Abstract
Recent evidence suggests that the gut microbiota contributes to the pathogenesis of hypertension (HTN). The gut microbiota is a highly dynamic organ mediating numerous physiological functions, which can be influenced by external factors such as diet. In particular, a major modifiable risk factor for HTN is dietary sodium intake. Sodium consumption in the United States is significantly greater than that recommended by the federal government and organizations such as the American Heart Association. Because of the emerging connection between the gut microbiota and HTN, the interaction between dietary sodium and gut microbiota has sparked interest. High-sodium diets promote local and systemic tissue inflammation and impair intestinal anatomy compared with low sodium intake in both human and animal studies. It is biologically plausible that the gut microbiota mediates the inflammatory response, as it is in constant interaction with the immune system and is necessary for proper maturation of immune cells. Recent rodent data demonstrate that dietary sodium disrupts gut microbial homeostasis as gut microbiota composition shifts with dietary sodium manipulation. In this review, we will focus on gut microbiota activity in HTN and the influence of high dietary sodium intake with an emphasis on the immune system, bacterial metabolites, and the circadian clock.
Keywords: blood pressure, dietary salt, gut microbiota, hypertension, sodium
INTRODUCTION
Hypertension (HTN) is the most prevalent risk factor for cardiovascular disease (CVD), afflicting nearly half of adults in the United States (70). HTN itself is a multifactorial disease, and one’s predisposition to it is influenced by inheritance (genetics), environment, and their interaction (50). Multiple environmental factors increase risk for HTN, including diet, inactivity, overweight and obesity, smoking, and psychological stress (7). Specifically, excess dietary sodium consumption is a prominent risk factor for HTN (7). Dietary Guidelines for Americans (DGA) recommend that sodium intake should be no more than 2,300 mg/day (67), whereas the American Heart Association reduces this further to 1,500 mg/day for optimal health and in particular for older adults, African Americans, and individuals with HTN (18). Both of these recommendations call for major diet modifications since the average US intake is 3,600 mg/day, ~1.5-fold greater than the DGA recommendation (5).
The gut microbiota, a rich community of microorganisms inhabiting the gastrointestinal (GI) tract, has been recognized as a dynamic organ imperative for host metabolism. Recent studies have established that alterations in gut microbiota can play a role in the development of HTN in rodents (1, 17, 39), whereas research in humans is in its infancy. Considering the importance of dietary sodium intake in HTN and the GI tract being in direct contact with ingested sodium, the potential interactions between dietary sodium and gut microbiota should not be overlooked (Fig. 1). Although other nutrients, such as fiber (45) and fructose (27), can affect BP through modulation of the gut microbiota and subsequent effects on various organ systems, this review will focus on the effects of sodium.
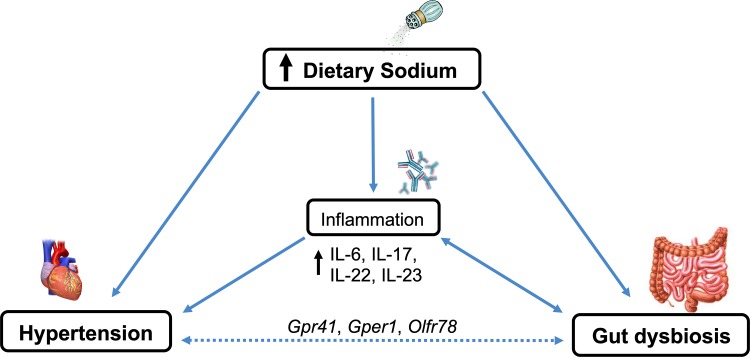
Fig. 1.
Interactions between dietary sodium, hypertension, and the gut microbiota. High dietary sodium consumption may contribute to gut dysbiosis, as it alters gut microbial composition, richness, and diversity. High dietary sodium also induces inflammation through increased production of inflammatory cytokines that can directly increase blood pressure (BP). Potential mechanism of BP increase via the gut microbiota is through G protein-coupled (Gpr) and olfactory (Olfr) receptors in the vasculature or through an inflammatory response. Gper, G protein-coupled estrogen receptor; IL, interleukin.
GUT MICROBIAL AND HOST INTERACTIONS
Human microbiota refers to the community of bacteria, viruses, archaea, and eukaryotic microbes that populate the human body. Even though the ratio of bacterial cells to human cells within the human body is ∼1:1 (59), the bacterial genome is ∼150 times greater than the human genome (54), suggesting the physiological importance of microbial and host interactions.
The regional abundance of microbiota populations varies along the GI tract. In the upper GI tract (e.g., the stomach), there are 102 bacteria present per gram of content. Bacterial abundance increases along the distal GI tract, reaching 1011 bacteria per gram of content in the colon (16). More than 2,900 bacterial species that reside in the human gut have been identified (4, 55), ranging from mutualistic (a term often used interchangeably with commensal) to opportunistic, which compete for resources to establish their niche and ensure survival. The gut microbiota is composed of several core phyla predominated by Bacteroidetes and Firmicutes, whereas Actinobacteria, Proteobacteria, and Verrucomicrobia are present in lower abundance (28). The gut microbiota interacts with multiple organ systems and mediates numerous physiological functions. In the gut, bacteria metabolize dietary fiber into various metabolites, such as short-chain fatty acids (SCFA) that would otherwise not be available to the host. Furthermore, gut bacteria stimulate GI tissue and immune system development. This particular relation between bacterial and host cells is symbiotic or mutualistic, as the host provides necessary resources for bacterial species to establish their niche, such as space, nutrients, and favorable environmental conditions (16). A complex immune system mechanism regulates the interaction between host and bacteria, monitoring bacterial abundance and diversity. The core microbiota is shared mostly among individuals but differs slightly from person to person. These differences in composition depend on a number of factors, such as genetics (66), dietary patterns (15), and geographical region (46). The gut microbiota is also highly dynamic and susceptible to transient changes resulting from antibiotic use or dietary alterations (16).
Research over the past two decades has focused on examining the role gut microbiota has in various infectious, autoimmune, and metabolic diseases, such as Clostridium difficile infection, inflammatory bowel disease (IBD), and obesity (16, 66). More recently, studies in rodents verified that changes in the gut microbiota composition take part in the onset of HTN (1, 17, 39), whereas research in humans remains scarce with only a few conducted studies. This review paper will discuss the current literature investigating the gut microbiota and HTN as well as the impact of sodium intake on gut microbiota.
GUT MICROBIAL AND HOST INTERACTIONS IN HYPERTENSION
It has become clear that HTN is associated with disruption in gut microbial composition (gut dysbiosis) (1, 17, 65, 72) and that bacterial metabolites play a role in blood pressure (BP) regulation. Observational data shows that hypertensive rodents (1, 17, 39, 47, 72) and humans (34, 39, 72) demonstrate gut dysbiosis compared with their normotensive counterparts, as seen by reduced microbial abundance, richness, and diversity and clear taxonomical distinction. Consequences of gut dysbiosis on intestinal milieu are detrimental, as dysbiosis reshapes intestinal mucosa in rodent models by thinning it and making it more permeable to pathogens, leading to persistent low-grade inflammation (2). Several bacterial taxa were observed to be different between hypertensive and normotensive groups in both animal and human studies (Table 1). Interestingly, a majority of taxa of which relative abundance is associated with BP (either HTN or normal BP) in rodents are of the order of Clostridiales (1, 8, 17, 72) and in humans of Clostridiales and Bacteroidales (34, 39). Furthermore, Kim et al. (34) demonstrated that Ruminococcus torques, Eubacterium siraeum, and Alistipes finegoldii can cause intestinal inflammation and are associated with high BP in contrast to Bacteroides thetaiotaomicron which has anti-inflammatory properties and is associated with lower BP. As the field progresses, data from future studies will allow for a precise outlining and targeting of crucial taxa associated with HTN.
Table 1.
Bacterial taxa altered in hypertension with their full classification
| Phylum/Class | Order | Family | Genus | Species | Reference |
|---|---|---|---|---|---|
| Rodent taxa positively associated with BP or increased in abundance in hypertension | |||||
| Bacteroidetes | |||||
| Bacteroidia | Bacteroidales | Barnesiellaceae | Bier et al. (8) | ||
| Firmicutes | |||||
| Clostridia | Clostridiales | Christensenellaceae | Bier et al. (8) | ||
| Clostridiaceae | Durgan et al. (17) | ||||
| Eubacteriaceae | Anaerofustis | Bier et al. (8) | |||
| Peptococcaceae | Dehalobacterium | Durgan et al. (17) | |||
| Bacilli | Lactobacillales | Lactobacillaceae | Lactobacillus | Adnan et al. (1) | |
| Streptococcaceae | Streptococcus | Yang et al. (72) | |||
| Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | Holdemania | Durgan et al. (17) | |
| Turicibacter | Toral et al. (65), Yang et al. (72) | ||||
| Proteobacteria | |||||
| Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Erwinia | Bier et al. (8) | |
| Pseudomonadales | Bier et al. (8) | ||||
| Human taxa positively associated with BP or increased in abundance in hypertension | |||||
| Actinobacteria | |||||
| Actinobacteria | Actinomycetales | Actinomycetaceae | Actinomyces | Li et al. (39) | |
| Bacteroidetes | |||||
| Bacteroidia | Bacteroidales | Porphyromonadaceae | Porphyromonas | Li et al. (39) | |
| Prevotellaceae | Prevotella | Li et al. (39) | |||
| Rikenellaceae | Alistipes | finegoldii | Kim et al. (34) | ||
| Firmicutes | |||||
| Clostridia | Clostridiales | Ruminococcaceae | Ruminococcus | torques | Kim et al. (34) |
| Eubacteriaceae | Eubacterium | siraeum | Kim et al. (34) | ||
| Proteobacteria | |||||
| Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Klebsiella | Li et al. (39) | |
| Rodent taxa negatively associated with BP or increased in abundance in normal BP | |||||
| Bacteroidetes | |||||
| Bacteroidia | Bacteroidales | S24–7 | S24–7_g (unclassified) | Toral et al. (65) | |
| Firmicutes | |||||
| Clostridia | Clostridiales | Clostridiaceae | Adnan et al. (1) | ||
| Lachnospiraceae | Anaerostipes | Bier et al. (8) | |||
| Coprococcus | Yang et al. (72) | ||||
| Pseudobutyrivibrio | Yang et al. (72) | ||||
| Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | Coprobacillus | Adnan et al. (1) | |
| Holdemania | Adnan et al. (1) | ||||
| Human taxa negatively associated with BP or increased in abundance normal BP | |||||
| Actinobacteria | |||||
| Actinobacteria | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | Li et al. (39) | |
| Bacteroidetes | |||||
| Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | thetaiotaomicron | Kim et al. (34) |
| Firmicutes | |||||
| Clostridia | Clostridiales | Clostridiaceae | Faecalibacterium | Li et al. (39) | |
| Lachnospiraceae | Butyrivibrio | Li et al. (39) | |||
| Roseburia | Li et al. (39) | ||||
Bacterial taxa found to be altered in the original publication are in boldface.
Research in rodents demonstrates that gut microbiota takes part in the pathogenesis of HTN. Fecal microbiota transplant (FMT) from hypertensive patients to germ-free mice results in increased systolic and diastolic BP and reduced bacterial diversity (39). Even more captivating is that FMT from hypertensive rats elevates systolic BP in normotensive rats (1, 65), whereas the reverse attenuates systolic BP in hypertensive rats (65). In addition to ameliorating BP in hypertensive rats, FMT from normotensive rats improved vascular relaxation, likely due to reduced oxidative stress and inflammation in the vasculature (65). In particular, NADPH oxidase, which produces the superoxide anion, and T helper-17 (TH-17) cells that produce proinflammatory interleukin (IL)-17 were outlined as essential for FMT-induced BP increase and vascular impairment (65). These findings demonstrate immense malleability of the gut microbiota in both healthy and dysbiotic states and delineate mechanisms through which the gut microbiota may affect the cardiovascular system.
Another common model for investigating microbial influence on BP is the angiotensin II (ANG II)-induced hypertensive rodent. Germ-free mice infused with ANG II have lower BP, lower levels of reactive oxygen species in the vasculature, and a reduced transcription factor for circulating IL-17 synthesis compared with conventionally (i.e., microbiota intact) raised mice infused with ANG II (32). Conventionally raised rats with ANG II-induced HTN have reduced microbial richness compared with controls despite no change in evenness and diversity indices (72). Subsequent treatment with the anti-inflammatory antibiotic minocycline reduces BP in these rats and shifts the gut microbial composition toward that seen in controls, further suggesting that ANG II mediated a deleterious shift in the gut microbiota composition (72). Interestingly, different antibiotics exhibit distinct effects on BP, depending on the rat strain, indicating that precaution should be taken when administering antibiotics to hypertensive rodents and potentially humans (22).
Taken together, these findings suggest that gut microbiota may facilitate a rise in BP and immune system activation. Although the mechanisms have not been elucidated, one of the potential molecular pathways may be activation of SCFA receptors and increased renin secretion in the kidney. Further research is warranted to confirm these findings in rodents and translate to humans.
Short-Chain Fatty Acids and Blood Pressure Regulation
SCFAs, metabolites exclusively produced by gut bacteria upon fermentation of dietary fiber, have emerged as potential regulators of BP. Three primary SCFAs, acetate, propionate, and butyrate, account for 80% of total SCFA production (13). Treatment with SCFA may be beneficial, as it reduces BP in rodents (23, 34, 51). Ganesh et al. (23) detected 48% lower colonic acetate levels in rats with obstructive sleep apnea (OSA)-induced HTN compared with controls and successfully reversed HTN with colonic acetate infusion. HTN was also reversed with Clostridium butyricum and resistant corn starch treatment, both of which lead to increased production of acetate and butyrate (23). Furthermore, intraperitoneal butyrate infusion attenuated ANG II-induced HTN in mice by shifting the microbial profile and improving cardiac and vascular function (34). Finally, propionate administered in drinking water to mice infused with ANG II reduced systolic and diastolic BP, systemic inflammation, and cardiac damage and improved vascular function (6). These data demonstrate that SCFAs are beneficial in rodent models of HTN.
Several G protein-coupled receptors (GPCRs) and an olfactory receptor for SCFAs form a complex network for BP regulation. Natarajan et al. (51) demonstrated that the G protein-coupled receptor 41 (Gpr41) is expressed in the vascular endothelium of blood vessels, as denuded vessels (without endothelium) do not express it. The Gpr41-knockout mouse presents with isolated systolic HTN compared with wild-type. Furthermore, the BP of Gpr41-knockout mice did not change when mice were fed a diet high in salt, indicating that these mice were salt resistant. However, since the Gpr41 receptor is also present in other tissues such as the sympathetic ganglia (35), it is necessary to further explore this topic considering the sustained sympathetic nervous system activation seen in HTN. Gpr42, another GPCR, appears to be a gene duplicate of Gpr41 and a pseudogene (11). A third GPCR, Gpr43, belongs to the same homologous family as Gpr41. SCFA propionate is the strongest ligand for both receptors, although they respond to acetate and butyrate as well. Finally, murine Olfr78 and its human ortholog OR51E2 are olfactory receptors found in the renal afferent arteriole and vascular smooth muscle cells (53) that respond to acetate and propionate (52). Unlike Gpr41, activation of Olfr78 triggers hormonal effects (51), as it increases renin secretion from the juxtaglomerular apparatus in the nephron, leading to a rise in BP. Similarly, Olfr78-knockout mice present with hypotension upon infusion with propionate in vivo (53). Most research provides evidence that SCFAs from gut microbiota appear to alter BP through the receptors Gpr41 and Olfr78. Recently, Waghulde et al. (68) demonstrated another GPCR involved in BP regulation: G protein-coupled estrogen receptor 1 (Gper1). Salt-sensitive Gper1−/− rats present with a lower BP and less gut dysbiosis compared with wild-type. These effects were abolished when fecal microbiota from wild type was transplanted to Gper1−/− rats, as BP exceeded that of wild-type animals, indicating that the BP changes were likely due to an altered microbiota. Furthermore, there were implications for sex differences, as female rats’ BP rose after transplantation compared with wild-type, whereas in male rats there was no change. Gper1 is a receptor for estrogen and aldosterone (24); however, SCFAs might be another potential mechanism of Gper1 action, as mesenteric arteries of Gper1−/− have impaired relaxation when exposed to SCFA acetate, and in particular butyrate, but not propionate (68). This role of Gper1 on SCFAs requires further investigation.
Similar to rodents, observational data demonstrate that hypertensive humans have reduced butyrate production, as evidenced by a lower abundance of butyrate-producing bacteria (Butyricimonas, Butyricicoccus, Eubacterium, Anaerostipes, Coprococcus, Roseburia, Faecalibacterium, Subdoligranulum, and Fusobacterium) and butyrate-generating enzymes (butyrate kinase and acetate-CoA transferase) compared with a nonhypertensive cohort (34). However, interventions using SCFAs in humans have been equivocal with studies reporting reductions in diastolic BP (57) but no change in systolic BP following butyrate supplementation (10, 57). These findings may be confounded by differing baseline BP in these studies (10, 57). Butyrate supplementation also did not change serum nor fecal butyrate levels, which was likely due to low (2%) systemic availability of colonic-derived butyrate, its high colonic uptake, and interconversion of butyrate to acetate and propionate (9). Nonetheless, in these few studies investigating the effect of SCFAs on BP in humans, it appears that SCFA supplements may have limited benefit due to their low uptake into the bloodstream.
Probiotics and Blood Pressure Regulation
Considering microbial actions in the gut, a number of studies have yielded positive outcomes of probiotic interventions in HTN (33). Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (26). In a meta-analysis conducted by Khalesi et al. (33), the authors concluded that probiotics reduce BP to only a modest degree. On average, single-strain probiotics reduce systolic BP and diastolic BP 4 and 2 mmHg, respectively, whereas multi-strain probiotics reduce it on average by 6 and 3 mmHg, respectively. The greatest effectiveness seems to be achieved if a dose of 1011 colony forming units/day or larger is administered for ≥8 wk. Most common species of bacteria used include Lactobacillus spp., Bifidobacterium spp., Enterococcus spp., and Streptococcus spp. It is important to note that probiotic consumption is not effective in lowering BP in normotensive or individuals with pre-HTN, as significant outcomes are seen only when BP is >130/85 mmHg (33).
MODULATION OF GUT MICROBIOTA BY DIETARY SODIUM
Both short- and long-term dietary patterns influence the gut microbiota (14, 15). Of late, because of the potential connection between the gut microbiota and BP, dietary sodium intake has emerged as an area of interest. However, interactions between dietary sodium intake and the gut microbiota are just beginning to be explored.
Of all the organ systems in the body, the GI tract is the first to encounter sodium. The sodium-proton exchanger 3 (NHE3), found in the renal proximal tubule and along the GI tract, regulates sodium and water absorption (25). Linz et al. (42) demonstrated that administration of a NHE3 inhibitor increased fecal sodium and water content, decreased urinary sodium excretion, and lowered BP in spontaneously hypertensive rats. The inhibitor used in the study has low oral bioavailability (<1%) (42), indicating that the effects take place predominantly in the GI tract. This suggests that inhibition of NHE3 in the gut could be a therapeutic target for salt-sensitive HTN. Investigations into the microbiota of the NHE3-knockout mouse have shown increased bacterial abundance as well as shifts in bacterial phyla and/or genera in both luminal and mucosal sites from the ileum to the distal colon compared with wild-type (19). In particular, Firmicutes were present in lower abundance and Bacteriodetes in greater abundance in the terminal ileum, cecum, and distal colon. A prominent increase in abundance was noted with a mutualistic Bacteriodetes member, Bacteroides thetaiotaomicron, in the terminal ileum. Although these results indicate positive bacterial composition outcomes with NHE3 inhibition, other research reports that NHE3 deficiency induces irritable bowel disease-like symptoms, gut dysbiosis, and an inflammatory immune system response (37). Hence, it remains unclear whether NHE3 inhibition is a viable therapeutic intervention for those with HTN, how it changes bacterial composition, and what its effect is on other health outcomes.
Gut Microbial and Immune System Interactions
The gut microbiota is in constant interaction with the immune system, and rodent models have shown that high-salt diets promote tissue inflammation and autoimmune disease (36). In particular, a high salt intake in mice increased plasma levels of the proinflammatory cytokines IL-6 and IL-23 (73). This has also been shown in males, as consumption of 12 g/day of salt (4,600 mg of sodium) for 30–60 days increased production of IL-6 and IL-23 compared with 6 (2,300 mg of sodium) and 9 g/day (3,500 mg of sodium) (73). More recently, a high sodium intake, and specifically the sodium ion, has been shown to activate TH-17 cells (36, 71), major modulators of the immune response that secrete the proinflammatory IL-17 and IL-22, suggesting that they contribute to HTN development. IL-17-knockout mice infused with ANG II have lower BP compared with wild-type mice in response to the same infusion (44). TH17 cells are abundant in gut-associated tissues in the presence of gut microbiota (30), and their maturation is dependent on gut microbiota-induced cytokines, particularly IL-1β (60). Their development is induced by certain mutualistic bacteria, such as those from the genus Arthromitus, which are known to enhance resistance to pathogenic bacteria (29). Furthermore, germ-free mice present with greater numbers of anti-inflammatory T-regulatory cells, which are inversely correlated to TH17 cells in these animals. Altogether, it appears that sodium-induced immune activation of TH17 cells and specific interleukins is likely mediated by the intestinal microbiota.
Gut Microbial Composition and High Dietary Sodium
High dietary salt intake modulates microbial composition (Table 2) (8, 48, 69, 71) and function (48) in rodent models and humans. A high-salt diet alters the gut microbiota profile by rapidly depleting Lactobacillus spp. in both mice (48, 69, 71) and humans (71). In particular, depletion of Lactobacillus murinus, a species found in the mouse gut but not humans, was reversed when mice were returned to a normal salt diet after 14 days of high salt (71). Furthermore, feeding salt-sensitive mice a high-salt diet and supplementing it with Lactobacillus murinus or Lactobacillus reuteri reduced BP and TH17 cell activation, suggesting that commensal bacteria can influence BP regulation (71). In a small cohort of healthy humans, TH17 cells were increased, and 90% of the Lactobacillus spp. was depleted following 14 days of high sodium (5,500 mg/day), indicating a low resilience of the Lactobacillus spp. to sodium. Lactobacillus species confer benefits to the host as they impede pathogen growth and activation, exhibit anti-inflammatory properties, and optimize gut microbial structure and composition (58). Interestingly, Lactobacillus is not a dominant genus in the gut, as it is found in only 40% of humans (71). Furthermore, Lactobacillus is lower in abundance in Westernized societies that consume diets high in sodium compared with nonindustrialized, agriculture-based societies (46), suggesting that restoring gut Lactobacillus spp. abundance may have health implications, but more research is warranted.
Table 2.
Bacterial taxa altered by dietary sodium with their full classification
| Phylum/Class | Order | Family | Genus | Species | Reference |
|---|---|---|---|---|---|
| Rodent taxa reduced in abundance following a high sodium diet (3.15–5% Na; 14 days to 8 wk) | |||||
| Actinobacteria | |||||
| Actinobacteria | Actinomycetales | Micrococcaceae | Rothia | Wilck et al. (71) | |
| Bacteroidetes | |||||
| Bacteroidia | Bacteroidales | Rikenellaceae | Alistipes | Wang et al. (69) | |
| S24–7 | Wang et al. (69) | ||||
| Firmicutes | |||||
| Bacili | Lactobacillales | Lactobacillaceae | Lactobacillus | Wilck et al. (71) Wang et al. (69) Miranda et al. (48) |
|
| Lactobacillus | murinus | Wilck et al. (71) | |||
| Clostridia | Clostridiales | Clostridiaceae | Unclassified | Miranda et al. (48) | |
| Lachnospiraceae | Anaerostipes | Bier et al. (8) | |||
| Clostridium XIVa | Wilck et al. (71) | ||||
| Johnsonella | Wilck et al. (71) | ||||
| Oscillospiraceae | Oscillibacter | Wilck et al. (71) | |||
| Ruminococcaceae | Wang et al. (69) | ||||
| Family unassigned | Pseudoflavonifractor | Wilck et al. (71) | |||
| Human taxa reduced in abundance following a high sodium diet (5,500 mg Na/day; 14 days) | |||||
| Firmicutes | |||||
| Bacili | Lactobacillales | Lactobacillaceae | Lactobacillus | Wilck et al. (71) | |
| Rodent taxa increased in abundance following a high-sodium diet | |||||
| Actinobacteria | |||||
| Actinobacteria | Actinomycetales | Corynebacteriaceae | Bier et al. (8) | ||
| Bacteroidetes | |||||
| Bacteroidia | Bacteroidales | Prevotellaceae | Wilck et al. (71) | ||
| Rikenellaceae | Alistipes | Wilck et al. (71) | |||
| Firmicutes | |||||
| Clostridia | Clostridiales | Christensenellaceae | Bier et al. (8) | ||
| Lachnospiraceae | Roseburia | Wang et al. (69), Miranda et al. (48) | |||
| Oscillospiraceae | Oscillospira | Miranda et al. (48) | |||
| Oscillibacter | Wang et al. (69) | ||||
| Ruminococcaceae | Ruminococcus | Wang et al. (69) | |||
| Proteobacteria | |||||
| Betaproteobacteria | Burkholderiales | Sutterellaceae | Parasutterella spp | Wilck et al. (71) | |
| Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | Erwinia | Bier et al. (8) | |
| Verrucomicrobia | |||||
| Verrucomicrobiae | Verrucomicrobiales | Verrucomicrobiaceae | Akkermansia | Wilck et al. (71) | |
Bacterial taxa found to be altered in the original publication are in boldface.
In addition to Lactobacillus, genera in the Clostridiales order have shown to be greatly affected by high salt, as reductions of several genera have been noted in rodent models (Table 2) (8, 48, 69, 71). Conversely, the family Christensenellaceae in the order of Clostridiales was increased along with the genus Erwinia and family Corynebacteriaceae following 8 wk of high salt (8). Family Lachnospiraceae (48, 69) and genera Ruminococcae (69) and Oscillospira increased upon a 4- and 8-wk-long high-salt diet that also exacerbated colitis in mice (48). These data suggest that several taxa in class Clostridia are elevated on a high-salt diet and exacerbate colitis in these mice. Mice on a normal salt diet demonstrate higher abundance of Lactobacillus and lower abundance of Lachnospiraceae and Ruminococcae (69). Wang et al. (69) observed greater intragroup variation in bacterial species when mice were fed normal salt compared with high salt, suggesting that a high-salt diet is associated with lower microbial diversity. It is important to note that bacterial species have different salt tolerances. Bacterial resistance to salt is an important survival strategy in stressful environments such as increased extracellular osmolarity due to greater concentration of sodium ions. Several genes in the human gut microbiome have been detected as salt tolerance genes [salt metagenome (SMG), including SMG 3, SMG 5, SMG 9, and SMG 25] whose metabolic activity increases in high salt environments (12). Identification of these genes has implications in the development of novel therapeutics (for example, functional foods, dietary supplements, and drugs) that target specific members of the gut microbiota in conditions induced or mediated by high salt intake (12).
Short-Chain Fatty Acids and High Dietary Sodium
Concentrations of SCFAs are altered under high-salt conditions. In response, colonocytes may increase butyrate uptake as a protective mechanism. Mice fed high salt demonstrate a higher abundance of butyrate-producing Roseburia (69) and lower intestinal levels of SCFA butyrate (48). A reduction in Lactobacillus spp. may in part be responsible for accompanying lower butyrate levels (48) due to bacterial cross-feeding between lactate-producing bacteria and lactate-utilizing, butyrate-producing bacteria (43). In contrast to butyrate levels, fecal contents of acetate and propionate (8) as well as plasma acetate (47) are reported to be higher under high-salt conditions in rodents. Because acetate and propionate are strong ligands for the SCFA receptor Olfr78 that stimulates renin secretion and increases BP (51, 52), this suggests different physiological roles of SCFAs, as acetate and propionate may act to increase BP and butyrate may offer protection. These findings are in conflict with Ganesh et al. (23), who demonstrated a BP reduction in OSA hypertensive rodents upon acetate infusion, indicating that more mechanistic insight is warranted.
Altogether, evidence of sodium-induced immune system activation suggests a disturbance in gut homeostasis, which is confirmed by changes in gut microbial composition and function. Specific mechanisms, including immune cell signaling and the NHE3 and SCFA pathways, have yet to be elucidated. Identification of specific salt tolerance genes may offer novel therapeutics to target the gut microbiota in conditions caused or exacerbated by high salt consumption.
Secondary Bile Acids and Dietary Sodium
Bile acids are an integral part of energy metabolism. Metabolizing primary bile acids to secondary involves several reactions mediated by specific bacteria (20). Primary bile acids chenodeoxycholic acid (CDCA) and cholic acid (CA) are synthesized in the liver from cholesterol and conjugated with glycine or taurine. Most are reabsorbed in the distal ileum to be recycled; however, bile acids that are not reabsorbed are metabolized in the colon by the gut bacteria to secondary bile acids deoxycholic acid (DCA; formed from CA) and lithocholic acid (LCA; formed from CDCA). Secondary bile acids are ligands for G protein-coupled bile acid receptor 1 (GPBAR1; also known as M-BAR, TGR5, or BG37) that is expressed along the gastrointestinal tract as well as in macrophages and T cells. GPBAR1 activation increases expression of cystathionine γ-lyase (CSE), an enzyme necessary for generation of the vasodilator hydrogen sulfide (21).
Bile acid profiles differ between wild-type and germ-free rats, with tissues and plasma of germ-free animals being dominated by taurine-conjugated bile acids with negligible amounts of glycine-conjugated and unconjugated acids. Administration of antibiotics to wild-type rats results in a bile acid profile similar to that of germ-free rats, indicating that bile acid metabolism is another avenue of microbiome-host interactions. Levels of taurine are increased in these animals compared with wild-type, likely due to the reduced number of bacteria that usually metabolize taurine to sulfate compounds (63). Interestingly, germ-free mice also present with low CSE activity and decreased levels of hydrogen sulfide in plasma and GI tissues (61), indicating integration of bile acid metabolism and hydrogen sulfide production that may be mediated by the gut microbiota. Intracolonic administration of a hydrogen sulfide donor induces peripheral vasodilation, resulting in lowered BP (64). These systemic effects of gut-derived hydrogen sulfate likely result in mechanisms dependent on the liver or the enteric nervous system.
To date, there is no research linking bile acids, dietary sodium, and the gut microbiota. Potential mechanism of dietary sodium affecting bile acid metabolism could be through manipulation of gut microbiota composition. As mentioned earlier, dietary sodium depletes Lactobacillus spp. and bacteria from the order Clostridiales, both integral to the secondary bile acid metabolism (20). Taken together, there is potential for gut microbiota-mediated effects of bile acids on BP regulation, but the mechanisms need to be elucidated.
CIRCADIAN RHYTHM OF SODIUM EXCRETION, BLOOD PRESSURE REGULATION, AND THE GUT MICROBIOTA
The circadian clock regulates rhythmic oscillations of physiological processes during the course of the day. It consists of the central clock found in the suprachiasmatic nucleus of the brain and peripheral clocks found in other organs such as the liver and kidneys (3). Disruptions in the main circadian clock proteins [CLOCK, BMAL1, Period (Per), and Cryptochrome (Cry)] are likely contributing to the pathophysiology of HTN (56). CLOCK and Per isoform 1 (Per1) regulate sodium reabsorption in the kidneys, as mice with lower expression of those proteins excrete more sodium and have a lower BP. Hypertensive humans have increased mRNA expression of Per1 compared with their normotensive counterparts (56). Diurnal patterns of natriuresis are greater during the active period and independent of food and water intake, emphasizing circadian clock command (49). Johnston et al. (31) demonstrated impaired natriuresis in salt-sensitive rats following an acute salt load that was dependent on time of day. The sodium excretion response was also delayed in salt-sensitive rats compared with controls (31). Importantly, 24-h sodium excretion rates were comparable between time of day when salt load was administered and independent of BP changes. Sutton et al. (62) observed greater reduction in systolic and diastolic BP in men with prediabetes when food was consumed during a 6-h window in the first half of the day (by 3 PM) compared with a 12-h feeding period. These changes occurred independent of weight loss, and these findings may be due to increased natriuresis earlier in the day consistent with the circadian rhythm.
A molecular clock has recently been established in the gut microbiota. Germ-free mice have an impaired central and peripheral (hepatic) circadian rhythm compared with conventionally raised mice, indicating an integral role of the gut microbiota in the circadian rhythm of the host (38). Their greatest fecal bacterial load is in the beginning of the active period and the lowest load at the onset of the inactive period, driven by variation in Bacteroidetes (40). Bacterial load can be attenuated with deletion of Bmal1. Furthermore, although deletion of Bmal1 does not suppress overall diurnal oscillations, diurnal oscillations of the relative and absolute abundances of Bacteroides and Lacotobacillaceae spp. are abolished (40). Altogether, 17% of operational taxonomic units and 20% of functional pathways in the murine gut exhibit diurnal oscillations (41). Accordingly, concentrations of SCFAs propionate and butyrate and butyrate-generating enzyme butyryl-CoA/acetate CoA-transferase exhibit diurnal patterns (38). Diet can also influence the circadian clock such as mice fed a high-fiber diet or whose diet was supplemented with acetate presented with upregulated control of the clock (45). Knowing that SCFAs and dietary components take part in BP regulation, it is suitable to suggest that these diurnal signals are integral to BP regulation mechanisms. Further research should elucidate molecular pathways of the gut microbiota-mediated BP control.
CONCLUSIONS AND FUTURE DIRECTIONS
Gut microbial activity interacts with numerous organ systems, thus influencing various autoimmune and metabolic diseases. Research in rodents and humans demonstrates that altered gut microbial composition is evident in HTN and can also be evoked with diets high in salt. Although certain mechanistic pathways are being revealed, much remains to be elucidated about the role of the gut microbiota on BP regulation and the interplay with high-sodium diets. Metabolites produced by bacteria in the gut, specifically SCFAs, bind to GPCRs in the vasculature and initiate BP and vascular responses. Future experimental research should focus on this vastly unexplored area on the role of excess sodium consumption on the gut microbiota and subsequent actions on the cardiovascular system and other organs implicated in the development of HTN.
GRANTS
This work was supported by an Institutional Development Award Center of Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant no. P20-GM-113125.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.S. and S.L.L. conceived and designed research; K.S. prepared figures; K.S. and S.L.L. drafted manuscript; K.S. and S.L.L. edited and revised manuscript; K.S. and S.L.L. approved final version of manuscript.
REFERENCES
- 1.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 49: 96–104, 2017. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, Billard E, Barnich N. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep 6: 19032, 2016. doi: 10.1038/srep19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht U, Eichele G. The mammalian circadian clock. Curr Opin Genet Dev 13: 271–277, 2003. doi: 10.1016/S0959-437X(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 4.Almeida A, Mitchell AL, Boland M, Forster SC, Gloor GB, Tarkowska A, Lawley TD, Finn RD. A new genomic blueprint of the human gut microbiota. Nature 568: 499–504, 2019. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey RL, Parker EA, Rhodes DG, Goldman JD, Clemens JC, Moshfegh AJ, Thuppal SV, Weaver CM. Estimating sodium and potassium intakes and their ratio in the American diet: data from the 2011-2012 NHANES. J Nutr 146: 745–750, 2016. doi: 10.3945/jn.115.221184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, Haase N, Kräker K, Hering L, Maase M, Kusche-Vihrog K, Grandoch M, Fielitz J, Kempa S, Gollasch M, Zhumadilov Z, Kozhakhmetov S, Kushugulova A, Eckardt KU, Dechend R, Rump LC, Forslund SK, Müller DN, Stegbauer J, Wilck N. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation 139: 1407–1421, 2019. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 139: e56–e528, 2019. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 8.Bier A, Braun T, Khasbab R, Di Segni A, Grossman E, Haberman Y, Leibowitz A. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients 10: E1154, 2018. doi: 10.3390/nu10091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM, Annaert P, Delcour JA, Verbeke KA. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol 595: 541–555, 2017. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouter K, Bakker GJ, Levin E, Hartstra AV, Kootte RS, Udayappan SD, Katiraei S, Bahler L, Gilijamse PW, Tremaroli V, Stahlman M, Holleman F, van Riel NAW, Verberne HJ, Romijn JA, Dallinga-Thie GM, Serlie MJ, Ackermans MT, Kemper EM, Willems van Dijk K, Backhed F, Groen AK, Nieuwdorp M. Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects. Clin Transl Gastroenterol 9: e155, 2018. doi: 10.1038/s41424-018-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 12.Culligan EP, Sleator RD, Marchesi JR, Hill C. Metagenomics and novel gene discovery: promise and potential for novel therapeutics. Virulence 5: 399–412, 2014. doi: 10.4161/viru.27208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28: 1221–1227, 1987. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107: 14691–14696, 2010. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 14: 20–32, 2016. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RM Jr. Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Hypertension 67: 469–474, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63: 2960–2984, 2014. [Erratum in: J Am Coll Cardiol 63: 3027–3028, 2014.] doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Engevik MA, Aihara E, Montrose MH, Shull GE, Hassett DJ, Worrell RT. Loss of NHE3 alters gut microbiota composition and influences Bacteroides thetaiotaomicron growth. Am J Physiol Gastrointest Liver Physiol 305: G697–G711, 2013. doi: 10.1152/ajpgi.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorucci S, Distrutti E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol Med 21: 702–714, 2015. doi: 10.1016/j.molmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Fiorucci S, Zampella A, Cirino G, Bucci M, Distrutti E. Decoding the vasoregulatory activities of bile acid-activated receptors in systemic and portal circulation: role of gaseous mediators. Am J Physiol Heart Circ Physiol 312: H21–H32, 2017. doi: 10.1152/ajpheart.00577.2016. [DOI] [PubMed] [Google Scholar]
- 22.Galla S, Chakraborty S, Cheng X, Yeo J, Mell B, Zhang H, Mathew AV, Vijay-Kumar M, Joe B. Disparate effects of antibiotics on hypertension. Physiol Genomics 50: 837–845, 2018. doi: 10.1152/physiolgenomics.00073.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM Jr, Durgan DJ. Prebiotics, Probiotics, and Acetate Supplementation Prevent Hypertension in a Model of Obstructive Sleep Apnea. Hypertension 72: 1141–1150, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gros R, Ding Q, Liu B, Chorazyczewski J, Feldman RD. Aldosterone mediates its rapid effects in vascular endothelial cells through GPER activation. Am J Physiol Cell Physiol 304: C532–C540, 2013. doi: 10.1152/ajpcell.00203.2012. [DOI] [PubMed] [Google Scholar]
- 25.He P, Yun CC. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol 2010: 1–10, 2010. doi: 10.1155/2010/238080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11: 506–514, 2014. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 27.Hsu CN, Lin YJ, Hou CY, Tain YL. Maternal administration of probiotic or prebiotic prevents male adult rat offspring against developmental programming of hypertension induced by high fructose consumption in pregnancy and lactation. Nutrients 10: E1229, 2018. doi: 10.3390/nu10091229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature 486: 207–214, 2012. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485–498, 2009. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov II, Frutos RL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4: 337–349, 2008. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston JG, Speed JS, Jin C, Pollock DM. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am J Physiol Renal Physiol 311: F991–F998, 2016. doi: 10.1152/ajprenal.00103.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, Schüler R, Finger S, Knorr M, Lagrange J, Brandt M, Waisman A, Kossmann S, Schäfer K, Münzel T, Reinhardt C, Wenzel P. Gut microbiota promote angiotensin ii-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc 5: e003698, 2016. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 64: 897–903, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond) 132: 701–718, 2018. doi: 10.1042/CS20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108: 8030–8035, 2011. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522, 2013. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laubitz D, Harrison CA, Midura-Kiela MT, Ramalingam R, Larmonier CB, Chase JH, Caporaso JG, Besselsen DG, Ghishan FK, Kiela PR. Reduced Epithelial Na+/H+ Exchange Drives Gut Microbial Dysbiosis and Promotes Inflammatory Response in T Cell-Mediated Murine Colitis. PLoS One 11: e0152044, 2016. doi: 10.1371/journal.pone.0152044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17: 681–689, 2015. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5: 14, 2017. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci USA 112: 10479–10484, 2015. doi: 10.1073/pnas.1501305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang X, FitzGerald GA. Timing the Microbes: The Circadian Rhythm of the Gut Microbiome. J Biol Rhythms 32: 505–515, 2017. doi: 10.1177/0748730417729066. [DOI] [PubMed] [Google Scholar]
- 42.Linz D, Wirth K, Linz W, Heuer HO, Frick W, Hofmeister A, Heinelt U, Arndt P, Schwahn U, Böhm M, Ruetten H. Antihypertensive and laxative effects by pharmacological inhibition of sodium-proton-exchanger subtype 3-mediated sodium absorption in the gut. Hypertension 60: 1560–1567, 2012. doi: 10.1161/HYPERTENSIONAHA.112.201590. [DOI] [PubMed] [Google Scholar]
- 43.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294: 1–8, 2009. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 44.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 135: 964–977, 2017. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 46.Martínez I, Stegen JC, Maldonado-Gómez MX, Eren AM, Siba PM, Greenhill AR, Walter J. The gut microbiota of rural papua new guineans: composition, diversity patterns, and ecological processes. Cell Reports 11: 527–538, 2015. doi: 10.1016/j.celrep.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 47.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 47: 187–197, 2015. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda PM, De Palma G, Serkis V, Lu J, Louis-Auguste MP, McCarville JL, Verdu EF, Collins SM, Bercik P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 6: 57, 2018. doi: 10.1186/s40168-018-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore-Ede MC, Herd JA. Renal electrolyte circadian rhythms: independence from feeding and activity patterns. Am J Physiol 232: F128–F135, 1977. doi: 10.1152/ajprenal.1977.232.2.F128. [DOI] [PubMed] [Google Scholar]
- 50.Munger RG, Prineas RJ, Gomez-Marin O. Persistent elevation of blood pressure among children with a family history of hypertension: the Minneapolis Children’s Blood Pressure Study. J Hypertens 6: 647–653, 1988. doi: 10.1097/00004872-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics 48: 826–834, 2016. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pluznick JL. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr Hypertens Rep 19: 25, 2017. doi: 10.1007/s11906-017-0722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65, 2010. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 38: 996–1047, 2014. doi: 10.1111/1574-6976.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richards J, Diaz AN, Gumz ML. Clock genes in hypertension: novel insights from rodent models. Blood Press Monit 19: 249–254, 2014. doi: 10.1097/MBP.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roshanravan N, Mahdavi R, Alizadeh E, Ghavami A, Rahbar Saadat Y, Mesri Alamdari N, Alipour S, Dastouri MR, Ostadrahimi A. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; A randomized, double-blind, placebo-controlled trial. J Cardiovasc Thorac Res 9: 183–190, 2017. doi: 10.15171/jcvtr.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salvetti E, O’Toole PW. The genomic basis of lactobacilli as health-promoting organisms. Microbiol Spectr 5: 5, 2017. doi: 10.1128/microbiolspec.BAD-0011-2016. [DOI] [PubMed] [Google Scholar]
- 59.Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 164: 337–340, 2016. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 60.Shaw MH, Kamada N, Kim YG, Núñez G. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med 209: 251–258, 2012. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen X, Carlström M, Borniquel S, Jädert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 60: 195–200, 2013. doi: 10.1016/j.freeradbiomed.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 27: 1212–1221.e3, 2018. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci USA 108, Suppl 1: 4523–4530, 2011. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomasova L, Dobrowolski L, Jurkowska H, Wróbel M, Huc T, Ondrias K, Ostaszewski R, Ufnal M. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide 60: 50–58, 2016. doi: 10.1016/j.niox.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Toral M, Robles-Vera I, de la Visitación N, Romero M, Sánchez M, Gómez-Guzmán M, Rodriguez-Nogales A, Yang T, Jiménez R, Algieri F, Gálvez J, Raizada MK, Duarte J. Role of the immune system in vascular function and blood pressure control induced by faecal microbiota transplantation in rats. Acta Physiol (Oxf) 227: e13285, 2019. doi: 10.1111/apha.13285. [DOI] [PubMed] [Google Scholar]
- 66.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature 457: 480–484, 2009. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.United States Department of Health and Human Services, United States Department of Agriculture 2015–2020 Dietary Guidelines for Americans (Online) https://health.gov/dietaryguidelines/2015/guidelines/ [7 Oct 2019].
- 68.Waghulde H, Cheng X, Galla S, Mell B, Cai J, Pruett-Miller SM, Vazquez G, Patterson A, Vijay Kumar M, Joe B. Attenuation of microbiotal dysbiosis and hypertension in a CRISPR/Cas9 gene ablation rat model of GPER1. Hypertension 72: 1125–1132, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C, Huang Z, Yu K, Ding R, Ye K, Dai C, Xu X, Zhou G, Li C. High-salt diet has a certain impact on protein digestion and gut microbiota: a sequencing and proteome combined study. Front Microbiol 8: 1838, 2017. doi: 10.3389/fmicb.2017.01838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 71: e127–e248, 2018. [Erratum in: J Am Coll Cardiol 71: 2275–2279, 2018.] doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Frätzer C, Krannich A, Gollasch M, Grohme DA, Côrte-Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ, Müller DN. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551: 585–589, 2017. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension 65: 1331–1340, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yi B, Titze J, Rykova M, Feuerecker M, Vassilieva G, Nichiporuk I, Schelling G, Morukov B, Choukèr A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res 166: 103–110, 2015. doi: 10.1016/j.trsl.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]