Abstract
Type I PKA regulatory α-subunit (RIα; encoded by the Prkar1a gene) serves as the predominant inhibitor protein of the catalytic subunit of cAMP-dependent protein kinase (PKAc). However, recent evidence suggests that PKA signaling can be initiated by cAMP-independent events, especially within the context of cellular oxidative stress such as ischemia-reperfusion (I/R) injury. We determined whether RIα is actively involved in the regulation of PKA activity via reactive oxygen species (ROS)-dependent mechanisms during I/R stress in the heart. Induction of ex vivo global I/R injury in mouse hearts selectively downregulated RIα protein expression, whereas RII subunit expression appears to remain unaltered. Cardiac myocyte cell culture models were used to determine that oxidant stimulus (i.e., H2O2) alone is sufficient to induce RIα protein downregulation. Transient increase of RIα expression (via adenoviral overexpression) negatively affects cell survival and function upon oxidative stress as measured by increased induction of apoptosis and decreased mitochondrial respiration. Furthermore, analysis of mitochondrial subcellular fractions in heart tissue showed that PKA-associated proteins are enriched in subsarcolemmal mitochondria (SSM) fractions and that loss of RIα is most pronounced at SSM upon I/R injury. These data were supported via electron microscopy in A-kinase anchoring protein 1 (AKAP1)-knockout mice, where loss of AKAP1 expression leads to aberrant mitochondrial morphology manifested in SSM but not interfibrillar mitochondria. Thus, we conclude that modification of RIα via ROS-dependent mechanisms induced by I/R injury has the potential to sensitize PKA signaling in the cell without the direct use of the canonical cAMP-dependent activation pathway.
NEW & NOTEWORTHY We uncovered a previously undescribed phenomenon involving oxidation-induced activation of PKA signaling in the progression of cardiac ischemia-reperfusion injury. Type I PKA regulatory subunit RIα, but not type II PKA regulatory subunits, is dynamically regulated by oxidative stress to trigger the activation of the catalytic subunit of PKA in cardiac myocytes. This effect may play a critical role in the regulation of subsarcolemmal mitochondria function upon the induction of ischemic injury in the heart.
Keywords: A-kinase anchoring protein 1, ischemia-reperfusion injury, mitochondria, oxidative stress, PKA regulatory subunit RIα
INTRODUCTION
Ischemic heart disease is the leading cause of death worldwide, resulting from damage to heart tissues following deprivation of normal blood flow (8). Ischemia-reperfusion (I/R) is an acute ischemic heart disease pathology associated with oxidative damage that occurs upon rapid restoration of blood flow after a myocardial infarction (21). Therefore, oxidation of cellular signaling proteins such as eukaryotic protein kinases and protein phosphatases (PPs) has become a topic of importance because of the oxidative nature of this disease pathology. Given previous examples of eukaryotic protein kinase pathways that can be noncanonically activated via oxidative stress in the heart such as Ca2+/calmodulin-dependent kinase II (22, 34), this study has aimed to assess the role of cAMP-dependent protein kinase (PKA) signaling within the context of cardiac I/R injury.
The catalytic subunit of PKA (PKAc) itself is a target of oxidation. In a series of publications by Humphries et al. (13–15), it was shown that Cys199, a reactive cysteine found near the active site of PKAc and adjacent to the activation-loop phosphorylation site Thr197, plays a critical role in regulating PKAc activity. Given findings from these studies, it was proposed that PKAc activity undergoes a “biphasic” response to oxidation: lower doses or shorter exposure time of oxidation treatment led to heightened PKAc activity, whereas higher dose or prolonged exposure time to oxidant lead to PKAc inhibition (15). This biphasic response is thought to be mediated by initial oxidation and thus inhibition of PPs at lower levels of oxidant stress (as PPs have been shown to be particularly oxidant sensitive) (38), followed by PKAc oxidation at Cys199 and therefore inactivation upon high levels of oxidant. While this previous work clearly outlines a role for oxidation in the regulation of PKA signaling, the potential involvement of other PKA-associated proteins during oxidative stress was not assessed.
The regulatory subunits of PKA (R-subunits, RI and RII each with α- and β-isoforms) (6, 18, 32) are well characterized regarding their cAMP-dependent mechanism for inhibiting PKAc under nonstimulated conditions (36, 37). The RIα isoform is a target of interest due to unique biochemical features that may lead to noncanonical (i.e., cAMP independent) mechanisms for PKA activation. The dimerization/docking domain of RIα has two highly conserved cysteine residues (Cys18/Cys39 in human RIα) that have been shown to generate interprotein disulfide bonds between antiparallel monomers of RIα, thus allowing for stabilization of the RIα homodimer via covalent linkages (2, 19). Previous studies have shown that these disulfide bonds are redox sensitive both in vitro and within cells and tissues, where treatment with oxidants promotes increased disulfide bond formation (2, 3). Recent work suggests that exposure to oxidants like hydrogen peroxide (H2O2) and nitric oxide in cardiac systems contributes to cAMP-independent mechanisms for activating PKAc (3, 4). Using mass spectrometry-based proteomic comparisons of cAMP-enriched heart proteins, Aye et al. (1) have shown that human patients suffering from dilated cardiomyopathy have significantly downregulated expression of regulatory subunit proteins RIα and RIIα, indicating that loss of regulatory subunit expression may play a role in the pathophysiology of chronic heart disease. Furthermore, Gao et al. (10) have shown a potential role for RIα during oxidative signaling in the heart, where ischemic injury led to a loss of RIα inhibition of p90 ribosomal S6 kinase 1 activity.
All these findings indicate that RIα may be a critical regulator of stress signaling in the heart, where loss of PKAc activity regulation could serve as a mechanism for enacting drastic cell signaling changes under oxidative conditions. Therefore, the aim of this work was to characterize the effect of cardiac I/R injury upon RIα protein oxidation and subsequent activation of PKAc signaling. Also, given the inherent role of mitochondria in oxidative stress regulation, this study partly focuses on the effects of PKA signaling upon the dynamics of differing mitochondria subpopulations in cardiac myocytes.
METHODS
Ethics statement.
Animals were treated in compliance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals, and protocols were approved by the Veterans Affairs San Diego Healthcare System Institutional Animal Care and Use Committee and by the animal ethics committee of the University of Iowa.
Materials.
Antibodies at given dilutions for PKAc, RIα, RIIα, and phospho-RRX(S/T) PKAc substrate were from BD Biosciences (San Jose, CA); antibodies for cytochrome-c oxidase (COXIV), green fluorescent protein (GFP), hypoxia-inducible factor 1α (HIF-1α), and optic atrophy protein 1 (Opa1) were from Abcam (Cambridge, MA); antibodies for Actin and GAPDH were from Santa Cruz Biotech (Dallas, TX); and antibodies for cleaved poly-ADP ribose polymerase (Cl PARP), cardiac troponin I (cTnI), and phospho-cTnI (p-cTnI) were from Cell Signaling (Danvers, MA). Antibody used to detect dimerized RIα was developed in house. Horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX). Other chemicals and reagents were obtained from Sigma (St. Louis, MO) unless otherwise stated.
Cell culture.
L6 cells (ATCC Cat. No. CRL-1458) were cultivated with Dulbecco's modified Eagle's medium modified to contain 4 mM l-glutamine, 4,500 mg/L glucose, 1 mM sodium pyruvate, and 1,500 mg/L sodium bicarbonate and supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin-streptomycin. Cells (0.8 × 106) were seeded in each well of six-well plate, and the same strategies of virus transfection and H2O2 treatment to those of experiments with adult mouse ventricular myocytes (AMVMs) were used. Cell cultures were maintained at 37°C with 5% CO2. Apoptosis assay, mitochondrial respiration, and Western blot analysis were performed three times in duplicate with cell passage of 2, 4, and 5.
Animals.
Wild-type male C57BL/6 mice (Jackson, Bar Harbor, ME) of age 8–12 wk were used for all ex vivo I/R injury experiments, adult mouse ventricular myocyte isolations, and mitochondrial subpopulation analysis. Animals were kept on a 12-h:12-h light/dark cycle in a temperature-controlled room with ad libitum access to food and water. Male A-kinase anchoring protein 1 knockout (AKAP1-KO) mice were group housed in a colony maintained with a standard 12-h:12-h light/dark cycle and given food and water ad libitum. Experiments were performed with age-matched animals for transmission electron microscopy (TEM) experiments.
Ex vivo I/R injury using Langendorff perfusion.
Intact heart tissue from pentobarbital sodium (80 mg/kg)-anesthetized mice was excised, and retrograde-perfusion with an oxygen-saturated perfusion buffer was initiated using a Langendorff perfusion apparatus as previously described (33). Global ischemia was induced via the prevention of perfusion flow to the heart for a period of 25 min. After 25 min ischemia, reperfusion was induced by reintroduction of perfusion flow to the heart for various times (between 0 and 45 min). After completion of I/R injury protocols, the heart was immediately removed from perfusion flow and subsequent procedures were initiated. Heart perfusion on separate Langendorff apparatuses was randomized for ischemic versus nonischemic groups, and blinding was used for subsequent biochemical manipulation of perfused hearts.
Preparation of adult mouse ventricular myocytes.
Young (8–12 wk aged) adult male C57BL/6 mice were treated with heparin (100 units) to prevent blood clotting and then anesthetized with pentobarbital sodium (100 mg/kg) before hearts were excised and retrograde perfused with Joklik medium (Sigma-Aldrich, Cat. No. 56449C) containing 1.2 mg/mL collagenase II enzyme to digest extracellular matrix material for isolation of ventricular AMVMs. Isolated cells were gradually acclimatized to 100 μΜ CaCl2 concentration and then exchanged into plating media, consisting of M199 media (Thermo Fisher, Waltham, MA) supplemented with 4% fetal bovine serum and 1% penicillin-streptomycin antibiotic solution, before being plated onto 35-mm dishes or six-well plates coated with 0.01 mg/mL mouse laminin protein (in 1× PBS). After incubation for 2 h to allow cell adherence, plating media was exchanged for maintenance media, consisting of M199 media (Thermo Fisher, Waltham, MA) supplemented with 1% culture-grade BSA and 1% penicillin-streptomycin antibiotic solution, to allow overnight culture.
AMVM cell treatments.
For AMVM cell treatments with H2O2, adherent cells were exposed to maintenance media supplemented with the desired concentration of H2O2 for the indicated time. After treatment, cell media was removed and the cells were then rinsed three times with ice-cold PBS before conducting cell lysis in 20 mM Tris (pH 7.4), 1 mM EGTA, and 0.1% Triton X-100. For adenoviral overexpression experiments, AMVMs were exposed to adenovirus (1 × 106 vp/mL) for 1 h followed by subsequent media change and overnight incubation. Infected AMVMs were then treated with oxidant stress to test the consequence of RIα overexpression upon AMVM cell-survival signaling. Given the exacerbated cell stress associated with adenoviral infection, lower doses of H2O2 (10 μM) were used as the oxidative stress condition.
Western blot analysis.
SDS-PAGE samples were prepared using 5× Laemelli buffer, containing 100 mM Tris·HCl (pH 7.0), 10% SDS, 50% glycerol, and 0.01% bromophenol blue, supplemented either with or without 100 mM dithiothreitol (DTT), for reducing versus nonreducing conditions, respectively. Protein concentrations were normalized between samples, and SDS-PAGE was then performed using NuPAGE 4–12% gradient gels in 2-(N-morpholino)ethanesulfonic acid (MES)-running buffer, consisting of 55 mM MES, 10 mM Tris-base, 1 mM EDTA, and 0.1% SDS. Gels were resolved at 150 V for 70 min and then subsequently transferred to 0.45 µM PVDF membranes (200 mA/blot for 60 min). Membranes were blocked using 5% milk protein (or with 5% BSA for phosphoblots) in 1× PBS -0.05% Tween (PBST) for 60 min, and primary antibodies were then incubated overnight at 4°C in PBST or 1× TBS-0.1% Tween 20 (TBST) at the following specified concentrations: PKAc (1:2,000), RIα (1:500), RIIα (1:1,000), phospho-RRX(S/T) PKAc substrate (1:1,000), COXIV (1:20,000), GFP (1:1,000), HIF-1α (1:1,000), Opa1 (1:1,000), Actin (1:200), GAPDH (1:200), cleaved PARP (1:1,000), cTnI (1:1,000) and p-cTnI (1:1,000), and in-house RIα (1:1,000). COX IV was used as a loading control throughout the study, Opa1 was used as a qualitative indicator of oxidative stress, and GAPDH was used as a cytosolic marker. HRP-conjugated secondary antibodies (1:5000 dilution) were used for chemiluminescence imaging of immunoblot samples. Immunoblot images were acquired using UVP imager system and analyzed using ImageJ software.
Coomassie staining.
PVDF membrane was put into Coomassie blue stain (0.125%) overnight. Membrane was destained with methanol-acetic acid (50:7) for 5 min twice. Membrane was air dried and image taken.
Detection of oxidized RIα protein.
SDS-PAGE of protein lysates was performed under nonreducing conditions (i.e., using samples prepared without DTT), followed by immunoblot analysis using a previously developed in-house antibody that recognizes both monomer and dimer forms of RIα. In this procedure, dimer bands of RIα are considered oxidized because of the requirement of covalent disulfide bonds to maintain dimerization upon denaturing yet nonreducing conditions.
Generation of adenoviral vectors.
Adenoviral RIα (Ad-RIα) and adenoviral GFP (Ad-GFP) were generated in house by amplifying human RIα cDNA by PCR (contained within the pEGFP-N1 vector) and then subsequently subcloning the genetic material (via the BamHI and XbaI sites) into the multicloning site reading frame of the Ad-shuttle adenoviral expression vector.
Apoptosis assay.
After washing with 1× PBS, L6 cells (1 × 106) were resuspended in 1× annexin binding buffer (Biovision) and incubated in the dark for 15 min at room temperature with 5 μL of annexin V-cyanine-5 (Cy5) (Biovision) and 1 μL (100 μg/mL) of propidium iodide (PI; Invitrogen), after which 400 μL of 1× annexin binding buffer was added. Annexin V-Cy5 and PI fluorescence were measured by flow cytometry.
Seahorse mitochondrial respiration.
The XF96e extracellular flux analyzer (Agilent-Seahorse XF Technology) was used to evaluate mitochondrial respiration in L6 cells. Briefly, cells were seeded at 20,000 cells/well on a 96-well tissue culture plate 20 h before the measurement. The oxygen consumption rate (OCR) was measured before and after the sequential injection of oligomycin (a complex V inhibitor), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP, a protonophore), and antimycin A and rotenone (complex III and I inhibitors) through ports of the Seahorse Flux Pak cartridges to achieve final concentrations of 1, 1, and 0.5 µM, respectively. From this, the basal OCR, oxygen consumption linked to ATP production, maximal respiration, and spare capacity were determined.
Isolation of subsarcolemmal mitochondria and interfibrillary mitochondria.
Cardiac subsarcolemmal mitochondria (SSM) and interfibrillary mitochondria (IFM) populations were isolated using a method modified from Palmer et al. (27). Chappell-Perry buffer was used (CP1), consisting of (in mM) 100 KCl, 50 MOPS, 1 EGTA, 5 MgSO4, and 1 ATP (pH 7.4) and CP2, same as CP1 but supplemented with 1 mg/mL BSA, for mitochondrial isolation. Upon heart tissue homogenization, samples were centrifuged at 600 g using a preparatory centrifuge (Beckman Coulter, Brea, CA) to clear nuclear/cytoskeletal pellet that contains the IFM fraction. The resulting supernatant was spun at 8,000 g for 15 min, then resuspended in 2 mL CP1, and centrifuged at the same condition to isolate the SSM fraction. For isolation of IFM, the first pellet was resuspended in 3 mL CP1 and then treated with 0.5 mL of 5 mg/mL trypsin enzyme for 15 min (on ice, with constant stirring) to digest the sarcomeric proteins and release the IFM fraction from the cytoskeletal components. Trypsin digest was quenched by adding 3 mL CP2, and the sample was centrifuged at 600 g to remove contaminants. The resulting supernatant was spun at 8,000 g for 15 min, then resuspended in 2 mL CP1, and centrifuged at the same condition to isolate the IFM fraction. Metabolically active mitochondria fractions were then suspended in 150 μL CP1 and frozen at −80°C, or directly resuspended in lysis buffer, consisting of 20 mM Tris (pH 7.4), 1 mM EGTA, and 0.1% Triton X-100, for biochemical studies. Purified SSM and membrane fractions were isolated via ultracentrifugation on a discontinuous Percoll gradient as previously described (9).
Transmission electron microscopy.
Mouse heart muscle was perfusion fixed and shipped overnight with cold packs in solution containing 2.5% glutaraldehyde + 2% paraformaldehyde in 0.1 M sodium cacodylate (pH 7.4). The muscle tissue was then diced and washed with ice-cold 0.1 M sodium cacodylate 3 × 10 min on ice followed by postfixation in 1% osmium tetroxide and 0.8% potassium ferrocyanide in 0.1 M sodium cacodylate for 3 h on ice. After three washes in ice-cold double-distilled H2O for 10 min each, the tissue was stained in 2% uranyl acetate for 2 h. Samples were dehydrated in an ethanol series of ice-cold 20, 50, 70, and 90% and then washed three times in 100% ethanol at 25°C for 10 min each. Samples were infiltrated in 50% ethanol-50% Durcupan ACM (Fluka/Sigma) for 3 h at 25°C with agitation, followed by three changes of 100% Durcupan for 8 h each at 25°C with agitation. The Durcupan resin was polymerized at 60°C for 2 days under vacuum. Sectioning was performed using a Leica ultramicrotome. Ultrathin (80 nm) sections were poststained with uranyl acetate (5 min) and lead salts (2 min) before imaging using a FEI spirit transmission electron microscope operated at 120 kV. The magnification was ×4,440 corresponding to a pixel resolution of 2.9 nm. The mitochondrial profile area was measured using the ImageJ area tool on TEM images taken randomly all at the same magnification. To avoid bias, all the mitochondria in an image were measured.
Statistics.
All data are presented as means ± SD or SE. GraphPad Prism 7 software (GraphPad Software, San Diego, CA) was used for all statistical analysis. Statistical analyses were performed by unpaired Student’s t-test (2-tailed testing with Welch’s correction), Holm-Sidak method, one-way ANOVA, and two-way ANOVA where appropriate. Normality of data was determined via the Shapiro-Wilk test. Statistical significance was defined as P < 0.05 or α = 0.05.
RESULTS
RIα expression is reduced upon global ex vivo cardiac I/R injury.
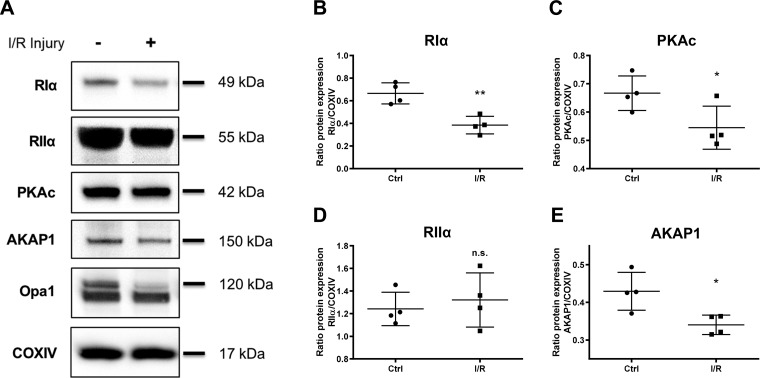
Using an established ex vivo perfused heart model of cardiac I/R injury, we characterized the global expression of PKA-associated proteins in heart ventricular tissue. Immunoblot analysis was conducted on samples treated either with control (Ctrl) perfusion or with 25 min of ischemia followed by 30 min of reperfusion injury (Fig. 1A). RIα protein was most significantly downregulated in its global expression upon exposure to I/R stress (Fig. 1B; mean ratio Riα/COXIV relative protein expression 0.3845 for I/R vs. 0.6657 for Ctrl; P = 0.0039). PKAc was mildly, yet significantly reduced in expression at 30 min of reperfusion injury (Fig. 1C; mean ratio PKAc/COXIV relative protein expression 0.5547 for I/R vs. 0.6667 for Ctrl; P = 0.0487). However, RIIα protein expression was not changed compared with control samples (Fig. 1D; mean ratio RIIα/COXIV relative protein expression 1.242 for I/R vs. 1.321 for Ctrl; P = 0.5978). AKAP1, a mitochondria-localized scaffold for PKA (26), also decreased in expression at the 30 min-reperfusion time point (Fig. 1E; mean ratio AKAP1/COXIV relative protein expression 0.3404 for I/R vs. 0.4293 for Ctrl; P = 0.0297). COXIV protein was used as a loading control throughout the study. In addition, immunoblot analysis of proteolytic cleavage for the inner mitochondrial membrane protein Opa1, as denoted by a loss in the 120-kDa full-length form of the protein, was used in this study as a qualitative indicator of mitochondrial stress upon I/R injury. These results illustrate that RIα, but not RIIα protein, appears to be specifically downregulated upon reperfusion injury in the heart.
Fig. 1.
Type I cAMP-dependent protein kinase (PKA) regulatory α-subunit (RIα) expression is reduced upon global ex vivo cardiac ischemia-reperfusion (I/R) injury. A: immunoblot analysis of tissue homogenate from adult mouse hearts exposed to ex vivo global I/R injury. Control perfusion heart samples were compared with hearts treated with 25 min ischemia followed by 30 min of reperfusion injury. Opa1, optic atrophy protein 1; COXIV, cytochrome-c oxidase. Corresponding scatter plots show protein expression quantification for RIα (B; P = 0.0039), catalytic subunit of PKA (PKAc) (C; P = 0.0487), RIIα (D; P = 0.5978), and A-kinase anchoring protein 1 (AKAP1) (E; P = 0.0297). Data are presented as means ± SD; n = 4 biological replicates. *P < 0.05; **P < 0.01; n.s., not significant.
Oxidative stress alone induces RIα downregulation in AMVMs.
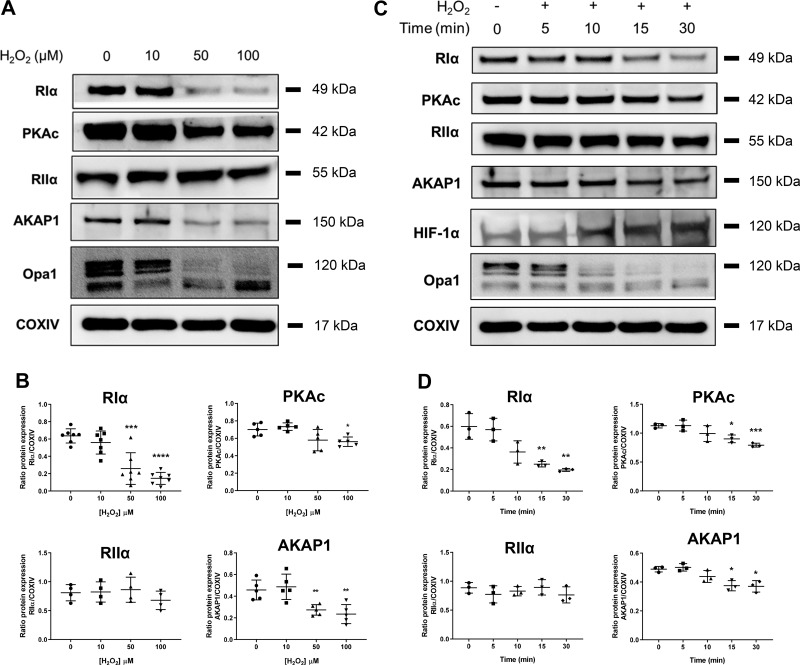
Given the oxidative nature of I/R injury, we moved to isolated primary AMVM culture models to assess the independent effect of oxidative stress upon RIα protein downregulation at the level of the myocyte. AMVMs were treated with the oxidant H2O2 as a simplified model of ROS-mediated injury. The effect of increasing dose of oxidant was tested within a 30-min time frame, where RIα expression was significantly decreased at higher concentrations (50–100 μM) of H2O2 (Fig. 2, A and B; mean ratio RIα/COXIV relative protein expression 0.1460 for 100 µM H2O2 vs. 0.6360 for 0 µM; P < 0.0001). In comparison to the ex vivo model of I/R injury, treatment with higher oxidant doses mimicked many of the findings in whole heart: we observed that 1) PKAc protein expression is partially decreased (mean ratio PKAc/COXIV relative protein expression 0.5611 for 100 µM H2O2 vs. 0.7003 for 0 µM; P = 0.0105), 2) RIIα subunit expression appeared to be unchanged for all the doses tested (mean ratio RIIα/COXIV relative protein expression 0.6781 for 100 µM H2O2 vs. 0.8091 for 0 µM; P = 0.2659), and 3) AKAP1 protein followed a similar degree of protein downregulation as RIα (mean ratio AKAP1/COXIV relative protein expression 0.2348 for 100 µM H2O2 vs. 0.4576 for 0 µM; P = 0.0044). In this experiment, Opa1 protein cleavage was again used as a qualitative indicator of oxidative stress.
Fig. 2.
Oxidative stress alone induces type I cAMP-dependent protein kinase (PKA) regulatory α-subunit (RIα) downregulation in adult mouse ventricular myocytes (AMVMs). A: immunoblot analysis of AMVM cell cultures treated for 30 min with the oxidant H2O2 at the indicated micromolar concentrations. Opa1, optic atrophy protein 1; COXIV, cytochrome-c oxidase. B: quantification of RIα, catalytic subunit of PKA (PKAc), RIIα, and A-kinase anchoring protein 1 (AKAP1) expression from H2O2 dose-response experiments in A. Data are presented as means ± SD; n = 4–7 biological replicates. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with 0-µM treatment. C: immunoblot analysis of AMVM cell cultures treated with 100 μΜ H2O2 for the indicated time periods. D: quantification of RIα, PKAc, RIIα, and AKAP1 expression from 100 μM H2O2 time-course experiments from C. Data are presented as means ± SD; n = 3 biological replicates. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with 0-min time point.
A time course of oxidant treatment (5, 10, 15, and 30 min) in AMVMs was also performed using 100 μM concentration treatment of H2O2 (Fig. 2, C and D). RIα protein is gradually decreased as a function of increased exposure time to oxidant, with significant decrease observed at 15 and 30 min of treatment (mean ratio RIα/COXIV relative protein expression 0.2491 for 15 min, 0.1957 for 30 min vs. 0.4576 for 0 min Ctrl; 15 min, P = 0.0075; 30 min, P = 0.0043). Similar results were observed for PKAc where slight, but significant, reduction in expression is observed at 15 and 30 min of oxidant stress (mean ratio PKAc/COXIV relative protein expression 0.9008 for 15 min, 0.7923 for 30 min vs. 1.128 for 0 min Ctrl; 15 min, P = 0.0126; 30 min, P = 0.0004). Levels of RIIα subunit protein expression were not significantly changed in this model as well mean ratio RIIα/COXIV relative protein expression 0.7629 for 30 min vs. 0.8854 for 0 min Ctrl; P = 0.2840). Induction of oxidative stress was validated by the loss of AKAP1 as well (mean ratio AKAP1/COXIV relative protein expression 0.3745 for 15 min, 0.3696 for 30 min vs. 0.4887 for 0 min Ctrl; 15 min, P = 0.0154; 30 min, P = 0.0197). Once again, qualitative analysis of Opa1 cleavage was used in this experiment to indicate oxidative stress upon H2O2 treatment and additionally by the appearance of HIF-1α expression with increasing time of oxidant exposure. Taken together, these data suggest that cell stress caused by ROS is sufficient to specifically downregulate RIα expression, but not RII regulatory subunit in cardiac myocytes.
RIα oxidation is correlated with PKAc activation.
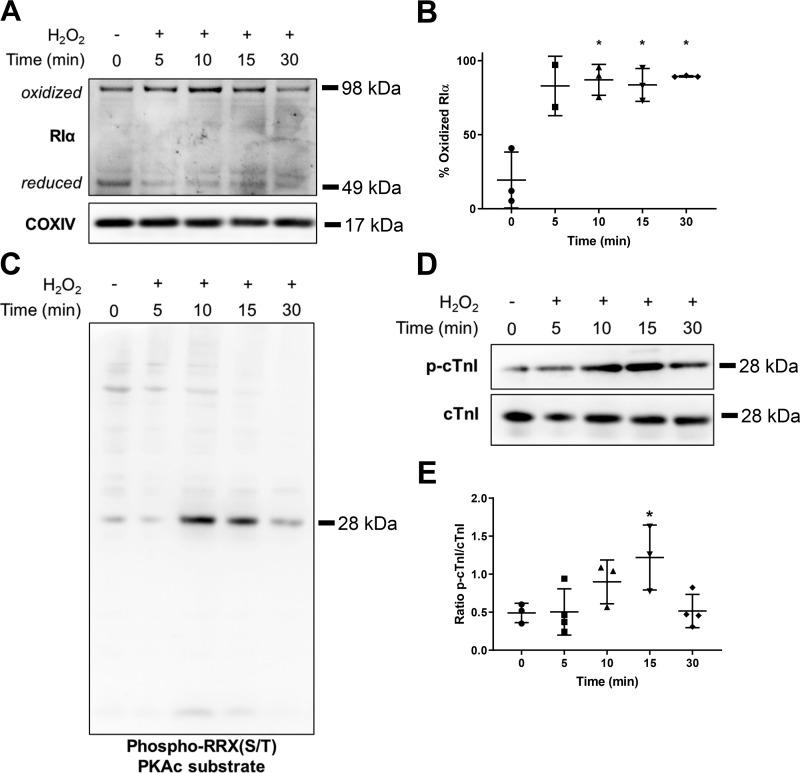
Because of the oxidant-sensitive nature of RIα dimerization/docking domain, we also assessed the extent of RIα protein oxidation within a H2O2 stress time-course regimen in AMVM cultures, where immunoblot analysis under nonreducing conditions allows detection of oxidized RIα subunit dimers (Fig. 3A; see Detection of oxidized RIα protein under methods). Under control treatment, RIα protein was expressed in a mixed population of reduced and oxidized protein. However, upon oxidant treatment, the vast majority of RIα protein was observed as oxidized in a dimeric form, where significant differences were observed after 10 min of H2O2 stress (Fig. 3, A and B; %oxidized RIα 86.99% for 10 min, 83.58% for 15 min, 89.34% for 30 min vs. 19.38% for 0 min Ctrl; 10 min, P = 0.0111; 15 min, P = 0.0123; 30 min, P = 0.0234). In accordance with data under reducing conditions, total RIα protein expression was reduced with oxidant stress longer than 15 min. Using an antibody that targets phosphorylated substrates of PKAc [phospho-RRX(S/T) PKAc substrate], we observed substantial phosphorylation signal, correlated with a band of molecular mass ~28 kDa, occurring at 10- and 15-min time points of H2O2 stress (Fig. 3C). Further analysis with specific antibody probes for known PKAc substrates showed that phosphorylation of cTnI is significantly increased at the 15-min time point (Fig. 3, D and E; mean ratio p-cTnI/cTnI 0.4905 for 15 min vs. 1.1219 for 0 min Ctrl; P = 0.0472). These findings indicate that PKAc activity appears to be directly correlated with the oxidation of RIα protein.
Fig. 3.
Oxidation of type I cAMP-dependent protein kinase (PKA) regulatory α-subunit (RIα) is correlated with increased catalytic subunit of PKA (PKAc) activity. A: immunoblot analysis comparing the extent of RIα protein oxidation upon time-course treatment with 100 μΜ Η2Ο2 in adult mouse ventricular myocytes cell cultures. COXIV, cytochrome-c oxidase. Dimer bands are considered oxidized due to the requirement of covalent disulfide bonds to maintain dimerization upon denaturing conditions of nonreducing SDS-PAGE. B: quantification of RIα protein oxidation, expressed as a percentage of oxidized RIα compared with total RIα protein (n = 2 to 3 biological replicates). Data are presented as means ± SD. *P < 0.05 compared with 0-min time point. C: immunoblot analysis comparing phospho-RRX(S/T) PKAc substrate phosphorylation within a time-course treatment with 100 μM H2O2 (n = 4 biological replicates). D: immunoblot analysis of cardiac troponin I (cTnI) phosphorylation within a time-course treatment with 100 µM H2O2. E: quantification of cTnI phosphorylation (p-cTnI) from D (n = 3–4 biological replicates). Data are presented as means ± SD. *P < 0.05 compared with 0-min time point.
Overexpression of RIα increases apoptosis upon oxidative stress in AMVMs.
To determine the downstream effect of oxidant-mediated RIα protein loss and subsequent PKAc activation upon cell fate in cardiomyocytes, we performed oxidative stress treatments on AMVMs treated with adenovirus to induce exogenous overexpression of human RIα (Ad-RIα) or green fluorescent protein (Ad-GFP) control (Fig. 4A). RIα protein expression was significantly increased in the Ad-RIα control treatment group (mean ratio RIα/COXIV relative protein expression 0.4001 for Ad-RIα Ctrl vs. 0.6190 for Ad-GFP Ctrl; P = 0.0281), but the Ad-RIα with oxidant stress group was not significantly different as compared with the Ad-GFP control group (Fig. 4B). Analysis of the apoptosis marker Cl PARP showed that Ad-RIα cells treated with oxidant had significant increase in cleaved PARP signal compared with Ad-GFP Ctrl samples (Fig. 4C; mean ratio Cl PARP/COXIV relative protein expression 0.5006 for Ad-RIα Ctrl vs. 0.02374 for Ad-GFP Ctrl; P = 0.0247). Interestingly, RIα overexpression without oxidant stress showed modest yet not significant increase in PARP cleavage as well (mean ratio Cl PARP/COXIV relative protein expression 0.1117 for Ad-RIα Ctrl vs. 0.02374 for Ad-GFP Ctrl; P = 0.0507). Analysis of cTnI phosphorylation in this model showed that while RIα overexpression significantly decreases phosphorylated cTnI as compared with Ad-GFP control samples (mean ratio p-cTnI/cTnI 0.4080 for Ad-RIα Ctrl vs. 0.7177 for Ad-GFP Ctrl; P = 0.0365), induction of oxidant stress led to increased phosphorylation in both GFP- and RIα-overexpressing cells, with no significant difference between the two oxidant-stressed groups (Fig. 4, D and E; mean ratio p-cTnI/cTnI 1.047 for Ad-GFP H2O2, 1.058 for Ad-RIα H2O2 vs. 0.7177 for Ad-GFP Ctrl; Ad-GFP H2O2, P = 0.0317; Ad-RIα H2O2, P = 0.0421).
Fig. 4.
Overexpression of type I cAMP-dependent protein kinase (PKA) regulatory α-subunit (RIα) in adult mouse ventricular myocytes (AMVMs) increases apoptosis upon oxidative stress. COXIV, cytochrome-c oxidase. A: immunoblot analysis of AMVM cell cultures first infected with adenovirus to overexpress regulatory α-subunit (RΙα) or green fluorescent protein (GFP) controls (Ad-RΙα and Ad-GFP, respectively) and then exposed to oxidant stress (10 µM H2O2) for 30 min. B: quantification of RIα expression from data in A (n = 4 biological replicates). Data are presented as means ± SD. *P < 0.05 compared with “Ad-GFP control (Ctrl)” condition. C: quantification of cleaved poly-ADP ribose polymerase (Cl PARP) expression from data in A (n = 4 biological replicates). Data are presented as means ± SD. *P < 0.05 compared with “Ad-GFP Ctrl” condition. D: immunoblot analysis of cardiac troponin I phosphorylation (p-TnI) from AMVM cells treated similarly as in A. E: quantification of p-cTnI data from D (n = 3 biological replicates). Data are presented as means ± SD. *P < 0.05; **P < 0.01.
Overexpression of RIα in L6 myoblasts increases apoptosis and decreases mitochondrial function upon oxidative stress.
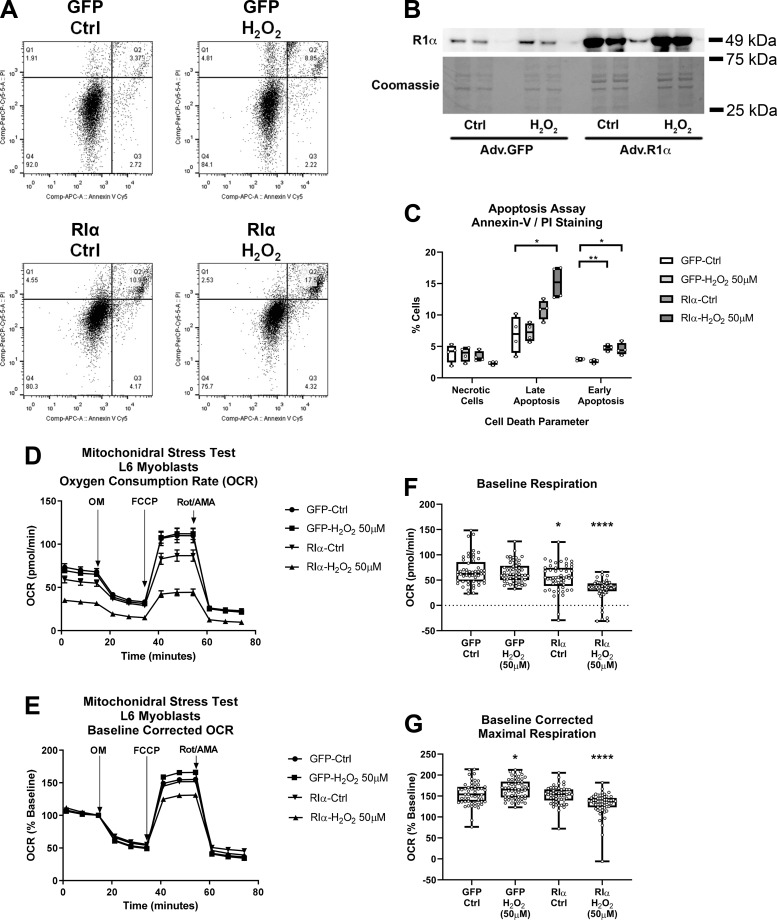
To further explore the effects of RIα overexpression upon heart cell function, we also assessed adenoviral overexpression of RIα combined with oxidative stress in a L6 rat myoblast cell model such that both flow cytometry analysis of apoptosis (annexin-V stain) and necrosis (PI stain) could be performed (Fig. 5, A–C). Immunoblot analysis confirmed RIα overexpression in these cells similar to that in AMVMs (Fig. 5B). Upon quantification of flow cytometry results, both Ad-RIα Ctrl and Ad-RIα H2O2 groups showed significant increases in annexin-V+/PI− stained (i.e., early apoptotic) cells, and Ad-RIα H2O2 showed significant increases in annexin-V+/PI+ stained (i.e., late apoptotic) cells, thus corroborating data from the AMVM model that RIα overexpression induces apoptosis, especially upon oxidative injury (Fig. 5C; mean cells “early apoptosis” for Ad-RIα Ctrl 4.795%, Ad-RIα H2O2 4.568% vs. Ad-GFP Ctrl 2.970%; Ad-RIα Ctrl, adjusted P = 0.00107; Ad-RIα H2O2, adjusted P = 0.035; mean cells “late apoptosis” for Ad-RIα H2O2 15.18% vs. Ad-GFP Ctrl 6.878%; Ad-RIα H2O2, adjusted P = 0.016). Measurement of cellular respiration was also assessed in this cell model using the Seahorse extracellular flux analyzer (Fig. 5, D–G). Performance of the “mitochondrial stress test” assay showed that RIα overexpression caused significant decreases in basal oxygen consumption rate (OCR) and effect that is more pronounced upon oxidative stress (Fig. 5F; mean baseline OCR for Ad-RIα Ctrl 54.90 pmol/min, Ad-RIα H2O2 31.55 vs. 68.11 pmol/min Ad-GFP Ctrl; Ad-RIα Ctrl, P = 0.0148; Ad-RIα H2O2, P < 0.0001). Upon addition of the uncoupling agent FCCP, RIα-overexpressing cells treated with oxidant showed significant decreases in baseline-corrected maximal (i.e., uncoupled) respiratory capacity (Fig. 5G; mean baseline-corrected maximal OCR for Ad-RIα H2O2 131.0 vs. 155.0 pmol/min Ad-GFP Ctrl; P < 0.0001). Taken together with experiments conducted in AMVMs, evidence gathered from the L6 myoblast model implies that RIα protein expression may have a critical role in regulation of prosurvival (i.e., cardioprotective) responses in cardiomyocytes upon oxidative injury.
Fig. 5.
Overexpression of type I cAMP-dependent protein kinase (PKA) regulatory α-subunit (RIα) in L6 myoblasts increases apoptosis and decreases mitochondrial function upon oxidative stress. A: representative scatter plot flow cytometry analysis of L6 myoblast cell cultures first infected with adenovirus to overexpress RΙα or green fluorescent protein (GFP) controls and then exposed to oxidant stress (50 µM H2O2) for 30 min, followed by staining with annexin-V and propidium iodide (PI) to detect apoptotic and necrotic cells. B: representative immunoblot and Coomassie stain analysis of RΙα expression from L6 myoblast cell cultures analyzed in A (n = 4 replicates). Adv, adenovirus. C: quantification of cell death parameters from data in A (necrotic cells, annexin-V−/PI+; late apoptosis, annexin-V+/PI+; early apoptosis, annexin-V+/PI−) (n = 4 replicates). Data are presented as means ± SD. *P < 0.05 compared with “GFP control (Ctrl)” condition, and **P < 0.01 compared with “GFP Ctrl” condition. D: Seahorse extracellular flux time-course analysis of mitochondrial “stress test” assays, assessing oxygen consumption rate (OCR) in GFP- or RIα-overexpressing L6 myoblasts treated with or without 50 µM H2O2. FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone; OM, oligomycin; Rot/AMA, rotenone/antimycin A. E: baseline corrected time-course analysis from data in D. F: quantification of baseline OCR data from D (n = 53–57 technical replicates summed over 3 independent experiments. Data are presented as means ± SE. *P < 0.05 and ****P < 0.0001 compared with “GFP Ctrl” condition. G: quantification of baseline corrected maximal respiration data from E (n = 53–57 technical replicates summed over 3 independent experiments). Data are presented as means ± SE. *P < 0.05 and ****P < 0.0001 compared with “GFP Ctrl” condition.
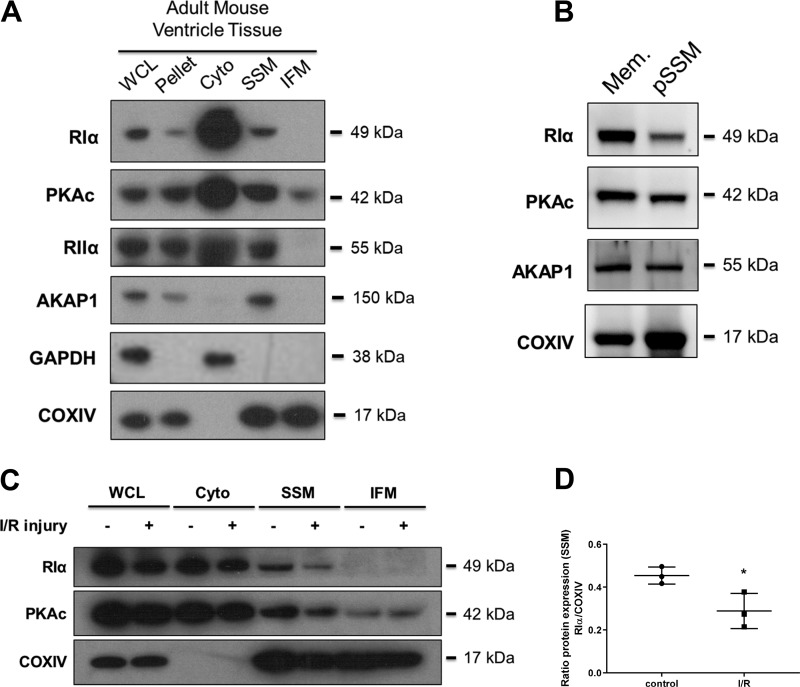
PKA proteins are enriched in SSM, and RIα expression is decreased in SSM after I/R injury.
Considering mitochondria play a crucial role in the regulation of cell function, especially under oxidative stress conditions, we moved to characterize the localization of PKA proteins within unique subpopulations of mitochondria in the heart, namely SSM and interfibrillar mitochondria (IFM). While majority of the catalytic and regulatory subunit PKA proteins are found in cytosolic fractions, we observed that RIα, PKAc, and RIIα were all enriched within SSM fractions of the untreated heart tissue (Fig. 6A). Interestingly, PKAc was the only protein shown to have any expression within the IFM fraction. We also observed that AKAP1 was also expressed only in the SSM fraction and not found in IFM. Further purification of SSM using density gradient ultracentrifugation methods showed that RIα, PKAc, and AKAP1 are all enriched with the purified mitochondrial fraction, indicating that the presence of these proteins in our crude SSM fractions is not merely due to membranous/vesicular contaminants (Fig. 6B). When performing mitochondrial fractionation upon I/R-treated mouse heart ventricle tissue, it was observed that while RIα protein decreased in global expression in this model, the fold-change of decreased RIα protein expression is most dramatic within the SSM fraction (Fig. 6, C and D; mean ratio RIα/COXIV relative SSM protein expression for I/R 0.2886 vs. 0.4538 for Ctrl; P = 0.0349). PKAc levels appear to be not significantly altered in the IFM fraction upon I/R injury. Given these findings, it is possible that oxidant-mediated decrease of RIα localization at the mitochondria may play a critical role in the regulation of apoptosis upon I/R injury.
Fig. 6.
cAMP-dependent protein kinase (PKA) proteins are specifically enriched in subsarcolemmal mitochondria (SSM), and type I PKA regulatory α-subunit (RIα) expression is decreased in SSM with ischemia-reperfusion (I/R) injury. A: immunoblot analysis of PKA-associated proteins from untreated adult mouse heart ventricular tissue fractionated by differential centrifugation to separate mitochondrial subpopulations. AKAP1, A-kinase anchoring protein 1; COXVI, cytochrome-c oxidase; WCL, whole cell lysate; Pellet, nuclear/cytoskeleton pellet; Cyto, cytosol. B: immunoblot analysis of PKA-associated proteins within SSM subjected to density gradient ultracentrifugation. Mem, purified membrane; pSSM, purified SSM. C: immunoblot analysis of RΙα, catalytic subunit of PKA (PKAc), and COXIV from fractionated adult mouse heart ventricular tissue, comparing hearts that received either control perfusion or I/R injury. D: quantification of RIα expression from data in C, expressing changes of SSM-localized protein either with or without I/R injury stimulus (n = 3 biological replicates). Data are presented as means ± SD. *P < 0.05, compared on control condition.
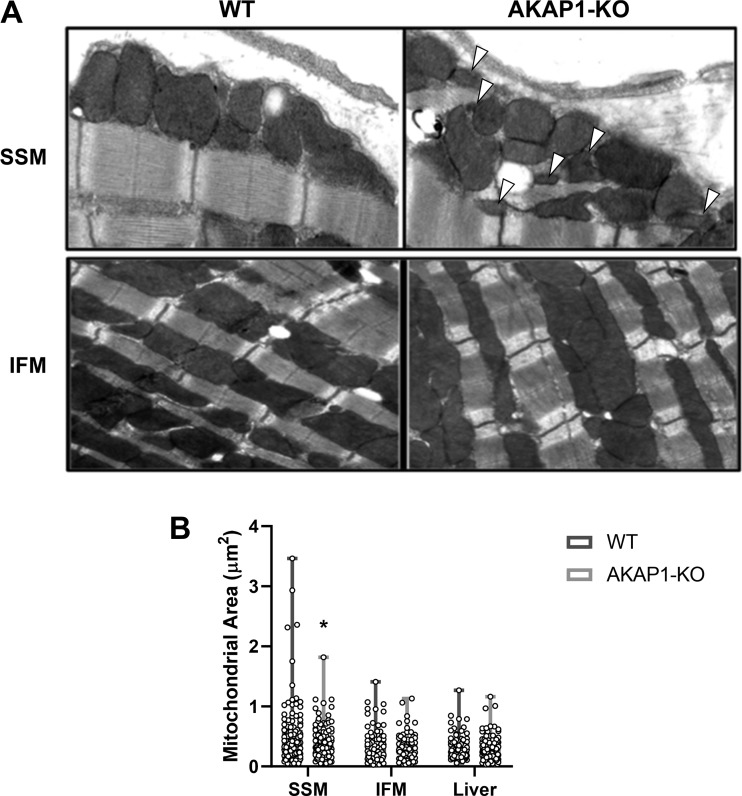
SSM morphology is perturbed in AKAP1-KO mice.
Because both RIα and AKAP1 are preferentially localized within SSM in mouse hearts and because both these proteins appear to be sensitive to ischemic stress, analysis of SSM and IFM morphology was conducted via transmission electron microscopy (TEM) of mouse heart tissues, comparing WT and AKAP1-KO mice (Fig. 7A). Upon quantification of mitochondria area in these tissues, SSM from AKAP1-KO mice were significantly decreased in size compared with wild-type controls, whereas IFM were not changed in morphology between the two groups (Fig. 7B; mean mitochondrial area for AKAP1-KO 0.39 vs. WT 0.50 µm2; P = 0.0331). Liver mitochondria were also examined as a noncardiac organ control (TEM data not shown). The presence of morphological abnormality only within the SSM fraction of the AKAP1-KO mice further suggested that PKA signaling localized to this peripheral pool of mitochondria may play a crucial role in exacting a response to ischemic stress in the heart.
Fig. 7.
Subsarcolemmal mitochondria (SSM) ultrastructure is perturbed in A-kinase anchoring protein 1 knockout (AKAP1-KO) mice. A: image analysis from transmission electron microscopy (TEM) performed on untreated mouse heart tissues from either wild-type (WT) or AKAP1-KO mice. Arrowheads indicate areas of decreased mitochondrial area. B: quantification of mitochondrial area (μm2) for heart SSM, heart interfibrillar mitochondria (IFM), and liver mitochondria, comparing WT and AKAP1-KO mice (n = 84–124 technical replicates summed over 10 biological replicates). Data are presented as means ± SE. *P < 0.05, compared with WT.
DISCUSSION
To summarize, this study integrates theories from previous work in the field and novel findings to suggest a possible role for oxidation of RIα and regulation of PKA signaling in the context of cardiac I/R injury. Our work indicates that the oxidation-dependent loss of RIα may function to activate PKAc to control cardiac cell fate upon oxidative stress. Although these observations are corroborated in multiple models, the major limitation of this study is a lack of evidence to elucidate the cellular mechanism by which RIα protein expression is decreased. Because this phenomenon occurs in such a rapid time frame, we presume that the loss of RIα protein is manifested at the level of protein synthesis and degradation, rather than transcriptionally regulated effects that would require longer time periods. Future studies are needed to examining mRNA expression of RIα in similar models of cardiac oxidative injury to verify this hypothesis. To support the inference of regulation at the level of protein expression, previous publications have shown that RIα may have alternative regulatory mechanisms for higher protein turnover. The linker-hinge domain of RIα includes a “PEST sequence” motif (noted for enrichment of proline, glutamate, serine, and threonine residues) that has been correlated with higher protein degradation rates (20). This protein domain, especially within cryptic regions of the sequence that interact with PKAc upon holoenzyme formation, has also been shown to be a target for both ubiquitination and protease-mediated proteolysis (17). With these findings in consideration, forthcoming efforts are needed to focus on uncovering the mechanism by which RIα is selectively downregulated in cardiomyocyte cells. Also, to validate the theory that loss of RIα may prime PKAc for oxidation and deactivation, forthcoming experiments should be aimed to assess the relative degree of PKAc oxidation within the models of I/R injury and oxidative stress in the heart.
Our research has also implicated a possible role for PKA signaling localized within SSM to regulate response to ischemic injury in the heart. In this study we demonstrated that localization of PKA signaling proteins within mitochondrial subcompartments (i.e., SSM and IFM) of cardiomyocytes is dynamically regulated upon oxidative stress in the heart. Despite these findings, we cannot yet conclude from our data whether the effect of ROS-mediated loss of RIα has a direct outcome in regulating SSM function. Thus, the mechanism by which type I PKA signaling localized to SSM affects mitochondria and overall cardiomyocyte function remains not fully understood. Many studies have focused on the involvement of PKA signaling localized to mitochondria in the development and progression of heart disease phenotypes. PKAc itself is known to regulate signaling intrinsic to mitochondrial function, whether in terms of modulation of oxidative phosphorylation (OXPHOS) (12, 28, 35), control of membrane fission/fusion and mitochondrial-dependent apoptosis processes (7, 11, 16, 30), or phosphorylation of mitochondrial proteins (30); however, the role of regulatory subunits proteins is rarely explored in these studies.
To explain these PKA-dependent mitochondrial signaling phenomena, characterizing the means of compartmentalization of PKAc and other PKA signaling proteins to mitochondria remains an ongoing challenge. The discovery of mitochondrial AKAPs including AKAP1 (23) and sphingosine kinase interacting protein (SKIP or SPHKAP) (24) gives a rationale for PKA localization to mitochondria. RNAi-mediated knockdown of AKAP1 in rat neurons has been shown to increase sensitivity to oxidative damage (25), thus spurring investigation of the AKAP1-KO mouse model for cardiac ischemia. It was observed here that SSM have perturbed morphology in AKAP1-KO mice, highlighting the potential role in PKA signaling regulation in the maintenance of mitochondrial function in the heart. However, the localization of RIα and related proteins within AKAP1-KO mice was not explored in this study, and therefore the intricacies of type I PKA-dependent signaling regimes in these animals remain unknown. Future studies are needed to investigate the relationship between RIα and AKAP1 in cardiac mitochondria, as it is likely that the interaction between these proteins fosters mitochondrial localization of PKA proteins to the outer mitochondrial membrane of SSM. AKAP1 has been shown to undergo hypoxia-mediated protein downregulation via targeting by seven in absentia homolog 2 (Siah2), a hypoxia-sensitive E3-ligase protein (5). We suspect that oxidant-dependent RIα loss could be directly correlated with Siah2-mediated AKAP1 loss. In contradiction of our findings, a recent report from Schiattarella et al. (31) asserts that AKAP1 knockout affects SSM and IFM equivalently in the in vivo model of myocardial infarction. Future efforts are needed to ascertain the significance of the differences between these two opposing data sets.
To add further complexity to the understanding of PKA signaling localized to mitochondria in the heart, a previous study from our laboratories has shown that A-kinase interacting protein 1 (AKIP1) is a critical regulator of response to I/R injury, manifested by protection of mitochondrial physiological function (29). As the role of AKIP1 was not explored in this study, future efforts are needed to further consider the possible interdependent nature of type I PKA signaling with AKIP1 in the heart. An important unanswered question is whether RIα loss may possibly facilitate AKIP1-mediated cardioprotection. The observed behaviors of these two proteins in cardiac I/R injury, where RIα protein decrease occurs within the same time frame and dosing regimen of oxidant stress that AKIP1 expression is increased, are highly suggestive that these two effects are correlated. No study to date has provided evidence of a direct interaction of AKIP1 with a PKA regulatory subunit, so identifying a relationship between these two proteins would be a novel and informative discovery, especially within the context of ischemic heart disease pathologies.
In conclusion, intervention upon type I PKA signaling may be a potential avenue for therapeutics within the clinical setting of treating ischemic heart disease. If indeed oxidation and specific downregulation of RIα protein (coupled with subsequent activation of PKAc) plays a critical role in cardiomyocyte prosurvival response, it is possible that targeting this pathway during an ischemic event could lead to preferential outcomes in the onset myocardial infarction. However, a better understanding of the spatiotemporal aspect of this stress event will be crucial for directly intervening upon this disease pathology in the clinic. While our evidence may suggest this assertion is true in the setting of acute oxidative damage, further study is required to assess this effect within both in vivo models of I/R as well as chronic disease conditions occurring over longer timescales (e.g., heart failure models). Considering similar findings regarding specific downregulation of regulatory subunit protein have been shown in examples of human heart disease (17), future efforts in the field should be focused on differentiating the effects of PKA signaling in both acute and chronic conditions of oxidative stress in the heart.
GRANTS
This work was supported by National Institutes of Health Grants GM-34921 (to S. S. Taylor), DK-54441 (to S. S. Taylor), HL-091071 (to H. H. Patel), HL-107200 (to H. H. Patel), and NS-056244 (to S. Strack) and Veterans Affairs Grant BX001963 (to H. H. Patel).
DISCLOSURES
H. H. Patel has equity as a founder in CavoGene LifeSciences Holdings, LLC.
AUTHOR CONTRIBUTIONS
K.J.H. and M.S. conceived and designed research; K.J.H., J.M.S., Y.S., M.S., and G.A.P. performed experiments; K.J.H., Y.S., and G.A.P. analyzed data; K.J.H. and Y.S. interpreted results of experiments; K.J.H. prepared figures; K.J.H. drafted manuscript; K.J.H., Y.S., S.S.T., and H.H.P. edited and revised manuscript; K.J.H., J.M.S., Y.S., M.S., G.A.P., S.S., S.S.T., and H.H.P. approved final version of manuscript
ACKNOWLEDGMENTS
We thank Dr. Atsushi Miyanohara for generating adenoviral constructs used in this study, Victoria Hoznek for assisting with cell culture and immunoblotting procedures, and Emily Walker for performing immunoblot densitometry analysis.
REFERENCES
- 1.Aye TT, Soni S, van Veen TA, van der Heyden MA, Cappadona S, Varro A, de Weger RA, de Jonge N, Vos MA, Heck AJ, Scholten A. Reorganized PKA-AKAP associations in the failing human heart. J Mol Cell Cardiol 52: 511–518, 2012. doi: 10.1016/j.yjmcc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Banky P, Huang LJ, Taylor SS. Dimerization/docking domain of the type Ialpha regulatory subunit of cAMP-dependent protein kinase. Requirements for dimerization and docking are distinct but overlapping. J Biol Chem 273: 35048–35055, 1998. doi: 10.1074/jbc.273.52.35048. [DOI] [PubMed] [Google Scholar]
- 3.Brennan JP, Bardswell SC, Burgoyne JR, Fuller W, Schröder E, Wait R, Begum S, Kentish JC, Eaton P. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem 281: 21827–21836, 2006. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- 4.Burgoyne JR, Eaton P. Transnitrosylating nitric oxide species directly activate type I protein kinase A, providing a novel adenylate cyclase-independent cross-talk to β-adrenergic-like signaling. J Biol Chem 284: 29260–29268, 2009. doi: 10.1074/jbc.M109.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlucci A, Adornetto A, Scorziello A, Viggiano D, Foca M, Cuomo O, Annunziato L, Gottesman M, Feliciello A. Proteolysis of AKAP121 regulates mitochondrial activity during cellular hypoxia and brain ischaemia. EMBO J 27: 1073–1084, 2008. doi: 10.1038/emboj.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clegg CH, Cadd GG, McKnight GS. Genetic characterization of a brain-specific form of the type I regulatory subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci USA 85: 3703–3707, 1988. doi: 10.1073/pnas.85.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darshi M, Mendiola VL, Mackey MR, Murphy AN, Koller A, Perkins GA, Ellisman MH, Taylor SS. ChChd3, an inner mitochondrial membrane protein, is essential for maintaining crista integrity and mitochondrial function. J Biol Chem 286: 2918–2932, 2011. doi: 10.1074/jbc.M110.171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson SM, Ferdinandy P, Andreadou I, Bøtker HE, Heusch G, Ibáñez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, Garcia-Dorado D, Action CC; CARDIOPROTECTION COST Action (CA16225) . multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol 73: 89–99, 2019. doi: 10.1016/j.jacc.2018.09.086. [DOI] [PubMed] [Google Scholar]
- 9.Fridolfsson HN, Kawaraguchi Y, Ali SS, Panneerselvam M, Niesman IR, Finley JC, Kellerhals SE, Migita MY, Okada H, Moreno AL, Jennings M, Kidd MW, Bonds JA, Balijepalli RC, Ross RS, Patel PM, Miyanohara A, Chen Q, Lesnefsky EJ, Head BP, Roth DM, Insel PA, Patel HH. Mitochondria-localized caveolin in adaptation to cellular stress and injury. FASEB J 26: 4637–4649, 2012. doi: 10.1096/fj.12-215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao X, Lin B, Sadayappan S, Patel TB. Interactions between the regulatory subunit of type I protein kinase A and p90 ribosomal S6 kinase1 regulate cardiomyocyte apoptosis. Mol Pharmacol 85: 357–367, 2014. doi: 10.1124/mol.113.090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, Scott JD, Korsmeyer SJ. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell 3: 413–422, 1999. doi: 10.1016/S1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- 12.Helling S, Vogt S, Rhiel A, Ramzan R, Wen L, Marcus K, Kadenbach B. Phosphorylation and kinetics of mammalian cytochrome c oxidase. Mol Cell Proteomics 7: 1714–1724, 2008. doi: 10.1074/mcp.M800137-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphries KM, Deal MS, Taylor SS. Enhanced dephosphorylation of cAMP-dependent protein kinase by oxidation and thiol modification. J Biol Chem 280: 2750–2758, 2005. doi: 10.1074/jbc.M410242200. [DOI] [PubMed] [Google Scholar]
- 14.Humphries KM, Juliano C, Taylor SS. Regulation of cAMP-dependent protein kinase activity by glutathionylation. J Biol Chem 277: 43505–43511, 2002. doi: 10.1074/jbc.M207088200. [DOI] [PubMed] [Google Scholar]
- 15.Humphries KM, Pennypacker JK, Taylor SS. Redox regulation of cAMP-dependent protein kinase signaling: kinase versus phosphatase inactivation. J Biol Chem 282: 22072–22079, 2007. doi: 10.1074/jbc.M702582200. [DOI] [PubMed] [Google Scholar]
- 16.Keshwani MM, Kanter JR, Ma Y, Wilderman A, Darshi M, Insel PA, Taylor SS. Mechanisms of cyclic AMP/protein kinase A- and glucocorticoid-mediated apoptosis using S49 lymphoma cells as a model system. Proc Natl Acad Sci USA 112: 12681–12686, 2015. doi: 10.1073/pnas.1516057112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, Harper JW, Gygi SP. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell 44: 325–340, 2011. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DC, Carmichael DF, Krebs EG, McKnight GS. Isolation of a cDNA clone for the type I regulatory subunit of bovine cAMP-dependent protein kinase. Proc Natl Acad Sci USA 80: 3608–3612, 1983. doi: 10.1073/pnas.80.12.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.León DA, Herberg FW, Banky P, Taylor SS. A stable α-helical domain at the N terminus of the RIalpha subunits of cAMP-dependent protein kinase is a novel dimerization/docking motif. J Biol Chem 272: 28431–28437, 1997. doi: 10.1074/jbc.272.45.28431. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Hu JY, Schacher S, Schwartz JH. The two regulatory subunits of aplysia cAMP-dependent protein kinase mediate distinct functions in producing synaptic plasticity. J Neurosci 24: 2465–2474, 2004. doi: 10.1523/JNEUROSCI.4331-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu NB, Wu M, Chen C, Fujino M, Huang JS, Zhu P, Li XK. Novel Molecular Targets Participating in Myocardial Ischemia-Reperfusion Injury and Cardioprotection. Cardiol Res Pract 2019: 6935147, 2019. doi: 10.1155/2019/6935147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest 123: 1262–1274, 2013. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Taylor SS. A molecular switch for targeting between endoplasmic reticulum (ER) and mitochondria: conversion of a mitochondria-targeting element into an ER-targeting signal in DAKAP1. J Biol Chem 283: 11743–11751, 2008. doi: 10.1074/jbc.M710494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Means CK, Lygren B, Langeberg LK, Jain A, Dixon RE, Vega AL, Gold MG, Petrosyan S, Taylor SS, Murphy AN, Ha T, Santana LF, Tasken K, Scott JD. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc Natl Acad Sci USA 108: E1227–E1235, 2011. doi: 10.1073/pnas.1107182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrill RA, Dagda RK, Dickey AS, Cribbs JT, Green SH, Usachev YM, Strack S. Mechanism of neuroprotective mitochondrial remodeling by PKA/AKAP1. PLoS Biol 9: e1000612, 2011. doi: 10.1371/journal.pbio.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrill RA, Strack S. Mitochondria: a kinase anchoring protein 1, a signaling platform for mitochondrial form and function. Int J Biochem Cell Biol 48: 92–96, 2014. doi: 10.1016/j.biocel.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252: 8731–8739, 1977. [PubMed] [Google Scholar]
- 28.Prabu SK, Anandatheerthavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J Biol Chem 281: 2061–2070, 2006. doi: 10.1074/jbc.M507741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sastri M, Haushalter KJ, Panneerselvam M, Chang P, Fridolfsson H, Finley JC, Ng D, Schilling JM, Miyanohara A, Day ME, Hakozaki H, Petrosyan S, Koller A, King CC, Darshi M, Blumenthal DK, Ali SS, Roth DM, Patel HH, Taylor SS. A kinase interacting protein (AKIP1) is a key regulator of cardiac stress. Proc Natl Acad Sci USA 110: E387–E396, 2013. doi: 10.1073/pnas.1221670110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schauble S, King CC, Darshi M, Koller A, Shah K, Taylor SS. Identification of ChChd3 as a novel substrate of the cAMP-dependent protein kinase (PKA) using an analog-sensitive catalytic subunit. J Biol Chem 282: 14952–14959, 2007. doi: 10.1074/jbc.M609221200. [DOI] [PubMed] [Google Scholar]
- 31.Schiattarella GG, Cattaneo F, Pironti G, Magliulo F, Carotenuto G, Pirozzi M, Polishchuk R, Borzacchiello D, Paolillo R, Oliveti M, Boccella N, Avvedimento M, Sepe M, Lombardi A, Busiello RA, Trimarco B, Esposito G, Feliciello A, Perrino C. Akap1 deficiency promotes mitochondrial aberrations and exacerbates cardiac injury following permanent coronary ligation via enhanced mitophagy and apoptosis. PLoS One 11: e0154076, 2016. doi: 10.1371/journal.pone.0154076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott JD, Glaccum MB, Zoller MJ, Uhler MD, Helfman DM, McKnight GS, Krebs EG. The molecular cloning of a type II regulatory subunit of the cAMP-dependent protein kinase from rat skeletal muscle and mouse brain. Proc Natl Acad Sci USA 84: 5192–5196, 1987. doi: 10.1073/pnas.84.15.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.See Hoe LE, Schilling JM, Tarbit E, Kiessling CJ, Busija AR, Niesman IR, Du Toit E, Ashton KJ, Roth DM, Headrick JP, Patel HH, Peart JN. Sarcolemmal cholesterol and caveolin-3 dependence of cardiac function, ischemic tolerance, and opioidergic cardioprotection. Am J Physiol Heart Circ Physiol 307: H895–H903, 2014. doi: 10.1152/ajpheart.00081.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME. MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardiol 52: 1135–1144, 2012. doi: 10.1016/j.yjmcc.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan S, Spear J, Chandran K, Joseph J, Kalyanaraman B, Avadhani NG. Oxidative stress induced mitochondrial protein kinase A mediates cytochrome c oxidase dysfunction. PLoS One 8: e77129, 2013. doi: 10.1371/journal.pone.0077129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem 59: 971–1005, 1990. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 37.Taylor SS, Ilouz R, Zhang P, Kornev AP. Assembly of allosteric macromolecular switches: lessons from PKA. Nat Rev Mol Cell Biol 13: 646–658, 2012. doi: 10.1038/nrm3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright VP, Reiser PJ, Clanton TL. Redox modulation of global phosphatase activity and protein phosphorylation in intact skeletal muscle. J Physiol 587: 5767–5781, 2009. doi: 10.1113/jphysiol.2009.178285. [DOI] [PMC free article] [PubMed] [Google Scholar]