51), and attenuated sympathetic responses have been reported (58).
The notion of aberrant sympathetic control is also supported by the reduction of ventricular arrhythmia susceptibility in db/db mice after β-receptor blockade, whereas sole muscarinic blockade did not influence arrhythmogenicity, indicating predominantly sympathetically-induced ventricular arrhythmias. A shift in balance toward elevated sympathetic tone is described in patients with impaired glucose tolerance (74), in patients with prediabetes (55), and also in patients with overt diabetes during hypoglycemia (13). The regulation of sympathetic tone in db/db mice is altered as shown by continuous measurement of heart rate in freely moving mice (59).
In conclusion, we propose that the increased susceptibility of type 2 diabetic mouse hearts to ventricular arrhythmias is due to sympathetic dysfunction in the absence of overt degeneration of postganglionic nerve fibers. Sympathetic dysregulation is supported by the observations of increased asynchronous release at stellate ganglia, a heterogeneous loss of TH from the ventricular wall but not apex, and inhibition of ventricular arrhythmias in db/db mice after β-sympathetic receptor blockade. Although there is not one clear comprehensive proarrhythmogenic mechanism, these data support a growing body of evidence demonstrating the association of sympathetic nerve dysfunction in T2DM with cardiac arrhythmogenesis.
Limitations.
This study has several limitations. Although results from mice may not be directly translatable to humans, this mouse model, which uses a leptin receptor mutation, is similar to the clinical T2DM in patients and has been used extensively to study the influence of diabetes on the cardiovascular system (2, 13, 44). Mouse hearts are similar to human hearts in many aspects (14); however, it is known that cardiac neuroanatomy has interspecies variability (49) and that the vagal baseline tone is lower in mice compared with men. One major limitation is the altered substrate use in diabetes, as mainly free fatty acids are used instead of glucose. In our experimental setup, only glucose was used instead of free fatty acids. Sympathetic distributions may vary regionally so that left instead of right stellate ganglion stimulation may have induced different effects. Although an ex vivo experimental setup without direct input from sympathetic stellate ganglia was used, it has been shown that intracardiac parasympathetic and sympathetic neurons are intact and still able to modulate electrical function (6, 72). The described arrhythmias have been induced by programmed extrastimulus or burst pacing in a well-established and well accepted ex vivo setup (17) and were not measured in vivo or in a conscious or chronic model.
GRANTS
This work was supported by the DZHK (Centre for Cardiovascular Research) (FKZ 81Z2710104 to C. Meyer), the DFG (German Research Foundation) (project no. 316865582 to F. Flenner), UCLA Department of Medicine research start-up funds (to J. D. Tompkins), and the National Institutes of Health (NIH) SPARC Grant No. OT2OD023848 (to J. D. Tompkins and J. L. Ardell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J. and J.D.T. conceived and designed research; C.J., K.S., F.F., P.R., K.A.D.J., and J.D.T. performed experiments; C.J., K.S., F.F., H.J., and J.D.T. analyzed data; C.J. and J.D.T. interpreted results of experiments; C.J. and J.D.T. prepared figures; C.J. and J.D.T. drafted manuscript; C.J., C.M., J.L.A., and J.D.T. edited and revised manuscript; C.J., K.S., F.F., H.J., P.R., K.A.D.J., V.N., C.M., J.L.A., and J.D.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We kindly thank Dr. Scott John and Prof. Kalyanam Shivkumar for careful review of the manuscript and for the comments, corrections, and suggestions that ensued; Dr. Catherine Meyer-Schwesinger for graciously providing the PGP 9.5 antibody; the UKE microscopy imaging facility (University Hospital Centre Hamburg-Eppendorf) for providing microscopes; Prof. Schumacher (University Clinic Hamburg-Eppendorf, Hamburg, Germany); and Hartwig Wieboldt for excellent technical support.
REFERENCES
- 1.Allman KC, Stevens MJ, Wieland DM, Hutchins GD, Wolfe ER Jr, Greene DA, Schwaiger M. Noninvasive assessment of cardiac diabetic neuropathy by carbon-11 hydroxyephedrine and positron emission tomography. J Am Coll Cardiol 22: 1425–1432, 1993. doi: 10.1016/0735-1097(93)90553-D. [DOI] [PubMed] [Google Scholar]
- 2.Anzawa R, Bernard M, Tamareille S, Baetz D, Confort-Gouny S, Gascard JP, Cozzone P, Feuvray D. Intracellular sodium increase and susceptibility to ischaemia in hearts from type 2 diabetic db/db mice. Diabetologia 49: 598–606, 2006. doi: 10.1007/s00125-005-0091-5. [DOI] [PubMed] [Google Scholar]
- 3.Ardell JL, Andresen MC, Armour JA, Billman GE, Chen PS, Foreman RD, Herring N, O’Leary DS, Sabbah HN, Schultz HD, Sunagawa K, Zucker IH. Translational neurocardiology: preclinical models and cardioneural integrative aspects. J Physiol 594: 3877–3909, 2016. doi: 10.1113/JP271869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balkau B, Jouven X, Ducimetière P, Eschwège E. Diabetes as a risk factor for sudden death. Lancet 354: 1968–1969, 1999. doi: 10.1016/S0140-6736(99)04383-4. [DOI] [PubMed] [Google Scholar]
- 5.Bassil G, Chang M, Pauza A, Diaz Vera J, Tsalatsanis A, Lindsey BG, Noujaim SF. Pulmonary vein ganglia are remodeled in the diabetic heart. J Am Heart Assoc 7: e008919, 2018. doi: 10.1161/JAHA.118.008919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591: 4515–4533, 2013. doi: 10.1113/jphysiol.2013.259382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belke DD, Swanson EA, Dillmann WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes 53: 3201–3208, 2004. doi: 10.2337/diabetes.53.12.3201. [DOI] [PubMed] [Google Scholar]
- 8.Brack KE, Coote JH, Ng GA. The effect of direct autonomic nerve stimulation on left ventricular force in the isolated innervated Langendorff perfused rabbit heart. Auton Neurosci 124: 69–80, 2006. doi: 10.1016/j.autneu.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Campanucci V, Krishnaswamy A, Cooper E. Diabetes depresses synaptic transmission in sympathetic ganglia by inactivating nAChRs through a conserved intracellular cysteine residue. Neuron 66: 827–834, 2010. doi: 10.1016/j.neuron.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Cardinal R, Pagé P, Vermeulen M, Ardell JL, Armour JA. Spatially divergent cardiac responses to nicotinic stimulation of ganglionated plexus neurons in the canine heart. Auton Neurosci 145: 55–62, 2009. doi: 10.1016/j.autneu.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Cavaghan MK, Ehrmann DA, Polonsky KS. Interactions between insulin resistance and insulin secretion in the development of glucose intolerance. J Clin Invest 106: 329–333, 2000. doi: 10.1172/JCI10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiou CW, Zipes DP. Selective vagal denervation of the atria eliminates heart rate variability and baroreflex sensitivity while preserving ventricular innervation. Circulation 98: 360–368, 1998. doi: 10.1161/01.CIR.98.4.360. [DOI] [PubMed] [Google Scholar]
- 13.Chou CC, Ho CT, Lee HL, Chu Y, Yen TH, Wen MS, Lin SF, Lee CH, Chang PC. Roles of impaired intracellular calcium cycling in arrhythmogenicity of diabetic mouse model. Pacing Clin Electrophysiol 40: 1087–1095, 2017. doi: 10.1111/pace.13166. [DOI] [PubMed] [Google Scholar]
- 14.Choy L, Yeo JM, Tse V, Chan SP, Tse G. Cardiac disease and arrhythmogenesis: Mechanistic insights from mouse models. Int J Cardiol Heart Vasc 12: 1–10, 2016. doi: 10.1016/j.ijcha.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coote JH. Myths and realities of the cardiac vagus. J Physiol 591: 4073–4085, 2013. doi: 10.1113/jphysiol.2013.257758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coumel P. Cardiac arrhythmias and the autonomic nervous system. J Cardiovasc Electrophysiol 4: 338–355, 1993. doi: 10.1111/j.1540-8167.1993.tb01235.x. [DOI] [PubMed] [Google Scholar]
- 17.Curtis MJ, Hancox JC, Farkas A, Wainwright CL, Stables CL, Saint DA, Clements-Jewery H, Lambiase PD, Billman GE, Janse MJ, Pugsley MK, Ng GA, Roden DM, Camm AJ, Walker MJ. The Lambeth Conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther 139: 213–248, 2013. doi: 10.1016/j.pharmthera.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 18.da Rocha Fernandes J, Ogurtsova K, Linnenkamp U, Guariguata L, Seuring T, Zhang P, Cavan D, Makaroff LE. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res Clin Pract 117: 48–54, 2016. doi: 10.1016/j.diabres.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 19.El-Armouche A, Pohlmann L, Schlossarek S, Starbatty J, Yeh YH, Nattel S, Dobrev D, Eschenhagen T, Carrier L. Decreased phosphorylation levels of cardiac myosin-binding protein-C in human and experimental heart failure. J Mol Cell Cardiol 43: 223–229, 2007. doi: 10.1016/j.yjmcc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 502: 372–376, 2013. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faggioni M, Hwang HS, van der Werf C, Nederend I, Kannankeril PJ, Wilde AA, Knollmann BC. Accelerated sinus rhythm prevents catecholaminergic polymorphic ventricular tachycardia in mice and in patients. Circ Res 112: 689–697, 2013. doi: 10.1161/CIRCRESAHA.111.300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flenner F, Friedrich FW, Ungeheuer N, Christ T, Geertz B, Reischmann S, Wagner S, Stathopoulou K, Söhren KD, Weinberger F, Schwedhelm E, Cuello F, Maier LS, Eschenhagen T, Carrier L. Ranolazine antagonizes catecholamine-induced dysfunction in isolated cardiomyocytes, but lacks long-term therapeutic effects in vivo in a mouse model of hypertrophic cardiomyopathy. Cardiovasc Res 109: 90–102, 2016. doi: 10.1093/cvr/cvv247. [DOI] [PubMed] [Google Scholar]
- 23.Friedrichs K, Adam M, Remane L, Mollenhauer M, Rudolph V, Rudolph TK, Andrié RP, Stöckigt F, Schrickel JW, Ravekes T, Deuschl F, Nickenig G, Willems S, Baldus S, Klinke A. Induction of atrial fibrillation by neutrophils critically depends on CD11b/CD18 integrins. PLoS One 9: e89307, 2014. doi: 10.1371/journal.pone.0089307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res 116: 2005–2019, 2015. doi: 10.1161/CIRCRESAHA.116.304679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, Heethaar RM, Stehouwer CD. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care 24: 1793–1798, 2001. doi: 10.2337/diacare.24.10.1793. [DOI] [PubMed] [Google Scholar]
- 26.Ginter E, Simko V. Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol 771: 42–50, 2012. doi: 10.1007/978-1-4614-5441-0_6. [DOI] [PubMed] [Google Scholar]
- 27.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103: 137–149, 2014. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Guo L, Esler MD, Sari C, Phillips S, Lambert EA, Straznicky NE, Lambert GW, Corcoran SJ. Does sympathetic dysfunction occur before denervation in pure autonomic failure? Clin Sci (Lond) 132: 1–16, 2018. doi: 10.1042/CS20170240. [DOI] [PubMed] [Google Scholar]
- 29.Hall ME, Maready MW, Hall JE, Stec DE. Rescue of cardiac leptin receptors in db/db mice prevents myocardial triglyceride accumulation. Am J Physiol Endocrinol Metab 307: E316–E325, 2014. doi: 10.1152/ajpendo.00005.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoard JL, Hoover DB, Mabe AM, Blakely RD, Feng N, Paolocci N. Cholinergic neurons of mouse intrinsic cardiac ganglia contain noradrenergic enzymes, norepinephrine transporters, and the neurotrophin receptors tropomyosin-related kinase A and p75. Neuroscience 156: 129–142, 2008. doi: 10.1016/j.neuroscience.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jungen C, Scherschel K, Eickholt C, Kuklik P, Klatt N, Bork N, Salzbrunn T, Alken F, Angendohr S, Klene C, Mester J, Klöcker N, Veldkamp MW, Schumacher U, Willems S, Nikolaev VO, Meyer C. Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias. Nat Commun 8: 14155, 2017. doi: 10.1038/ncomms14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34: 29–34, 1974. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 33.Krishnaswamy A, Cooper E. Reactive oxygen species inactivate neuronal nicotinic acetylcholine receptors through a highly conserved cysteine near the intracellular mouth of the channel: implications for diseases that involve oxidative stress. J Physiol 590: 39–47, 2012. doi: 10.1113/jphysiol.2011.214007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langer A, Freeman MR, Josse RG, Armstrong PW. Metaiodobenzylguanidine imaging in diabetes mellitus: assessment of cardiac sympathetic denervation and its relation to autonomic dysfunction and silent myocardial ischemia. J Am Coll Cardiol 25: 610–618, 1995. doi: 10.1016/0735-1097(94)00459-4. [DOI] [PubMed] [Google Scholar]
- 35.Leibel RL, Chung WK, Chua SC Jr. The molecular genetics of rodent single gene obesities. J Biol Chem 272: 31937–31940, 1997. doi: 10.1074/jbc.272.51.31937. [DOI] [PubMed] [Google Scholar]
- 36.Low PA, Benrud-Larson LM, Sletten DM, Opfer-Gehrking TL, Weigand SD, O’Brien PC, Suarez GA, Dyck PJ. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care 27: 2942–2947, 2004. doi: 10.2337/diacare.27.12.2942. [DOI] [PubMed] [Google Scholar]
- 37.Mabe AM, Hoover DB. Remodeling of cardiac cholinergic innervation and control of heart rate in mice with streptozotocin-induced diabetes. Auton Neurosci 162: 24–31, 2011. doi: 10.1016/j.autneu.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Marques JL, George E, Peacey SR, Harris ND, Macdonald IA, Cochrane T, Heller SR. Altered ventricular repolarization during hypoglycaemia in patients with diabetes. Diabet Med 14: 648–654, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 39.Mitasíková M, Lin H, Soukup T, Imanaga I, Tribulová N. Diabetes and thyroid hormones affect connexin-43 and PKC-epsilon expression in rat heart atria. Physiol Res 58: 211–217, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Morrow JP, Katchman A, Son NH, Trent CM, Khan R, Shiomi T, Huang H, Amin V, Lader JM, Vasquez C, Morley GE, D’Armiento J, Homma S, Goldberg IJ, Marx SO. Mice with cardiac overexpression of peroxisome proliferator-activated receptor γ have impaired repolarization and spontaneous fatal ventricular arrhythmias. Circulation 124: 2812–2821, 2011. doi: 10.1161/CIRCULATIONAHA.111.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Movahed MR, Hashemzadeh M, Jamal M. Increased prevalence of ventricular fibrillation in patients with type 2 diabetes mellitus. Heart Vessels 22: 251–253, 2007. doi: 10.1007/s00380-006-0962-9. [DOI] [PubMed] [Google Scholar]
- 42.Olivas A, Gardner RT, Wang L, Ripplinger CM, Woodward WR, Habecker BA. Myocardial infarction causes transient cholinergic transdifferentiation of cardiac sympathetic nerves via gp130. J Neurosci 36: 479–488, 2016. doi: 10.1523/JNEUROSCI.3556-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsen KB, Axelsen LN, Braunstein TH, Sørensen CM, Andersen CB, Ploug T, Holstein-Rathlou NH, Nielsen MS. Myocardial impulse propagation is impaired in right ventricular tissue of Zucker diabetic fatty (ZDF) rats. Cardiovasc Diabetol 12: 19, 2013. doi: 10.1186/1475-2840-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, Gómez AM. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes 55: 608–615, 2006. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 45.Pohlmann L, Kröger I, Vignier N, Schlossarek S, Krämer E, Coirault C, Sultan KR, El-Armouche A, Winegrad S, Eschenhagen T, Carrier L. Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes. Circ Res 101: 928–938, 2007. doi: 10.1161/CIRCRESAHA.107.158774. [DOI] [PubMed] [Google Scholar]
- 46.Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 33: 1578–1584, 2010. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajab M, Jin H, Welzig CM, Albano A, Aronovitz M, Zhang Y, Park HJ, Link MS, Noujaim SF, Galper JB. Increased inducibility of ventricular tachycardia and decreased heart rate variability in a mouse model for type 1 diabetes: effect of pravastatin. Am J Physiol Heart Circ Physiol 305: H1807–H1816, 2013. doi: 10.1152/ajpheart.00979.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudchenko A, Akude E, Cooper E. Synapses on sympathetic neurons and parasympathetic neurons differ in their vulnerability to diabetes. J Neurosci 34: 8865–8874, 2014. doi: 10.1523/JNEUROSCI.0033-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rysevaite K, Saburkina I, Pauziene N, Noujaim SF, Jalife J, Pauza DH. Morphologic pattern of the intrinsic ganglionated nerve plexus in mouse heart. Heart Rhythm 8: 448–454, 2011. doi: 10.1016/j.hrthm.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnell O, Hammer K, Muhr-Becker D, Ziegler A, Weiss M, Tatsch K, Standl E. Cardiac sympathetic dysinnervation in Type 2 diabetes mellitus with and without ECG-based cardiac autonomic neuropathy. J Diabetes Complications 16: 220–227, 2002. doi: 10.1016/S1056-8727(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 52.Schrickel JW, Brixius K, Herr C, Clemen CS, Sasse P, Reetz K, Grohé C, Meyer R, Tiemann K, Schröder R, Bloch W, Nickenig G, Fleischmann BK, Noegel AA, Schwinger RH, Lewalter T. Enhanced heterogeneity of myocardial conduction and severe cardiac electrical instability in annexin A7-deficient mice. Cardiovasc Res 76: 257–268, 2007. doi: 10.1016/j.cardiores.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol 283: H976–H982, 2002. doi: 10.1152/ajpheart.00088.2002. [DOI] [PubMed] [Google Scholar]
- 54.Shaikh AS, Somani RS. Animal models and biomarkers of neuropathy in diabetic rodents. Indian J Pharmacol 42: 129–134, 2010. doi: 10.4103/0253-7613.66833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh JP, Larson MG, O’Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, Levy D. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol 86: 309–312, 2000. doi: 10.1016/S0002-9149(00)00920-6. [DOI] [PubMed] [Google Scholar]
- 56.Soltysinska E, Speerschneider T, Winther SV, Thomsen MB. Sinoatrial node dysfunction induces cardiac arrhythmias in diabetic mice. Cardiovasc Diabetol 13: 122, 2014. doi: 10.1186/s12933-014-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sosna J, Voigt S, Mathieu S, Kabelitz D, Trad A, Janssen O, Meyer-Schwesinger C, Schütze S, Adam D. The proteases HtrA2/Omi and UCH-L1 regulate TNF-induced necroptosis. Cell Commun Signal 11: 76, 2013. doi: 10.1186/1478-811X-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P; Toronto Consensus Panel on Diabetic Neuropathy . Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 27: 639–653, 2011. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 59.Stables CL, Auerbach DS, Whitesall SE, D’Alecy LG, Feldman EL. Differential impact of type-1 and type-2 diabetes on control of heart rate in mice. Auton Neurosci 194: 17–25, 2016. doi: 10.1016/j.autneu.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stöckigt F, Brixius K, Lickfett L, Andrié R, Linhart M, Nickenig G, Schrickel JW. Total beta-adrenoceptor knockout slows conduction and reduces inducible arrhythmias in the mouse heart. PLoS One 7: e49203, 2012. doi: 10.1371/journal.pone.0049203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stølen TO, Høydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, Larsen T, Rolim N, Condorelli G, Smith GL, Wisløff U. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res 105: 527–536, 2009. doi: 10.1161/CIRCRESAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F III, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis 28: 276–285, 2007. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjörnsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M. Excess mortality among persons with type 2 diabetes. N Engl J Med 373: 1720–1732, 2015. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 64.Tompkins JD, Vizzard MA, Parsons RL. Synaptic transmission at parasympathetic neurons of the major pelvic ganglion from normal and diabetic male mice. J Neurophysiol 109: 988–995, 2013. doi: 10.1152/jn.00354.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Treweek JB, Chan KY, Flytzanis NC, Yang B, Deverman BE, Greenbaum A, Lignell A, Xiao C, Cai L, Ladinsky MS, Bjorkman PJ, Fowlkes CC, Gradinaru V. Whole-body tissue stabilization and selective extractions via tissue-hydrogel hybrids for high-resolution intact circuit mapping and phenotyping. Nat Protoc 10: 1860–1896, 2015. doi: 10.1038/nprot.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tse G, Lai ET, Tse V, Yeo JM. Molecular and electrophysiological mechanisms underlying cardiac arrhythmogenesis in diabetes mellitus. J Diabetes Res 2016: 2848759, 2016. doi: 10.1155/2016/2848759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tse G, Lai ET, Yeo JM, Tse V, Wong SH. Mechanisms of electrical activation and conduction in the gastrointestinal system: lessons from cardiac electrophysiology. Front Physiol 7: 182, 2016. doi: 10.3389/fphys.2016.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tse G, Lai ET, Yeo JM, Yan BP. Electrophysiological mechanisms of Bayés syndrome: insights from clinical and mouse studies. Front Physiol 7: 188, 2016. doi: 10.3389/fphys.2016.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vaseghi M, Shivkumar K. Neuraxial modulation for ventricular arrhythmias: a new hope. Heart Rhythm 9: 1888–1889, 2012. doi: 10.1016/j.hrthm.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 70.Venardos K, De Jong KA, Elkamie M, Connor T, McGee SL. The PKD inhibitor CID755673 enhances cardiac function in diabetic db/db mice. PLoS One 10: e0120934, 2015. doi: 10.1371/journal.pone.0120934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 115: 387–397, 2007. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 72.Wengrowski AM, Wang X, Tapa S, Posnack NG, Mendelowitz D, Kay MW. Optogenetic release of norepinephrine from cardiac sympathetic neurons alters mechanical and electrical function. Cardiovasc Res 105: 143–150, 2015. doi: 10.1093/cvr/cvu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wheeler SG, Ahroni JH, Boyko EJ. Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 58: 131–138, 2002. doi: 10.1016/S0168-8227(02)00128-6. [DOI] [PubMed] [Google Scholar]
- 74.Wu JS, Yang YC, Lin TS, Huang YH, Chen JJ, Lu FH, Wu CH, Chang CJ. Epidemiological evidence of altered cardiac autonomic function in subjects with impaired glucose tolerance but not isolated impaired fasting glucose. J Clin Endocrinol Metab 92: 3885–3889, 2007. doi: 10.1210/jc.2006-2175. [DOI] [PubMed] [Google Scholar]
- 75.Wyse BM, Dulin WE. The influence of age and dietary conditions on diabetes in the db mouse. Diabetologia 6: 268–273, 1970. doi: 10.1007/BF01212237. [DOI] [PubMed] [Google Scholar]
- 76.Xue M, Xuan YL, Wang Y, Hu HS, Li XL, Suo F, Li XR, Cheng WJ, Yan SH. Exogenous nerve growth factor promotes the repair of cardiac sympathetic heterogeneity and electrophysiological instability in diabetic rats. Cardiology 127: 155–163, 2014. doi: 10.1159/000355535. [DOI] [PubMed] [Google Scholar]
- 77.Yamakawa K, So EL, Rajendran PS, Hoang JD, Makkar N, Mahajan A, Shivkumar K, Vaseghi M. Electrophysiological effects of right and left vagal nerve stimulation on the ventricular myocardium. Am J Physiol Heart Circ Physiol 307: H722–H731, 2014. doi: 10.1152/ajpheart.00279.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang B, Treweek JB, Kulkarni RP, Deverman BE, Chen CK, Lubeck E, Shah S, Cai L, Gradinaru V. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 158: 945–958, 2014. doi: 10.1016/j.cell.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yue P, Arai T, Terashima M, Sheikh AY, Cao F, Charo D, Hoyt G, Robbins RC, Ashley EA, Wu J, Yang PC, Tsao PS. Magnetic resonance imaging of progressive cardiomyopathic changes in the db/db mouse. Am J Physiol Heart Circ Physiol 292: H2106–H2118, 2007. doi: 10.1152/ajpheart.00856.2006. [DOI] [PubMed] [Google Scholar]
- 80.Yun JS, Park YM, Cha SA, Ahn YB, Ko SH. Progression of cardiovascular autonomic neuropathy and cardiovascular disease in type 2 diabetes. Cardiovasc Diabetol 17: 109, 2018. doi: 10.1186/s12933-018-0752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ziegler D, Zentai CP, Perz S, Rathmann W, Haastert B, Döring A, Meisinger C; KORA Study Group . Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care 31: 556–561, 2008. doi: 10.2337/dc07-1615. [DOI] [PubMed] [Google Scholar]