Abstract
Brain-derived neurotrophic factor (BDNF) is upregulated in the paraventricular nucleus of the hypothalamus (PVN) in response to hypertensive stimuli such as stress and hyperosmolality, and BDNF acting in the PVN plays a key role in elevating sympathetic activity and blood pressure. However, downstream mechanisms mediating these effects remain unclear. We tested the hypothesis that BDNF increases blood pressure, in part by diminishing inhibitory hypotensive input from nucleus of the solitary tract (NTS) catecholaminergic neurons projecting to the PVN. Male Sprague-Dawley rats received bilateral PVN injections of viral vectors expressing either green fluorescent protein (GFP) or BDNF and bilateral NTS injections of vehicle or anti-dopamine-β-hydroxylase-conjugated saporin (DSAP), a neurotoxin that selectively lesions noradrenergic and adrenergic neurons. BDNF overexpression in the PVN without NTS lesioning significantly increased mean arterial pressure (MAP) in awake animals by 18.7 ± 1.8 mmHg. DSAP treatment also increased MAP in the GFP group, by 9.8 ± 3.2 mmHg, but failed to affect MAP in the BDNF group, indicating a BDNF-induced loss of NTS catecholaminergic hypotensive effects. In addition, in α-chloralose-urethane-anesthetized rats, hypotensive responses to PVN injections of the β-adrenergic agonist isoprenaline were significantly attenuated by BDNF overexpression, whereas PVN injections of phenylephrine had no effect on blood pressure. BDNF treatment was also found to significantly reduce β1-adrenergic receptor mRNA expression in the PVN, whereas expression of other adrenergic receptors was unaffected. In summary, increased BDNF expression in the PVN elevates blood pressure, in part by downregulating β-receptor signaling and diminishing hypotensive catecholaminergic input from the NTS to the PVN.
NEW & NOTEWORTHY We have shown that BDNF, a key hypothalamic regulator of blood pressure, disrupts catecholaminergic signaling between the NTS and the PVN by reducing the responsiveness of PVN neurons to inhibitory hypotensive β-adrenergic input from the NTS. This may be occurring partly via BDNF-mediated downregulation of β1-adrenergic receptor expression in the PVN and results in an increase in blood pressure.
Keywords: β-adrenergic receptors, blood pressure, brain-derived neurotrophic factor, catecholamine, hypothalamus
INTRODUCTION
Increased sympathetic activity is a significant factor in the development of hypertension and a major contributor to cardiovascular risk factors such as stress and high-fat and high-salt diets (12, 29, 41, 71, 84). Neural control of blood pressure is mediated by a core network of hypothalamic and brain stem nuclei. Presympathetic neurons within the paraventricular nucleus of the hypothalamus (PVN) stimulate spinal sympathetic preganglionic neurons directly or via the rostral ventrolateral medulla (RVLM) (18, 72), while the nucleus of the solitary tract (NTS) plays an important role in processing sensory information, responding to blood pressure changes and regulating sympathetic nervous system activity through the baroreflex (2, 18). The PVN plays a critical role in integrating autonomic, neuroendocrine, and cardiovascular responses to stressful stimuli (7, 19, 81), and our recent findings indicate that brain-derived neurotrophic factor (BDNF), acting in the PVN through its high-affinity tropomyosin receptor kinase B (TrkB receptor), may be a novel regulator of stress-induced elevations in sympathetic activity and blood pressure (24, 66, 67).
BDNF is a member of the neurotrophin family, capable of excitatory neurotransmitter and neurotrophic functions to induce both short- and long-term adaptive alterations throughout the central nervous system (6, 47, 53). In the hypothalamus, BDNF modulates several homeostatic mechanisms, such as regulation of food intake and body weight (43, 55, 77), and activation of the hypothalamic-pituitary-adrenal (HPA) axis, which is responsible for the neuroendocrine aspects of the stress response (28, 37). In addition, BDNF mRNA and protein levels in the PVN are elevated in response to several hypertensive stimuli, such as chronic and acute stress, hyperosmolality, and repeated amphetamine administration (1, 30, 49, 57, 69), and we (24, 67) have recently shown that both long-term overexpression and acute microinjection of exogenous BDNF into the PVN lead to significant increases in sympathetic nervous system activity, blood pressure, and heart rate. Furthermore, we (66) have found that inhibition of BDNF signaling by blocking its high-affinity receptor TrkB in the PVN reduces acute stress-induced blood pressure elevations without affecting baseline cardiovascular function.
BDNF has been shown to promote angiotensin II signaling and elevate the expression of angiotensin receptor 1 (AT1R) in the PVN, which is associated with elevated blood pressure (4, 24, 25, 67), and BDNF is also known to stimulate the synthesis of arginine vasopressin (AVP) in the PVN (1, 28), which may promote sympathetic activity acting centrally within the PVN (23) or elevate blood pressure through peripheral actions (31). However, BDNF is also a known modulator of catecholaminergic neurotransmission (8, 9, 68), and within the hypothalamus BDNF has been shown to increase norepinephrine uptake and decrease its evoked release (60). The PVN receives substantial catecholaminergic input from NTS A2 and C2 neurons (16, 51, 63, 65), and although the role of these projections is not completely understood, several studies have indicated that these neurons exert a hypotensive action at baseline (20, 22, 35). In addition, NTS catecholaminergic neurons are thought to be activated during both acute and chronic stress (15, 76, 86) and to play a role in the HPA axis response to systemic stressors (59, 79). Thus, an increase in BDNF expression in the PVN in response to hypertensive stimuli may have a significant impact on cardiovascular regulation by interfering with catecholaminergic signaling.
Here, we set out to test the hypothesis that upregulation of BDNF in the PVN elevates blood pressure either by diminishing catecholaminergic β-receptor-mediated inhibitory input from the NTS or by augmenting α-receptor-mediated excitatory mechanisms. We used our previously published model (24) of vector-mediated upregulation of BDNF in the PVN to induce hypertension and investigated the interaction of BDNF and catecholaminergic signaling by selectively lesioning NTS catecholaminergic neurons by using the neurotoxin anti-dopamine-β-hydroxylase-conjugated saporin (DSAP) (44). In addition, cardiovascular responses to PVN microinjections of adrenergic receptor agonists and mRNA expression of adrenergic receptors were analyzed following overexpression of BDNF in the PVN. Better understanding of BDNF-mediated cardiovascular regulatory mechanisms within the PVN is crucial for elucidating the central circuitry involved in the development of hypertension and could eventually lead to the identification of novel therapeutic targets.
METHODS
All animal housing, handling, and surgical and experimental procedures were conducted within an Association for the Assessment and Accreditation of Laboratory Care International-accredited animal care facility at the University of Vermont, in accordance with the National Institutes of Health (NIH) Policy on Humane Care and Use of Laboratory Animals and the NIH Guide for the Care and Use of Laboratory Animals. Experiments were performed in male Sprague-Dawley (SD) rats obtained from Charles River (Saint-Constant, QC, Canada). Rats were housed individually with a 12:12-h light-dark cycle (lights on at 6:00 AM), with free access to food (standard chow) and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont.
Experimental Design
Experiment 1.
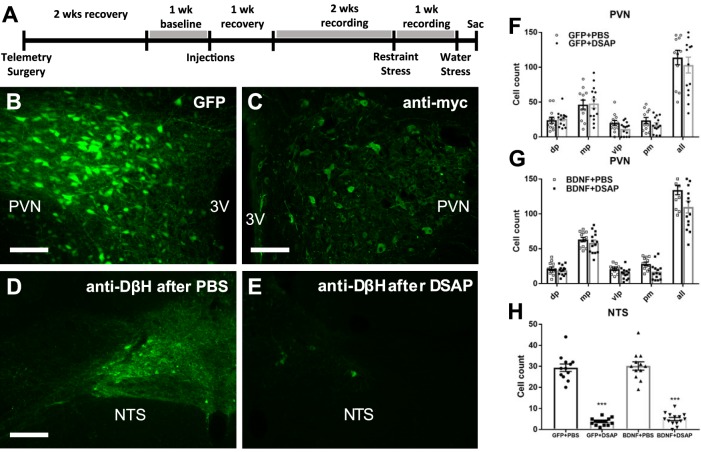
The aim of the first experiment was to determine how increased BDNF expression in the PVN affects catecholaminergic input from the NTS. Radiotelemetric transmitters were implanted for blood pressure measurement in 8-wk-old male SD rats, and animals were allowed to recover for 2 wk. Baseline cardiovascular parameters were recorded for 6 days, and then, adeno-associated viral vectors (AAV2, 1012 vps/mL, 200 nL/side) expressing GFP or myc epitope-tagged BDNF fusion protein (BDNFmyc) were injected bilaterally into the PVN. In addition, PBS (100 nL) or DSAP (22 ng in 100 nL of PBS), was injected bilaterally into the NTS as described previously (20). After a week of recovery, cardiovascular parameters were recorded for 3 wk. During this period, rats were subjected to an acute-restraint stress procedure 3 wk after brain injections and to an acute water stress procedure 4 wk after brain injections (Fig. 1A). At the end of the experiment, animals were deeply anesthetized with isoflurane and perfused transcardially with ice-cold PBS and 4% paraformaldehyde. PVN expressions of GFP and BDNFmyc as well as effects of DSAP lesioning in the NTS were verified with immunofluorescence (Fig. 1, B–H). In addition, percent volumetric density of dopamine β-hydroxylase (DβH)-positive vesicles, an indicator of catecholaminergic projections into the PVN, was determined within the PVN using immunofluorescence and confocal microscopy.
Fig. 1.
A: timeline of experiment 1. B and C: representative fluorescent images of coronal brain sections ~1.8 mm posterior from bregma showing PVN expression of GFP and BDNFmyc, respectively. BDNFmyc expression was detected with an anti-myc tag antibody and immunofluorescence; scale bars = 100 µm. D and E: representative fluorescence images showing DβH expression in the NTS in coronal brain sections at the level of the calamus scriptorius following PBS and DSAP injections, respectively; scale bars, 100 µm. F and G: number of GFP- and myc-positive cells was assessed in subnuclei of the PVN; dorsal parvocellular nuclei (dp), medial parvocellular nuclei (mp), ventrolateral parvocellular nuclei (vlp), and posterior magnocellular nuclei (pm) following AAV2-GFP or AAV2-BDNFmyc injections in the PVN and PBS or DSAP injections in the NTS. DSAP had no significant effect on vector mediated gene transduction in GFP or BDNF rats. GFP+PBS (n = 12), GFP+DSAP (n = 14), BDNF+PBS (n = 12), BDNF+DSAP (n = 14); n refers to number of PVN sides. H: number of DβH-positive neurons in the NTS following AAV2-GFP or AAV2-BDNFmyc injections in the PVN and PBS or DSAP injections in the NTS. DSAP significantly reduced the number of DβH-positive neurons in both the GFP and BDNF groups. 3V, third ventricle; PVN, paraventricular nucleus; NTS, nucleus of the solitary tract; GFP, green fluorescent protein; PBS, phosphate-buffered saline; BDNF, brain-derived neurotrophic factor; BDNFmyc, myc epitope-tagged BDNF; DβH, dopamine β-hydroxylase; DSAP, anti-dopamine-β-hydroxylase-conjugated saporin; AAV2, adeno-associated viral vector 2. ***P < 0.001 for DSAP (1-way ANOVA).
Experiment 2.
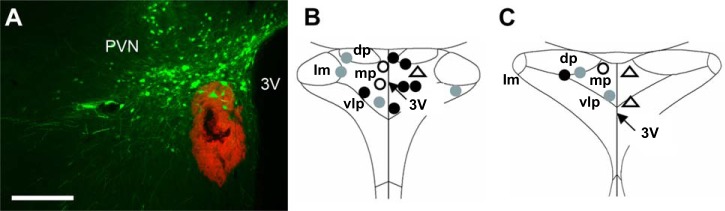
The goal of this second experiment was to determine the effect of BDNF on adrenergic receptor signaling in the PVN. Seven-week-old male SD rats received bilateral PVN injections of AAV2 viral vectors (1012 vps/mL, 200 nL/side) expressing GFP or BDNFmyc. Three weeks later, blood pressure and heart rate responses were recorded following unilateral PVN injections of isoprenaline (125 µM or 250 µM, 200 nL/side) a β-adrenergic agonist, under α-chloralose-urethane anesthesia. In addition, responses to unilateral PVN injections of phenylephrine (200 µM, 200 nL), an α1-agonist or vehicle [artificial cerebrospinal fluid (aCSF), 200 nL/side] were also recorded. At the end of the experiment, animals were deeply anesthetized and perfused transcardially with PBS and 4% paraformaldehyde. PVN expressions of GFP and BDNFmyc and drug injection sites of isoprenaline, phenylephrine, and aCSF, indicated by a red fluorescent microbead solution, were verified with immunofluorescence and fluorescent microscopy in coronal sections of the PVN (Fig. 2, A–C).
Fig. 2.
A: representative fluorescent image of a coronal brain section ~1.8 mm posterior to bregma showing PVN expression of GFP and the red fluorescent microbead solution that was mixed with injected drug solution to verify injection sites; scale bars, 250 µm. B and C: diagrams of the PVN ~1.8 mm and ~1.9 mm posterior to bregma showing locations of isoprenaline injections in rats previously injected with AAV2-GFP (gray circles) or AAV2-BDNFmyc (filled circles), and phenylephrine (open circles) and aCSF (open triangles) injections in untreated rats. 3V, third ventricle; lm, lateral magnocellular nuclei; dp, dorsal parvocellular nuclei; mp, medial parvocellular nuclei; vlp, ventrolateral parvocellular nuclei; PVN, paraventricular nucleus; GFP, green fluorescent protein; BDNF, brain-derived neurotrophic factor; BDNFmyc, myc epitope-tagged BDNF; AAV2, adeno-associated viral vector 2; aCSF, artificial cerebrospinal fluid.
Experiment 3.
The goal of the third experiment was to determine the effect of BDNF on adrenergic receptor expression in the PVN. Seven-week-old male SD rats received bilateral PVN injections of AAV2 viral vectors (1012 vps/mL, 200 nL/side) expressing GFP or BDNFmyc. Three weeks later, animals were euthanized, and the brains were quickly removed and snap-frozen. PVN and NTS tissue samples were isolated, mRNA levels of BDNF, corticotropin-releasing hormone (CRH) and α1a-, α1b-, α2a-, β1-, and β2-adrenergic receptors were determined in the PVN, and mRNA levels of tyrosine hydroxylase (TH) and DβH were determined in the NTS with quantitative real-time RT-PCR.
Surgical Procedures
Surgeries were performed using aseptic techniques under continuous isoflurane anesthesia (5% induction, 2–3% maintenance) delivered in oxygen. Depth of anesthesia was ensured by lack of a reflex response to pinch of the hindpaw. Carprofen (5 mg·kg−1·day−1 sc) was used for postsurgical analgesia administered at the beginning of surgery and for 2 days after surgery.
Telemetry transmitter implantation.
In the first experiment, radiotelemetric transducers (model HD-S10; Data Sciences International) were implanted into the descending aorta via a midline abdominal incision. The aorta was isolated and briefly occluded, and the tip of the catheter was inserted using a 21-gauge needle. Surgical glue (3M Vetbond Tissue Adhesive) and a nitrocellulose patch were applied to secure the catheter in place. The transducer was sutured to the abdominal muscle, and the incision closed in layers.
Stereotaxic viral vector and DSAP injections.
Rats were put under isoflurane anesthesia and placed in a stereotaxic frame, BDNFmyc and GFP viral vectors (1012 viral particles/mL, 200 nL/side) were injected bilaterally into the PVN using pipettes pulled from thin-walled borosilicate glass capillary tubes (OD, 1 mm; ID, 0.58 mm; tip diameter: ∼25 μm; World Precision Instruments Inc., Sarasota, FL) at the following stereotactic coordinates: 1.80 mm posterior to bregma, 1.70 mm lateral to the midline, and 7.65 mm ventral from the dorsal surface of the brain, with the micropipette tilted 10° laterally toward the midline. Virus stocks were injected over 5 min using a pneumatic pico pump (World Precision Instruments). The pipette was left in place for an additional 3 min before being withdrawn.
In experiment 1, PVN injections were combined with bilateral NTS injections of sterile PBS (for control) or DSAP (Advanced Targeting Systems, San Diego, CA), an immunotoxin that selectively lesions catecholaminergic neurons (44). The head was ventroflexed at an angle of ~30° to allow for surgical exposure of the dorsal surface of the hindbrain. The micropipette tip was lowered at the calamus scriptorius (rostral-caudal), 0.3 mm lateral from midline and 0.5 mm ventral to the surface of the medulla, and 100 nL of PBS or DSAP diluted to a concentration of 0.22 mg/mL in sterile PBS was injected over 5 min using a pneumatic pico pump. This concentration of DSAP was chosen since it has been used previously to lesion NTS catecholaminergic neurons without any significant impact on other catecholaminergic nuclei (20). The pipette was left in place for an additional 3 min before being withdrawn.
Assessment of cardiovascular responses to adrenergic receptor agonists.
In experiment 2, the left femoral artery and vein were catheterized under isoflurane anesthesia 3 wk after bilateral PVN injections of AAV2-GFP or AAV2-BDNFmyc. Rats were then placed in a stereotaxic frame, and isoflurane anesthesia was gradually switched to intravenous α-chloralose (60 mg kg−1 h−1) and urethane (800 mg kg−1 h−1) anesthesia administered through the femoral vein catheter over a 30-min period, during which, isoflurane was gradually reduced from 2.5% to 0%. Blood pressure, heart rate, toe-pinch, and eye blink reflexes were monitored closely to ensure the animal remained anesthetized. After complete withdrawal of isoflurane, anesthesia was maintained by intravenous α-chloralose (15 mg kg−1 h−1) and urethane (200 mg kg−1 h−1) infusion for the remainder of the experiment. After establishment of steady baseline blood pressure and heart rate for a minimum of 30 min, rats received unilateral PVN injections of isoprenaline hydrochloride (125 or 250 µM, 200 nL/side, Acros Organics, AC437210050), phenylephrine (200 µM, 200 nL/side; Fisher Scientific, AC207240100), or aCSF (200 nL/side). Blood pressure was monitored via the catheter placed in the left femoral artery, and heart rate was extracted from the pulsatile pressure wave by using Laboratory Chart Pro 8 (ADInstruments). To verify locations of the isoprenaline, phenylephrine, and aCSF injections in the PVN with fluorescent microscopy, 10% rhodamine-labeled fluorescent microspheres (0.04 μm; Molecular Probes) were mixed into the injection solution.
Viral Vector-Mediated Gene Transfer into the PVN and DSAP Lesioning of NTS Neurons
AAV2 viral vectors were used to elicit the expression of enhanced GFP and BDNFmyc, derived from rat Bdnf, constructed and packaged by Vector Biolabs (Philadelphia, PA). The expression of GFP and BDNFmyc was driven by a chicken β-actin promoter with human cytomegalovirus enhancer and a woodchuck posttranscriptional regulatory element, which enhanced the expression of transgenes present downstream of GFP and BDNFmyc. The BDNFmyc plasmid was a generous gift from Dr. Ronald Klein (LSU Health Sciences Center Shreveport, LA) and was used previously to protect retinal ganglion cells in a rat glaucoma model (45), and to study cardiovascular effects of BDNF in the PVN (24). In addition, full efficacy of BDNFmyc expression driven by the rat neuron-specific enolase promoter was confirmed previously both in vitro and in vivo (39).
PVN injections of AAV2-GFP and AAV2-BDNFmyc resulted in marked expressions of GFP and BDNFmyc in the PVN, as confirmed by fluorescent imaging and an antibody against the myc tag (Fig. 1, B and C). Only animals with bilateral GFP or BDNF expression in the PVN were included in the study, and analysis of the number of GFP- or myc-positive cells in the subnuclei of the PVN indicated that DSAP treatment in the NTS had no effect on GFP or BDNFmyc expression in the PVN (Fig. 1, F and G). In addition, analysis of DβH-positive neurons in the NTS indicated a significant DSAP-mediated reduction of catecholaminergic neurons in the NTS in both the GFP and BDNF groups, and vector treatment had no effect on the efficacy of DSAP lesioning (Fig. 1H).
Analysis of Resting Radiotelemetric Cardiovascular Data
Blood pressure, heart rate, and physical activity of the animals were analyzed with Dataquest A.R.T. Analysis software (Data Sciences International). Data were recorded every 10 min for 15 s and averaged between 8:00 AM and 4:00 PM to calculate daytime values and between 8:00 PM and 4:00 AM to calculate nighttime values for each animal. Spontaneous baroreflex sensitivity was calculated with the sequence technique with the freely available HemoLab software (http://www.haraldstauss.com/HaraldStaussScientific/hemolab/), as previously described (5). Blood pressure was recorded continuously at a sampling rate of 500 Hz for a minimum of 2 h between 8:00 AM and 10:00 PM before water stress experiments (3 wk after vector injections). Sampling rate of the data sets was first increased to 1,500 Hz with spline interpolation. Then, sequences defined as a minimum of three consecutive (beat by beat) increases or decreases in systolic blood pressure accompanied by likewise increases or decreases in the pulse interval, were identified. Sequences with increases in systolic blood pressure (“up sequences”) and decreases in systolic blood pressure (“down sequences”) were pooled. No time delay and no thresholds for changes in systolic blood pressure or pulse interval were used. However, only sequences with a correlation coefficient (R) for the linear correlation between systolic blood pressure and pulse interval of >0.8 were included in the analysis, and the slope of the linear correlation was taken as the gain of the baroreceptor-pulse interval reflex (5, 42).
Acute-Stress Procedures
Acute stress procedures were performed between 10:00 AM and 12:00 PM and were started after obtaining baseline blood pressure and heart rate recordings for a minimum of 30 min. For water stress, rats were placed in standard rat cages filled with 1-cm-deep water (room temperature, ∼25°C) for 15 min. Restraint stress was performed by placing animals in cylindrical plastic restrainers for 60 min. After the animals were returned to their home cages, blood pressure and heart rate were recorded for an additional 60-min recovery period. Blood pressure and heart rate data were exported with 3-min moving average from continuously recorded blood pressure data, and baseline values were calculated by averaging the baseline period after physical activity-related peaks from blood pressure and heart rate data sets were removed. Average blood pressure and heart rate changes from baseline (during stress and recovery periods), as well as amplitude and time delay of peak responses, were calculated.
Analysis of Cardiovascular Data from Anesthetized Animals
Blood pressure and heart rate were recorded using LabChart software version 8.0.7 (ADInstruments, Dunedin, NZ) at a 1,000-Hz sampling rate and condensed to 10-s moving averages for data analysis and presentation. Maximum and average changes in MAP and heart rate following aCSF, isoprenaline, and phenylephrine microinjections were evaluated.
Immunofluorescence
After experiments 1 and 2, animals were perfused with 400 mL of ice-cold PBS followed by 400 mL of ice-cold 4% paraformaldehyde in PBS. Brains were removed and post-fixed for 2 h in 4% paraformaldehyde and then equilibrated in 30% sucrose solution at 4°C. Coronal sections (40 µm) were cut on a microtome (Leica SM2000R) and mounted on Fisher Superfrost Plus slides. BDNFmyc and DβH were detected using the following primary antibodies: anti-c-Myc (Santa Cruz Biotechnology; 9E10, 1:200, overnight incubation at 4°C) and anti-DβH primary antibody (Millipore, Billerica, MA; 1:1,000, overnight incubation at 4°C). Secondary antibodies were donkey anti-mouse AF546 (Invitrogen; 1:200, 2-h incubation at room temperature) and donkey anti-rabbit A555 (Invitrogen; 1:200, 2-h incubation at room temperature). GFP, BDNFmyc, and DβH immunofluorescences in the PVN and NTS were detected with a fluorescent microscope (Nikon Eclipse 50i).
Confocal Microscopy and Image Analysis
PVN brain sections from experiment 1 were used to determine the effect of BDNF overexpression on volumetric density of DβH-positive vesicles located on axon terminals in the PVN. Z-stack images of PVN brain slices labeled with immunofluorescence as described above were taken using a Zeiss LSM 510 Meta confocal laser-scanning imaging system. The z-stacks were then deconvoluted using the deconvolution software AutoQuant X3 (Media Cybernetics, Inc.). Volumetric density of DβH-positive vesicles within the PVN was then calculated with the custom-written software Volumetry G7 (Grant Hennig, Dept. of Pharmacology Univ of Vermont). DβH immunoreactivity is limited to vesicles in the PVN, which appear as distinct points in the image. To precisely capture those points in the PVN, each image was thresholded such that all vesicles were captured and any background fluorescence was removed. DβH percent density was quantified in each slice of the z-stack of each side of the PVN. Then, the z-stack slice with the highest percent density was selected on each side of the PVN in each group and the mean percent density calculated for each experimental group.
Real-Time RT-PCR
Frozen forebrains and hindbrains were mounted on a cryostat, and rostral coronal sections were cut until reaching the level ~1.5 mm posterior of bregma for the PVN and ~14.1 mm posterior of bregma for the NTS. Then, tissue samples 0.75 mm in diameter and 0.5 mm deep were punched bilaterally from the region of the PVN or NTS under a microscope, with the third ventricle used as reference for the PVN and the midline and dorsal brain stem surface for the NTS. The depth of the punches was limited by a spacer glued onto the outside of the punch tool. Total RNA was extracted from brain punches with Qiashredder columns (Qiagen, Valencia, CA) and the RNeasy Micro Kit (Qiagen). Samples were treated with an RNase-free DNase Set (Qiagen) on the column to remove genomic DNA. RNA was quantified with a Qubit Fluorometer, and cDNA was synthesized with a high-capacity cDNA reverse transcription kit (Applied Biosystems) according to manufacturer’s instructions and stored at −20°C. All quantitative RT-PCR reactions were run in duplicate in eight-well optical-grade strips in a Prism 7000 Sequence Detection System (Applied Biosystems) and quantified with the cycle threshold (CT) method. The mRNA levels of BDNF and CRH, which are known to be elevated by BDNF (37), adrenergic receptors, and the reference genes β-actin (ActB) and 18S were analyzed with quantitative real-time RT-PCR using specific oligonucleotide primers and TaqMan probes (Applied Biosystems, Foster City, CA): BDNF, Rn02531967_s1; CRH, Rn01462137_m1; ActB, Rn00667869_m1; α1a, RN00567876_m1; α1b, RN01471343_m1; α2a, RN00562488_s1; β1, RN008244536_s1; β2, RN00560650_s1; 18S, Hs03003631_g1; DβH, Rn00565819_m1; and TH, Rn00562500_m1). Control reactions containing no template were run for each plate, and results were analyzed with the 2−ΔΔCT method.
Statistics
Baseline (day/night) telemetry data were analyzed using two-way repeated-measures ANOVA with Tukey's post hoc test. Peak and average changes in MAP and heart rate during acute stress and microinjection experiments, as well as mean DβH percent volumetric density, were analyzed by one-way ANOVA with Bonferroni’s multiple comparisons test. RT-PCR data were analyzed by unpaired t test. Statistical tests were performed using Prism 7.0 software (GraphPad, San Diego, CA). Results are expressed as means ± SE, and the criterion for statistical significance was P < 0.05.
RESULTS
Effects of BDNF and DSAP Treatments
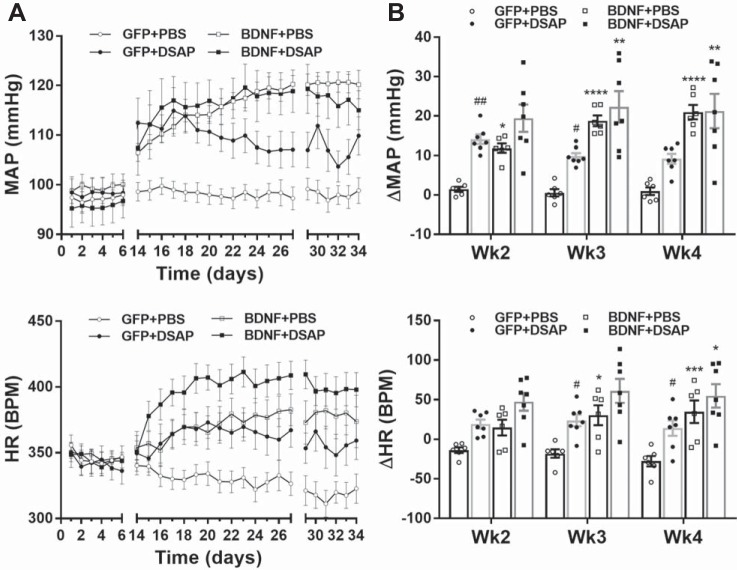
Results from our first experiment confirmed our previous findings (24) and showed that BDNF overexpression in the PVN without DSAP lesioning in the NTS significantly increased MAP and heart rate during both day- and nighttime compared with the GFP group (Fig. 3 and Table 1). In addition, we found that in GFP animals DSAP lesioning of NTS catecholaminergic neurons significantly increased MAP both during day- and nighttime, whereas DSAP lesioning in BDNF animals had no further effect on blood pressure (Fig. 3 and Table 1). Heart rate was significantly elevated by DSAP treatment in the GFP group during week 3 and week 4 both day- and nighttime, and while it tended to increase heart rate in the BDNF animals, the difference between BDNF+PBS and BDNF+DSAP groups was not statistically significant (Fig. 3 and Table 1). Furthermore, BDNF overexpression reduced spontaneous baroreflex sensitivity in rats receiving PBS injections into the NTS (P < 0.05), and DSAP reduced baroreflex sensitivity in GFP rats (P < 0.05). However, DSAP failed to have an effect in the BDNF group (P = 0.99; Table 1).
Fig. 3.
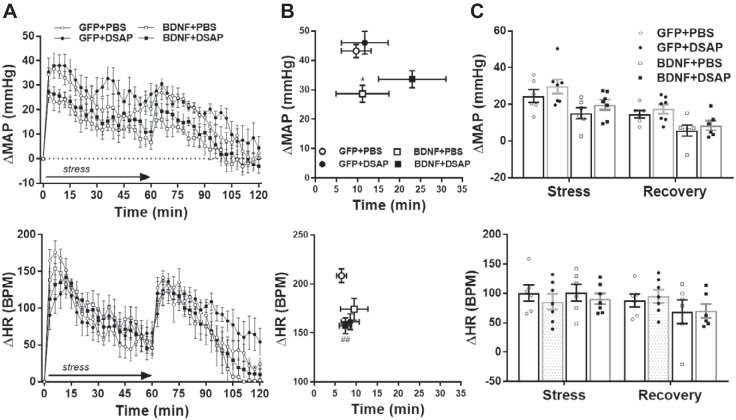
Radiotelemetric recordings of daytime mean arterial pressure (MAP) and heart rate [HR, in beats/min (bpm)]. Radiotelemetric parameters were recorded for 6 days before brain injections and during weeks 2–4 after brain injections. Recording was turned off during the postsurgical recovery phase (between days 6 and 14), and data from day 28 are omitted because animals were subjected to water stress on that day. Since viral vector and DSAP treatments had similar effects on daytime and nighttime parameters, only daytime parameters are shown (nighttime data are summarized in Table 1). A: daytime MAP (top) and HR (bottom) in GFP+PBS (n = 6), GFP+DSAP (n = 7), BDNF+PBS (n = 6), and BDNF+DSAP (n = 7) rats. B: average changes in daytime MAP (top) and HR (bottom) during weeks 2, 3, and 4 from pretreatment baseline period. PVN, paraventricular nucleus; NTS, nucleus of the solitary tract; GFP, green fluorescent protein; PBS, phosphate-buffered saline; BDNF, brain-derived neurotrophic factor; DSAP, anti-dopamine-β-hydroxylase-conjugated saporin. Results represent means ± SE. Two-way repeated-measures ANOVA on weekly averages indicated significant treatment effect for MAP and HR (P < 0.001), significant time effect for HR (P < 0.05), and significant treatment × time interaction for both MAP and HR (P < 0.01). Post hoc analysis indicated *P < 0.05 for GFP+PBS vs. BDNF+PBS, **P < 0.01 for GFP+DSAP vs. BDNF+DSAP, ***P < 0.001 for GFP+PBS vs. BDNF+PBS, ****P < 0.0001 for GFP+PBS vs. BDNF+PBS; #P < 0.05 for GFP+PBS vs. GFP+DSAP, ##P < 0.01 for GFP+PBS vs. GFP+DSAP.
Table 1.
Radiotelemetric parameters
| MAP, mmHg |
HR, beats/min | DIA, mmHg | SYS, mmHg | ACT, AU |
sBRS, ms/mmHg | |
|---|---|---|---|---|---|---|
| GFP+PBS-D-Wk0 | 97.2 ± 1.8 | 346 ± 8.6 | 79.2 ± 1.0 | 120.1 ± 2.9 | 1.01 ± 0.14 | |
| GFP+DSAP-D-Wk0 | 98.3 ± 2.8 | 342 ± 8.8 | 80.7 ± 2.5 | 120.9 ± 3.3 | 0.88 ± 0.10 | |
| BDNF+PBS-D-Wk0 | 99.5 ± 2.3 | 347 ± 3.2 | 81.7 ± 1.7 | 122.0 ± 3.1 | 0.90 ± 0.09 | |
| BDNF+DSAP-D-Wk0 | 95.7 ± 3.5 | 344 ± 7.4 | 78.1 ± 3.2 | 117.9 ± 3.8 | 0.93 ± 0.06 | |
| GFP+PBS-N-Wk0 | 103.8 ± 1.4 | 400 ± 8.5 | 85.7 ± 0.9 | 127.0 ± 2.5 | 3.74 ± 0.61 | |
| GFP+DSAP-N-Wk0 | 104.8 ± 3.0 | 398 ± 8.6 | 87.6 ± 2.6 | 127.6 ± 3.7 | 3.67 ± 0.38 | |
| BDNF+PBS-N-Wk0 | 105.0 ± 2.6 | 411 ± 4.7 | 87.6 ± 2.0 | 127.2 ± 3.5 | 3.12 ± 0.24 | |
| BDNF+DSAP-N-Wk0 | 101.2 ± 3.5 | 396 ± 7.6 | 83.7 ± 3.3 | 123.8 ± 3.6 | 3.39 ± 0.20 | |
| GFP+PBS-D-Wk3 | 97.8 ± 1.9 | 328 ± 8.6 | 80.1 ± 1.5 | 121.2 ± 2.7 | 0.77 ± 0.12 | 1.63 ± 0.15 |
| GFP+DSAP-D-Wk3 | 108.1 ± 3.2† | 366 ± 12† | 87.2 ± 2.8 | 134.7 ± 3.9 | 0.93 ± 0.08 | 1.16 ± 0.08† |
| BDNF+PBS-D-Wk3 | 118.2 ± 1.8*** | 378 ± 12* | 97.5 ± 1.6 | 145.9 ± 2.2 | 1.24 ± 0.15** | 1.12 ± 0.14* |
| BDNF+DSAP-D-Wk3 | 118.4 ± 4.3 | 406 ± 11 | 96.4 ± 4.3 | 145.2 ± 4.2 | 1.46 ± 0.12 | 1.06 ± 0.11 |
| GFP+PBS-N-Wk3 | 105.3 ± 1.4 | 383 ± 7.9 | 87.0 ± 1.5 | 129.4 ± 2.0 | 3.21 ± 0.50 | |
| GFP+DSAP-N-Wk3 | 116.2 ± 3.3† | 433 ± 13† | 95.0 ± 3.0 | 143.9 ± 4.0 | 3.30 ± 0.13 | |
| BDNF+PBS-N-Wk3 | 124.6 ± 1.4*** | 450 ± 13** | 103.0 ± 1.2 | 153.2 ± 2.0 | 3.56 ± 0.28 | |
| BDNF+DSAP-N-Wk3 | 125.2 ± 5.3 | 476 ± 13 | 102.5 ± 5.2 | 153.9 ± 5.4 | 3.82 ± 0.32 |
Values are means ± SE. HR, heart rate; DIA, diastolic pressure; SYS, systolic pressure; ACT, activity; sBRS, spontaneous baroreflex sensitivity; AU, arbitrary units; BDNF, brain-derived neurotrophic factor; DSAP, anti-dopamine-β-hydroxylase-conjugated saporin; GFP, green fluorescent protein; PBS, phosphate-buffered saline; D, daytime; N, nighttime; week 0 values represent average of parameters measured during the week preceding vector injections; week 3 values represent average of parameters measured during week 3 after vector injections. GFP+PBS, n = 6; GFP+DSAP, n = 7; BDNF+PBS, n = 6; BDNF+DSAP, n = 7.
P < 0.05 GFP+PBS vs. GFP+DSAP;
P < 0.05 GFP+PBS vs. BDNF+PBS;
P < 0.01 GFP+PBS vs. BDNF+PBS;
P < 0.001 GFP+PBS vs. BDNF+PBS (2-way repeated-measures ANOVA for MAP and HR, and 1-way ANOVA for sBRS).
In addition to its effects on cardiovascular function, BDNF overexpression also significantly reduced body weight gain, in agreement with our previous study (24). At baseline, the body weight of the GFP+PBS group was 428 ± 18 g, and of the BDNF+PBS group was 414 ± 11 g. At the end of the experiment, the GFP+PBS group was 554 ± 26 g and the BDNF+PBS group was 445 ± 17 g (P < 0.05). In contrast, DSAP lesioning of NTS catecholaminergic neurons had no effect on body weight gain in either GFP or BDNF rats. At baseline, the body weight of the GFP+DSAP group was 420 ± 12 g, and final body weight was 566 ± 12 g, compared with the BDNF+DSAP group, whose body weight at baseline was 411 ± 10 g, and final body weight was 448 ± 15 g. BDNF overexpression was also found to increase daytime locomotor activity at the end of week 3 compared with the GFP group (P < 0.01; Table 1).
Relative volumetric density of DβH-positive vesicles was quantified in PVN brain sections as an indicator of catecholaminergic projections to the PVN. DSAP treatment in GFP rats tended to reduce DβH-positive vesicle density in the PVN compared with the GFP+PBS group (P = 0.06), and it significantly reduced DβH-positive vesicle density compared with the BDNF+DSAP group (P < 0.05). No significant difference was seen in the BDNF+DSAP group compared with the BDNF+PBS group. Additionally, BDNF alone did not significantly alter DβH-positive vesicle density in the PVN compared with the GFP+PBS group (Fig. 4).
Fig. 4.
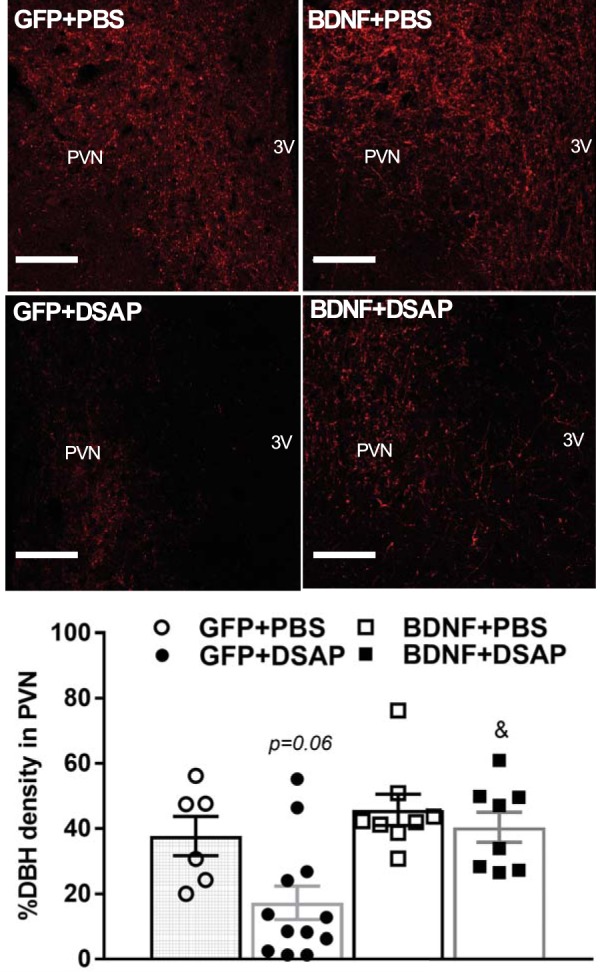
DβH-positive vesicles in the PVN of rats previously injected with AAV2-GFP or AAV2-BDNFmyc in the PVN and with PBS or DSAP in the NTS. Top: representative confocal images of DβH expression within the PVN in all experimental groups as detected with an anti-DβH antibody and immunofluorescence; scale bars, 100 µm. Bottom: maximum percent volumetric density of DβH-positive vesicles within the PVN, averaged for each experimental group; n refers to number of PVN sides [GFP+PBS (n = 6), GFP+DSAP (n = 12), BDNF+PBS (n = 8), BDNF+DSAP (n = 8)]. 3V, third ventricle; PVN, paraventricular nucleus; NTS, nucleus of the solitary tract; GFP, green fluorescent protein; PBS, phosphate-buffered saline; BDNF, brain-derived neurotrophic factor; DBH, dopamine β-hydroxylase; DSAP, anti-dopamine-β-hydroxylase-conjugated saporin; AAV2, adeno-associated viral vector 2. Results represent means ± SE. P = 0.06 for GFP+PBS vs. GFP+DSAP; &P < 0.05 for GFP+DSAP vs. BDNF+DSAP (1-way ANOVA).
Acute Water and Restraint Stress
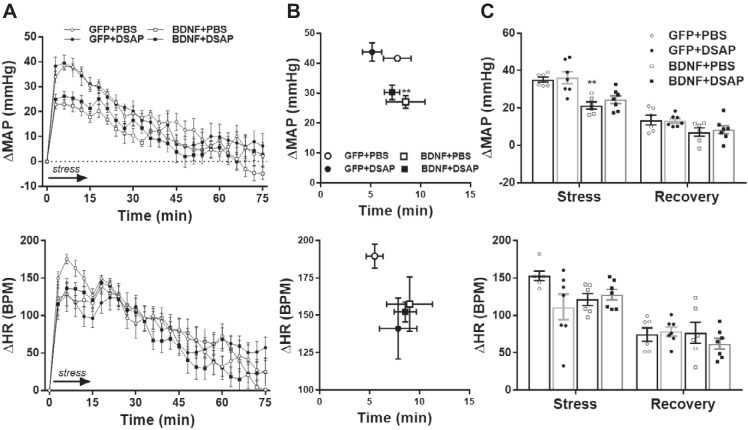
Water stress-induced peak pressor responses and average MAP increase during stress were significantly reduced in the BDNF+PBS group compared with the GFP+PBS group (P < 0.01), whereas amplitude of heart rate increases to water stress was unaffected by BDNF overexpression. DSAP treatment did not significantly alter the amplitude of pressor and tachycardic responses in either GFP or BDNF rats, and timings of peak MAP and heart rate responses were also similar in all four experimental groups (Fig. 5).
Fig. 5.
Radiotelemetric recordings of mean arterial pressure (MAP) and heart rate (HR) during acute water stress and poststress recovery in GFP+PBS (n = 6), GFP+DSAP (n = 7), BDNF+PBS (n = 6) and BDNF+DSAP (n = 7) rats. A: MAP (top) and HR (bottom) traces with 3-min moving average during stress (15 min, indicated by arrow) and during poststress recovery (60 min). B: amplitude and delay of peak MAP and HR responses. C: MAP and HR increases averaged during stress and poststress recovery. Prestress MAP and HR were 94 ± 3 mmHg and 308 ± 11 beats/min (bpm) in the GFP+PBS group, 101 ± 4 mmHg and 327 ± 9 beats/min in the GFP+DSAP group, 121 ± 4 mmHg and 366 ± 19 beats/min in the BDNF+PBS group, and 113 ± 5 mmHg and 378 ± 11 beats/min in the BDNF+DSAP group. GFP, green fluorescent protein; PBS, phosphate-buffered saline; BDNF, brain-derived neurotrophic factor; DSAP, anti-dopamine-β-hydroxylase-conjugated saporin. Results represent means ± SE. **P < 0.01 for GFP+PBS vs. BDNF+PBS (1-way ANOVA).
Restraint stress-induced peak pressor responses were significantly reduced in the BDNF+PBS group compared with the GFP+PBS group (P < 0.05), whereas amplitude of heart rate increases to restraint stress was unaffected by BDNF overexpression. In contrast, DSAP treatment significantly reduced maximum heart rate increase in the GFP group (P < 0.01) but not in the BDNF group. DSAP treatment did not alter the amplitude of pressor responses in either GFP or BDNF rats, and timings of peak MAP and heart rate responses were also similar in all four experimental groups (Fig. 6).
Fig. 6.
Radiotelemetric recordings of mean arterial pressure (MAP) and heart rate (HR) during acute restraint stress and poststress recovery in GFP+PBS (n = 6), GFP+DSAP (n = 7), BDNF+PBS (n = 6), and BDNF+DSAP (n = 7) rats. A: MAP (top) and HR (bottom) traces with 3-min moving average during stress (60 min, indicated by arrow) and during poststress recovery (60 min). B: amplitude and delay of peak MAP and HR responses. C: MAP and HR increases averaged during stress and poststress recovery. Prestress MAP and HR were 92 ± 3 mmHg and 311 ± 11 beats/min (bpm) in the GFP+PBS group, 101 ± 4 mmHg and 351 ± 14 beats/min in the GFP+DSAP group, 120 ± 2 mmHg and 372 ± 18 beats/min in the BDNF+PBS group, and 114 ± 3 mmHg and 380 ± 13 beats/min in the BDNF+DSAP group. GFP, green fluorescent protein; PBS, phosphate-buffered saline; BDNF, brain-derived neurotrophic factor; DSAP, anti-dopamine-β-hydroxylase-conjugated saporin. Results are represented as means ± SE. *P < 0.05, for GFP+PBS vs. BDNF+PBS, ##P < 0.01, GFP+PBS vs. GFP+DSAP (1-way ANOVA).
Effect of BDNF on Adrenergic Receptor Signaling in the PVN
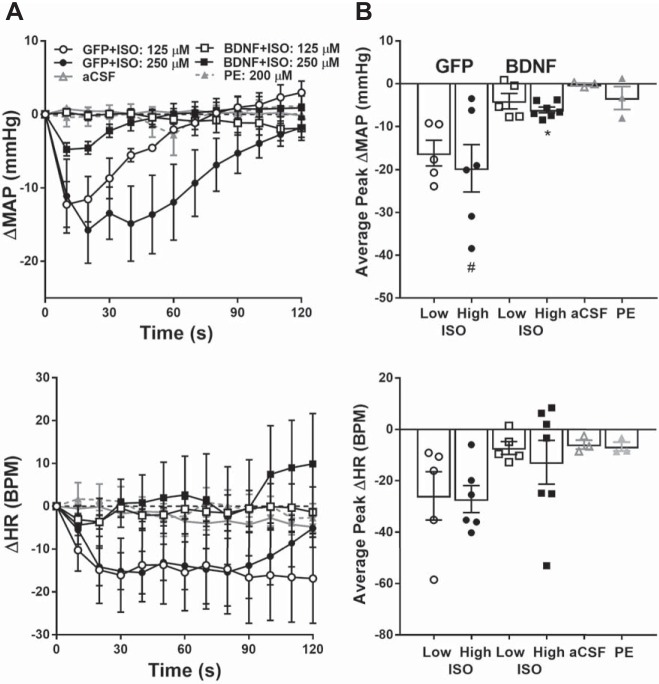
In our second experiment, we tested whether BDNF affects PVN adrenergic receptor-mediated cardiovascular responses. We found that stimulation of β-adrenergic receptors with isoprenaline dose-dependently lowered MAP in GFP rats and that BDNF overexpression in the PVN almost completely abolished these hypotensive responses to β-adrenergic receptor stimulation (P < 0.05; Fig. 7). Heart rate tended to decline in the GFP group in response to isoprenaline, but its effects were not statistically significant in any of the experimental groups (GFP+ISO high dose vs. aCSF, P = 0.24; GFP+ISO low dose vs. aCSF, P = 0.32). In contrast with isoprenaline, activation of α-adrenergic receptors in the PVN by phenylephrine injections did not significantly alter either MAP (P = 0.99) or heart rate (P = 0.99), and injection of aCSF had no effect on cardiovascular parameters either (Fig. 7).
Fig. 7.
Changes in mean arterial pressure (MAP; top) and heart rate (HR; bottom) in response to PVN injections of adrenergic receptor agonists and vehicle in anesthetized rats previously treated with AAV2-GFP (n = 6) or AAV2-BDNFmyc (n = 7). A: MAP and HR obtained with 10-s moving average over 120 s following isoprenaline (ISO) injection in GFP and BDNFmyc treated rats, and phenylephrine (PE; n = 3) or aCSF, artificial cerebrospinal fluid (aCSF; n = 3) injections in untreated rats. B: average peak MAP and HR responses to ISO, PE, and aCSF injections. Baseline MAP and HR were 89 ± 6 mmHg and 351 ± 15 beats/min (bpm) in GFP and 108 ± 4 mmHg and 398 ± 15 beats/min in BDNF rats, and 105 ± 3 mmHg and 353 ± 7 beats/min in untreated rats. PVN, paraventricular nucleus; GFP, green fluorescent protein; BDNF, brain-derived neurotrophic factor; BDNFmyc, myc epitope-tagged BDNF; AAV2, adeno-associated viral vector 2. Results represent means ± SE. Statistical analysis was done on peak changes in MAP and HR. #P < 0.05 for 250 µM ISO vs. aCSF, *P < 0.05, for GFP vs. BDNF (1-way ANOVA).
Expression of Components of Catecholaminergic Signaling
In our third experiment, we tested the effect of BDNF upregulation in the PVN on expressions of catecholamine biosynthesizing enzymes in the NTS and adrenergic receptors in the PVN.
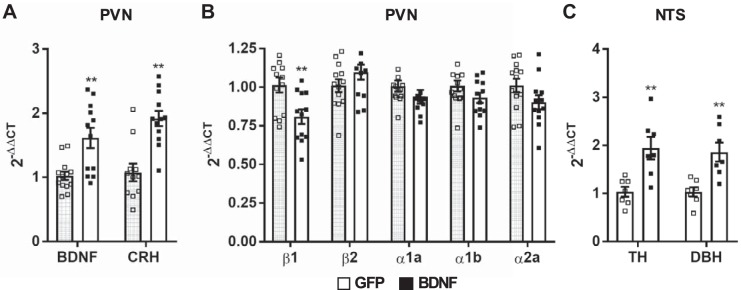
To verify vector mediated BDNF transduction and activation of BDNF signaling pathways in the PVN, BDNF and CRH mRNA expressions were determined, and both were found to be significantly elevated in BDNF rats compared with GFP rats (P < 0.01; Fig. 8A). Analysis of adrenergic receptor expression showed that β1-adrenergic receptor mRNA was significantly downregulated in the PVN of BDNF rats compared with GFP (P < 0.01), whereas expressions of α1a-, α1b-, α2a-, and β2-adrenergic receptors were unaffected by BDNF (Fig. 8B). Additionally, BDNF overexpression in the PVN also significantly upregulated TH (P < 0.01) and DβH (P < 0.01) mRNA expression in the NTS (Fig. 8C).
Fig. 8.
Expression of BDNF, CRH, and adrenergic receptor mRNA in the PVN, and TH and DβH mRNA in the NTS of rats previously injected with AAV2-GFP (n = 6) or AAV2-BDNFmyc (n = 6) in the PVN. A: significant increases in BDNF and CRH mRNA expressions verify successful vector mediated BDNF transduction and activation of BDNF-mediated signaling mechanisms. B: expressions of α1a-, α1b-, and α2a- and β1- and β2-adrenergic receptor mRNAs in GFP and BDNF rats. C: expressions of TH and DβH mRNAs in the NTS of AAV2-GFP (n = 7) and AAV2-BDNFmyc (n = 7) rats. PVN, paraventricular nucleus; NTS, nucleus of the solitary tract; GFP, green fluorescent protein; BDNF, brain-derived neurotrophic factor; BDNFmyc, myc epitope-tagged BDNF; TH, tyrosine hydroxylase; DBH, dopamine β-hydroxylase; AAV2, adeno-associated viral vector 2; CRH, corticotropin-releasing hormone. Results are expressed as 2−ΔΔCT (where CT is threshold cycle) normalized to the control group and presented as means ± SE. **P < 0.01 GFP vs. BDNF (unpaired t test).
DISCUSSION
Mounting evidence suggest that BDNF, acting in the PVN, is a key regulator of blood pressure and plays a major role in eliciting cardiovascular responses to stress and other hypertensive stimuli (1, 24, 30, 57, 66, 67, 69). Results from this study further emphasize the hypertensive actions of BDNF within the PVN and demonstrate that a potential mechanism mediating these effects is a downregulation of inhibitory/hypotensive input from NTS catecholaminergic neurons via β-adrenergic receptor signaling. This is supported by our findings that selective lesioning of NTS catecholaminergic neurons increased blood pressure in control (AAV2-GFP-treated) animals but failed to exert a hypertensive effect in animals that were subjected to BDNF overexpression in the PVN. Additionally, activating β-adrenergic receptors in the PVN markedly decreased blood pressure in control, AAV2-GFP-treated rats, whereas β-adrenergic receptor stimulation had a significantly reduced hypotensive effect after BDNF overexpression in the PVN. Diminished β-adrenergic receptor signaling was at least partially due to a downregulation of β1-adrenergic receptor mRNA expression in the PVN, whereas expression of other adrenergic receptors was unaffected by BDNF. Interestingly, we observed a BDNF-induced upregulation of catecholamine biosynthetic enzymes in the NTS and recruitment of catecholaminergic projections to the PVN in DSAP-treated animals, but it seems these compensatory mechanisms were unable to overcome reduced responsiveness of the PVN to these inputs.
BDNF, a member of the neurotrophin family, is a widely recognized regulator of neuronal function throughout the central nervous system (33, 46, 53), and previous reports have indicated that BDNF is involved in blood pressure control in several cardiovascular regulatory nuclei. Our previous studies have shown that both acute injection and chronic overexpression of BDNF in the PVN elevates blood pressure, heart rate, and sympathetic activity (24, 67), and BDNF expression has been found to be significantly elevated in the PVN in response to several hypertensive stimuli such as acute and chronic stress (28, 30, 57, 69), hyperosmolality (1), and repeated amphetamine administration (49). In addition, BDNF is an important modulator of stress-related neuroendocrine mechanisms such as CRH synthesis and activation of the HPA axis (28, 37). BDNF signaling within the supraoptic nucleus has also been shown to reduce GABAA-mediated inhibition of AVP neurons and to increase blood pressure in response to high salt intake in rats (13). In addition, BDNF microinjections into the RVLM and the medial NTS have also been found to elevate blood pressure (14, 83), and BDNF modulates baroreflex sensitivity in the NTS (3). Thus, BDNF seems to be a key regulator of many different aspects of cardiovascular regulation within both the hypothalamus and the brain stem. However, the downstream mechanisms mediating these cardiovascular actions of BDNF are not fully understood.
In this study, we investigated the potential modulatory role of BDNF on NTS-PVN catecholaminergic projections, since these neurons play a significant role in blood pressure regulation (20, 22, 35) and because BDNF is known to influence catecholaminergic signaling throughout the central nervous system (9, 60, 68). NTS A2 and C2 neurons provide the primary catecholaminergic input to the PVN (16, 65). However, the role of these NTS-PVN projections in cardiovascular regulation is not clear. For example, selective lesioning of NTS catecholaminergic neurons in rats led to an increase in blood pressure in some cases (20, 22, 35, indicating that these neurons exert an inhibitory/hypotensive role, but other studies indicated no effect on baseline blood pressure (34, 36, 75). Stimulation of adrenergic receptors in the PVN has also led to conflicting results. For example, PVN microinjections of the β-adrenergic receptor agonist fenoterol were shown to reduce blood pressure in Wistar-Kyoto (WKY) rats and spontaneously hypertensive rats but had no effect in Wistar rats (78). However, other studies found that microinjections of the β-adrenergic receptor agonist isoprenaline in WKY and SD rats had no effect on blood pressure (48, 82) but did increase baroreflex sensitivity in SD rats (82). On the other hand, PVN injections of the α1-adrenergic receptor agonist phenylephrine or the nonselective α-adrenergic receptor antagonist phentolamine significantly increased renal sympathetic nerve activity and heart rate without an effect on blood pressure (10, 48). Our experiments demonstrated that, in control GFP animals, selective catecholaminergic lesioning in the NTS induced a marked increase in blood pressure, whereas stimulation of β-adrenergic receptors in the PVN resulted in significant hypotensive responses. These findings support the idea that under baseline conditions in normotensive animals these catecholaminergic neurons exert an inhibitory role on hypertensive mechanisms and that these actions are mediated, at least in part, by β-receptor signaling in the PVN. However, in contrast to control GFP rats, DSAP lesioning in the NTS of BDNF rats did not significantly affect blood pressure. This finding is especially surprising, since catecholamine biosynthesizing enzymes TH and DβH were markedly upregulated in the NTS of BDNF rats, indicating an elevated activity of these catecholaminergic neurons. We propose that a plausible explanation for these results is that elevated BDNF levels in the PVN diminished sensitivity of PVN neurons to β-adrenergic receptor-mediated hypotensive input from the NTS. Thus, despite a compensatory increase in catecholamine biosynthesis in NTS neurons, DSAP lesioning had no further effect on the already elevated baseline blood pressure. This hypothesis is also supported by our findings that hypotensive responses to PVN injections of isoprenaline were significantly diminished in BDNF rats compared with GFP controls.
Reduced β-adrenergic signaling following overexpression of BDNF in the PVN can be partially explained by downregulation of β1-adrenergic receptor mRNA expression in the PVN as indicated by our results, but other mechanisms may also be involved. For example, BDNF increases the expression and release of AVP in the hypothalamus (1, 13, 80), and AVP can stimulate the recruitment of β-arrestins, which can inhibit β-adrenergic receptor signaling and enhance endocytosis of β-receptors (40, 50). Another potential mechanism of BDNF-mediated downregulation of β-receptor signaling may involve inhibition of G protein-coupled inwardly rectifying potassium (GIRK) channels. GIRK1–4 have been shown to be expressed in the PVN (38, 62), and a subset of PVN parvocellular neurons respond to β-adrenergic activation by GIRK channel-mediated hyperpolarization and inhibition (17). BDNF, on the other hand, has been shown to strongly inhibit the activity of GIRK1 and GIRK4 via activation of TrkB receptors (61), thus, BDNF may oppose β-adrenergic inhibition of PVN neurons by blocking β-receptor-mediated stimulation of GIRK channels. However, future studies are needed to address specifically how elevated BDNF expression alters β-receptor signaling in the PVN.
In addition to diminishing β-adrenergic signaling in the PVN, BDNF overexpression also increased the expression of catecholamine biosynthetic enzymes in the NTS and prevented DSAP-induced reductions in the density of DβH-positive vesicles in the PVN. The upregulation of TH and DβH in the NTS of BDNF animals may be an indirect response caused by increased blood pressure and baroreflex-mediated activation of NTS neurons, but it could also be caused by retrograde actions of BDNF on NTS-PVN neurons. Retrograde actions of BDNF are well documented in the central nervous system (64, 70), and BDNF is known to localize to noradrenergic nerve fibers and terminals (26), regulate the development of noradrenergic neurons (32), and stimulate TH gene expression (27, 87). Thus, BDNF released from PVN neurons could retrogradely enhance catecholamine biosynthesis in NTS neurons. Retrograde BDNF-mediated signaling may also be responsible for recruiting catecholaminergic projections from other nuclei after lesioning NTS A2/C2 neurons with DSAP. Such a mechanism could explain why DβH vesicle density in the PVN remained unaffected by DSAP lesioning of the NTS in BDNF rats but decreased in the GFP group despite similar reductions in NTS DβH-positive neuron number in both groups. However, the present study demonstrated an increase in TH and DβH mRNA only in the NTS and DβH percent volumetric vesicle density in the PVN, but changes in NTS neuronal activity or NTS TH, DβH protein expression were not measured. Future studies will be needed to determine the existence and function of these compensatory mechanisms.
BDNF and catecholaminergic signaling both contribute to neuroendocrine stress responses and baroreflex regulation (3, 28, 37, 82). Here, in correlation with our previous study (24), we found that BDNF overexpression in the PVN reduced MAP increases to both acute water and restraint stress without an effect on heart rate responses. We (66) have also shown previously that selective inhibition of TrkB signaling in the PVN reduces MAP elevations to acute stressors. These findings combined clearly demonstrate that upregulation of BDNF expression in the PVN (24, 66, 67) and subsequent activation of TrkB signaling cascades are required for eliciting blood pressure elevations to stress. Thus, both inhibition of TrkB receptors (66) and masking the stress-induced BDNF upregulation in the PVN by vector-mediated constitutive BDNF overexpression diminish stress-induced hypertensive responses. However, whereas TrkB inhibition fails to affect baseline blood pressure (66), vector-mediated BDNF overexpression increases it. One important mechanism mediating these actions of BDNF could be its ability to interact with angiotensin II signaling. Angiotensin II is known to play a role in mediating cardiovascular stress responses (7), and overexpression of BDNF in the PVN has been shown to upregulate the expression of AT1R (24). In addition, BDNF-mediated blood pressure increases can be partially attenuated by AT1R antagonists or by an angiotensin-converting enzyme inhibitor (24, 67). On the other hand, angiotensin II can also upregulate BDNF and TrkB receptor expression in cultured catecholaminergic cells, leading to a reduction in voltage-gated potassium currents and increases in neuronal excitability (4). Similarly, in chronic heart failure, elevated angiotensin II contributes to increased sympathoexcitation (88) and can increase neuronal excitability, in part by reducing voltage-gated potassium currents via BDNF signaling (73, 74, 89).
NTS catecholaminergic neurons are also involved in the regulation of acute and chronic stress responses. They can be activated in response to physiological and psychological stressors (21, 52) and may play a role in altering cardiovascular responses to stress via projections to the PVN or other forebrain regions (16, 58). In the current study, DSAP lesioning of the NTS in the GFP group was found to reduce heart rate responses to restraint stress but not to water stress and failed to affect blood pressure responses to either stressor. Furthermore, DSAP treatment had no effect on heart rate and blood pressure in the BDNF group during either stress paradigm. Thus, our results suggest a limited role of catecholaminergic NTS neurons in the regulation of acute cardiovascular stress responses regardless of the level of BDNF expression in the PVN.
The PVN is known to modulate baroreflex-regulated sympathetic outflow (54, 85), and neurons in the PVN have been shown to be activated by baroreflex challenges (11, 56). However, while BDNF has been shown to have a significant impact on baroreflex sensitivity in the NTS (3, 14), its actions on the baroreflex within the PVN have never been examined. Interestingly, our studies demonstrated that both inhibition of TrkB signaling (66) and upregulation of BDNF expression in the PVN (Table 1) reduced spontaneous baroreflex sensitivity. Further studies are needed to determine the underlying mechanisms, but trophic retrograde actions of BDNF on PVN-projecting NTS neurons could potentially be involved. Additionally, β-receptor signaling has been shown to modulate baroreflex sensitivity in the PVN (82); thus, BDNF may also alter baroreflex sensitivity by diminishing β-receptor signaling in PVN neurons.
Within the NTS, both catecholaminergic neurons and BDNF have been implicated in the regulation of baroreflex sensitivity. Baroreflex sensitivity was diminished both by lesioning NTS catecholaminergic neurons (34, 75) and by antagonizing TrkB receptors in the NTS (3). Furthermore, microinjections of function-blocking BDNF antibodies in the NTS have led to depressor responses and sympathoinhibition (14). Similarly, in this study, DSAP lesioning in the NTS was found to significantly reduce spontaneous baroreflex sensitivity in the GFP group, but the same treatment had no further effect on the already reduced baroreceptor reflex response seen in the BDNF group. Therefore, the effect of DSAP lesioning on baroreflex sensitivity was similar to its effects on MAP, with significant differences in the GFP group but not in the BDNF group.
In summary, our studies demonstrate that disruption of catecholaminergic signaling between the NTS and PVN contributes to the hypertensive effects of BDNF in the PVN. Lesioning NTS catecholaminergic neurons was found to increase blood pressure in GFP control rats but did not further elevate blood pressure following BDNF overexpression in the PVN. In addition, activating β-adrenergic receptors in the PVN significantly decreased blood pressure in the GFP control group but did not lower blood pressure in the BDNF group, and BDNF overexpression in the PVN also significantly reduced mRNA levels of adrenergic β1-adrenergic receptors in the PVN. These BDNF-mediated mechanisms may significantly contribute to stress-induced elevations in blood pressure and the development of stress-related cardiovascular diseases.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-133211 (to B. Erdos), start-up funds from the University of Vermont (UVM; to B. Erdos), and the UVM Cardiovascular Research Institute Bloomfield Early Career Professorship fund (to B. Erdos).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.T. and B.E. conceived and designed research; D.T., N.C.C., Z.E., and B.E. performed experiments; D.T., N.C.C., Z.E., G.W.H., and B.E. analyzed data; D.T. and B.E. interpreted results of experiments; D.T. and B.E. prepared figures; D.T. drafted manuscript; D.T. and B.E. edited and revised manuscript; D.T., N.C.C., Z.E., G.W.H., and B.E. approved final version of manuscript.
REFERENCES
- 1.Aliaga E, Arancibia S, Givalois L, Tapia-Arancibia L. Osmotic stress increases brain-derived neurotrophic factor messenger RNA expression in the hypothalamic supraoptic nucleus with differential regulation of its transcripts. Relation to arginine-vasopressin content. Neuroscience 112: 841–850, 2002. doi: 10.1016/S0306-4522(02)00128-8. [DOI] [PubMed] [Google Scholar]
- 2.Andresen MC, Kunze DL. Nucleus tractus solitarius--gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 3.Becker BK, Tian C, Zucker IH, Wang HJ. Influence of brain-derived neurotrophic factor-tyrosine receptor kinase B signalling in the nucleus tractus solitarius on baroreflex sensitivity in rats with chronic heart failure. J Physiol 594: 5711–5725, 2016. doi: 10.1113/JP272318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker BK, Wang HJ, Tian C, Zucker IH. BDNF contributes to angiotensin II-mediated reductions in peak voltage-gated K+ current in cultured CATH.a cells. Physiol Rep 3: e12598, 2015. doi: 10.14814/phy2.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia V, Rarick KR, Stauss HM. Effect of the data sampling rate on accuracy of indices for heart rate and blood pressure variability and baroreflex function in resting rats and mice. Physiol Meas 31: 1185–1201, 2010. doi: 10.1088/0967-3334/31/9/009. [DOI] [PubMed] [Google Scholar]
- 6.Blum R, Kafitz KW, Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel Na(V)1.9. Nature 419: 687–693, 2002. doi: 10.1038/nature01085. [DOI] [PubMed] [Google Scholar]
- 7.Busnardo C, Tavares RF, Correa FM. Angiotensinergic neurotransmission in the paraventricular nucleus of the hypothalamus modulates the pressor response to acute restraint stress in rats. Neuroscience 270: 12–19, 2014. doi: 10.1016/j.neuroscience.2014.03.064. [DOI] [PubMed] [Google Scholar]
- 8.Castren E, Thoenen H, Lindholm D. Brain-derived neurotrophic factor messenger RNA is expressed in the septum, hypothalamus and in adrenergic brain stem nuclei of adult rat brain and is increased by osmotic stimulation in the paraventricular nucleus. Neuroscience 64: 71–80, 1995. doi: 10.1016/0306-4522(94)00386-J. [DOI] [PubMed] [Google Scholar]
- 9.Chen MJ, Nguyen TV, Pike CJ, Russo-Neustadt AA. Norepinephrine induces BDNF and activates the PI-3K and MAPK cascades in embryonic hippocampal neurons. Cell Signal 19: 114–128, 2007. doi: 10.1016/j.cellsig.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Li DP, Pan HL. Presynaptic alpha1 adrenergic receptors differentially regulate synaptic glutamate and GABA release to hypothalamic presympathetic neurons. J Pharmacol Exp Ther 316: 733–742, 2006. doi: 10.1124/jpet.105.094797. [DOI] [PubMed] [Google Scholar]
- 11.Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 118: 797–807, 2003. doi: 10.1016/S0306-4522(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 12.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 55: 1026–1032, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 13.Choe KY, Han SY, Gaub P, Shell B, Voisin DL, Knapp BA, Barker PA, Brown CH, Cunningham JT, Bourque CW. High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron 85: 549–560, 2015. doi: 10.1016/j.neuron.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark CG, Hasser EM, Kunze DL, Katz DM, Kline DD. Endogenous brain-derived neurotrophic factor in the nucleus tractus solitarius tonically regulates synaptic and autonomic function. J Neurosci 31: 12318–12329, 2011. doi: 10.1523/JNEUROSCI.0746-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64: 477–505, 1995. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham ET Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol 274: 60–76, 1988. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 17.Daftary SS, Boudaba C, Tasker JG. Noradrenergic regulation of parvocellular neurons in the rat hypothalamic paraventricular nucleus. Neuroscience 96: 743–751, 2000. doi: 10.1016/S0306-4522(00)00003-8. [DOI] [PubMed] [Google Scholar]
- 18.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 19.Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32: 419–425, 2005. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 20.Daubert DL, McCowan M, Erdos B, Scheuer DA. Nucleus of the solitary tract catecholaminergic neurons modulate the cardiovascular response to psychological stress in rats. J Physiol 590: 4881–4895, 2012. doi: 10.1113/jphysiol.2012.232314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 14: 1143–1152, 2001. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- 22.Duale H, Waki H, Howorth P, Kasparov S, Teschemacher AG, Paton JF. Restraining influence of A2 neurons in chronic control of arterial pressure in spontaneously hypertensive rats. Cardiovasc Res 76: 184–193, 2007. doi: 10.1016/j.cardiores.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 23.El-Werfali W, Toomasian C, Maliszewska-Scislo M, Li C, Rossi NF. Haemodynamic and renal sympathetic responses to V1b vasopressin receptor activation within the paraventricular nucleus. Exp Physiol 100: 553–565, 2015. doi: 10.1113/expphysiol.2014.084426. [DOI] [PubMed] [Google Scholar]
- 24.Erdos B, Backes I, McCowan ML, Hayward LF, Scheuer DA. Brain-derived neurotrophic factor modulates angiotensin signaling in the hypothalamus to increase blood pressure in rats. Am J Physiol Heart Circ Physiol 308: H612–H622, 2015. doi: 10.1152/ajpheart.00776.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erdos B, Clifton RR, Liu M, Li H, McCowan ML, Sumners C, Scheuer DA. Novel mechanism within the paraventricular nucleus reduces both blood pressure and hypothalamic pituitary-adrenal axis responses to acute stress. Am J Physiol Heart Circ Physiol 309: H634–H645, 2015. doi: 10.1152/ajpheart.00207.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, McLean JH, Miller FD. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci 18: 2808–2821, 1998. doi: 10.1523/JNEUROSCI.18-08-02808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuchi M, Fujii H, Takachi H, Ichinose H, Kuwana Y, Tabuchi A, Tsuda M. Activation of tyrosine hydroxylase (TH) gene transcription induced by brain-derived neurotrophic factor (BDNF) and its selective inhibition through Ca(2+) signals evoked via the N-methyl-D-aspartate (NMDA) receptor. Brain Res 1366: 18–26, 2010. doi: 10.1016/j.brainres.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 28.Givalois L, Naert G, Rage F, Ixart G, Arancibia S, Tapia-Arancibia L. A single brain-derived neurotrophic factor injection modifies hypothalamo-pituitary-adrenocortical axis activity in adult male rats. Mol Cell Neurosci 27: 280–295, 2004. doi: 10.1016/j.mcn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Guyenet PG, Stornetta RL. The presympathetic cells of the rostral ventrolateral medulla (rvlm): anatomy, physiology and role in the control of circulation. In: Neural Mechanisms of Cardiovascular Regulation, edited by Dun NJ, Machado BH, Pilowsky PM. New York: Springer, 2004, chapt. 9, p. 187–218. [Google Scholar]
- 30.Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34: 833–843, 2009. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson KK, Byron KL. Vasopressin-induced vasoconstriction: two concentration-dependent signaling pathways. J Appl Physiol (1985) 102: 1402–1409, 2007. doi: 10.1152/japplphysiol.00825.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holm PC, Rodríguez FJ, Kresse A, Canals JM, Silos-Santiago I, Arenas E. Crucial role of TrkB ligands in the survival and phenotypic differentiation of developing locus coeruleus noradrenergic neurons. Development 130: 3535–3545, 2003. doi: 10.1242/dev.00565. [DOI] [PubMed] [Google Scholar]
- 33.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736, 2001. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh H, Alper RH, Buñag RD. Baroreflex changes produced by serotonergic or catecholaminergic lesions in the rat nucleus tractus solitarius. J Pharmacol Exp Ther 261: 225–233, 1992. [PubMed] [Google Scholar]
- 35.Itoh H, Buñag RD. Age-related reduction of reflex bradycardia in conscious rats by catecholaminergic nucleus tractus solitarius lesions. Mech Ageing Dev 67: 47–63, 1993. doi: 10.1016/0047-6374(93)90111-4. [DOI] [PubMed] [Google Scholar]
- 36.Itoh H, Buñag RD. Catecholaminergic nucleus tractus solitarius lesions in anesthetized rats alter baroreflexes differently with age. Mech Ageing Dev 64: 69–84, 1992. doi: 10.1016/0047-6374(92)90097-W. [DOI] [PubMed] [Google Scholar]
- 37.Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci USA 109: 1305–1310, 2012. doi: 10.1073/pnas.1114122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karschin C, Dissmann E, Stühmer W, Karschin A. IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci 16: 3559–3570, 1996. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein RL, Muir D, King MA, Peel AL, Zolotukhin S, Möller JC, Krüttgen A, Heymach JV Jr, Muzyczka N, Meyer EM. Long-term actions of vector-derived nerve growth factor or brain-derived neurotrophic factor on choline acetyltransferase and Trk receptor levels in the adult rat basal forebrain. Neuroscience 90: 815–821, 1999. doi: 10.1016/S0306-4522(98)00537-5. [DOI] [PubMed] [Google Scholar]
- 40.Klein U, Müller C, Chu P, Birnbaumer M, von Zastrow M. Heterologous inhibition of G protein-coupled receptor endocytosis mediated by receptor-specific trafficking of beta-arrestins. J Biol Chem 276: 17442–17447, 2001. doi: 10.1074/jbc.M009214200. [DOI] [PubMed] [Google Scholar]
- 41.Lambert EA, Lambert GW. Stress and its role in sympathetic nervous system activation in hypertension and the metabolic syndrome. Curr Hypertens Rep 13: 244–248, 2011. doi: 10.1007/s11906-011-0186-y. [DOI] [PubMed] [Google Scholar]
- 42.Laude D, Baudrie V, Elghozi JL. Applicability of recent methods used to estimate spontaneous baroreflex sensitivity to resting mice. Am J Physiol Regul Integr Comp Physiol 294: R142–R150, 2008. doi: 10.1152/ajpregu.00319.2007. [DOI] [PubMed] [Google Scholar]
- 43.Lebrun B, Bariohay B, Moyse E, Jean A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: a minireview. Auton Neurosci 126-127: 30–38, 2006. doi: 10.1016/j.autneu.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Madden CJ, Ito S, Rinaman L, Wiley RG, Sved AF. Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats using anti-DbetaH-saporin. Am J Physiol 277: R1063–R1075, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Martin KR, Quigley HA, Zack DJ, Levkovitch-Verbin H, Kielczewski J, Valenta D, Baumrind L, Pease ME, Klein RL, Hauswirth WW. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci 44: 4357–4365, 2003. doi: 10.1167/iovs.02-1332. [DOI] [PubMed] [Google Scholar]
- 46.Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci 29: 14185–14198, 2009. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci 11: 131–133, 2008. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- 48.Mendonça MM, Santana JS, da Cruz KR, Ianzer D, Ghedini PC, Nalivaiko E, Fontes MAP, Ferreira RN, Pedrino GR, Colugnati DB, Xavier CH. Involvement of GABAergic and adrenergic neurotransmissions on paraventricular nucleus of hypothalamus in the control of cardiac function. Front Physiol 9: 670, 2018. doi: 10.3389/fphys.2018.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meredith GE, Callen S, Scheuer DA. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res 949: 218–227, 2002. doi: 10.1016/S0006-8993(02)03160-8. [DOI] [PubMed] [Google Scholar]
- 50.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem 274: 32248–32257, 1999. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 51.Pacák K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 22: 502–548, 2001. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- 52.Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol 16: 89–150, 1995. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- 53.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14: 7–23, 2013. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 54.Patel KP, Schmid PG. Role of paraventricular nucleus (PVH) in baroreflex-mediated changes in lumbar sympathetic nerve activity and heart rate. J Auton Nerv Syst 22: 211–219, 1988. doi: 10.1016/0165-1838(88)90109-9. [DOI] [PubMed] [Google Scholar]
- 55.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol 131: 229–238, 1995. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 56.Polson JW, Mrljak S, Potts PD, Dampney RA. Fos expression in spinally projecting neurons after hypotension in the conscious rabbit. Auton Neurosci 100: 10–20, 2002. doi: 10.1016/S1566-0702(02)00143-1. [DOI] [PubMed] [Google Scholar]
- 57.Rage F, Givalois L, Marmigère F, Tapia-Arancibia L, Arancibia S. Immobilization stress rapidly modulates BDNF mRNA expression in the hypothalamus of adult male rats. Neuroscience 112: 309–318, 2002. doi: 10.1016/S0306-4522(02)00072-6. [DOI] [PubMed] [Google Scholar]
- 58.Riche D, De Pommery J, Menetrey D. Neuropeptides and catecholamines in efferent projections of the nuclei of the solitary tract in the rat. J Comp Neurol 293: 399–424, 1990. doi: 10.1002/cne.902930306. [DOI] [PubMed] [Google Scholar]
- 59.Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. Am J Physiol Regul Integr Comp Physiol 293: R1495–R1503, 2007. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- 60.Rodríguez Fermepin M, Trinchero M, Minetto J, Beltrán A, Fernández BE. Brain derived neurotrophic factor and neurotrophin-4 employ different intracellular pathways to modulate norepinephrine uptake and release in rat hypothalamus. Neuropeptides 43: 275–282, 2009. doi: 10.1016/j.npep.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Rogalski SL, Appleyard SM, Pattillo A, Terman GW, Chavkin C. TrkB activation by brain-derived neurotrophic factor inhibits the G protein-gated inward rectifier Kir3 by tyrosine phosphorylation of the channel. J Biol Chem 275: 25082–25088, 2000. doi: 10.1074/jbc.M000183200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saenz del Burgo L, Cortes R, Mengod G, Zarate J, Echevarria E, Salles J. Distribution and neurochemical characterization of neurons expressing GIRK channels in the rat brain. J Comp Neurol 510: 581–606, 2008. doi: 10.1002/cne.21810. [DOI] [PubMed] [Google Scholar]
- 63.Saphier D, Feldman S. Catecholaminergic projections to tuberoinfundibular neurones of the paraventricular nucleus. III. Effects of adrenoceptor agonists and antagonists. Brain Res Bull 26: 863–870, 1991. doi: 10.1016/0361-9230(91)90250-N. [DOI] [PubMed] [Google Scholar]
- 64.Sasi M, Vignoli B, Canossa M, Blum R. Neurobiology of local and intercellular BDNF signaling. Pflugers Arch 469: 593–610, 2017. [Erratum in Pflugers Arch 469: 611, 2017.] doi: 10.1007/s00424-017-1964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 4: 275–325, 1982. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 66.Schaich CL, Wellman TL, Einwag Z, Dutko RA, Erdos B. Inhibition of BDNF signaling in the paraventricular nucleus of the hypothalamus lowers acute stress-induced pressor responses. J Neurophysiol 120: 633–643, 2018. doi: 10.1152/jn.00459.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaich CL, Wellman TL, Koi B, Erdos B. BDNF acting in the hypothalamus induces acute pressor responses under permissive control of angiotensin II. Auton Neurosci 197: 1–8, 2016. doi: 10.1016/j.autneu.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scott AL, Zhang M, Nurse CA. Enhanced BDNF signalling following chronic hypoxia potentiates catecholamine release from cultured rat adrenal chromaffin cells. J Physiol 593: 3281–3299, 2015. doi: 10.1113/JP270725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith MA, Makino S, Kim SY, Kvetnansky R. Stress increases brain-derived neurotropic factor messenger ribonucleic acid in the hypothalamus and pituitary. Endocrinology 136: 3743–3750, 1995. doi: 10.1210/endo.136.9.7649080. [DOI] [PubMed] [Google Scholar]
- 70.Sobreviela T, Pagcatipunan M, Kroin JS, Mufson EJ. Retrograde transport of brain-derived neurotrophic factor (BDNF) following infusion in neo- and limbic cortex in rat: relationship to BDNF mRNA expressing neurons. J Comp Neurol 375: 417–444, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 71.Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol 9: 360–370, 2012. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 72.Stornetta RL. Neurochemistry of bulbospinal presympathetic neurons of the medulla oblongata. J Chem Neuroanat 38: 222–230, 2009. doi: 10.1016/j.jchemneu.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sumners C, Gelband CH. Neuronal ion channel signalling pathways: modulation by angiotensin II. Cell Signal 10: 303–311, 1998. doi: 10.1016/S0898-6568(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 74.Sumners C, Zhu M, Gelband CH, Posner P. Angiotensin II type 1 receptor modulation of neuronal K+ and Ca2+ currents: intracellular mechanisms. Am J Physiol Cell Physiol 271: C154–C163, 1996. doi: 10.1152/ajpcell.1996.271.1.C154. [DOI] [PubMed] [Google Scholar]
- 75.Talman WT, Snyder D, Reis DJ. Chronic lability of arterial pressure produced by destruction of A2 catecholaminergic neurons in rat brainstem. Circ Res 46: 842–853, 1980. doi: 10.1161/01.RES.46.6.842. [DOI] [PubMed] [Google Scholar]
- 76.Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol 388: 169–190, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 77.Toriya M, Maekawa F, Maejima Y, Onaka T, Fujiwara K, Nakagawa T, Nakata M, Yada T. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 22: 987–995, 2010. doi: 10.1111/j.1365-2826.2010.02039.x. [DOI] [PubMed] [Google Scholar]
- 78.Tsushima H, Fujimoto S, Matsuda T. Effects of beta 1- and beta 2-adrenoceptor agonists applied into the hypothalamic paraventricular nuclei of spontaneously hypertensive rats on urine production. Jpn J Pharmacol 64: 201–207, 1994. doi: 10.1254/jjp.64.201. [DOI] [PubMed] [Google Scholar]
- 79.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409, 2009. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veltmar A, Culman J, Qadri F, Rascher W, Unger T. Involvement of adrenergic and angiotensinergic receptors in the paraventricular nucleus in the angiotensin II-induced vasopressin release. J Pharmacol Exp Ther 263: 1253–1260, 1992. [PubMed] [Google Scholar]
- 81.Wamsteeker JI, Bains JS. A synaptocentric view of the neuroendocrine response to stress. Eur J Neurosci 32: 2011–2021, 2010. doi: 10.1111/j.1460-9568.2010.07513.x. [DOI] [PubMed] [Google Scholar]
- 82.Wang D, Feng H, Li YS, Qiu DL, Jin H, Jin QH. Β-adrenoceptors in the hypothalamic paraventricular nucleus modulate the baroreflex in conscious rats. Neurosci Lett 551: 43–46, 2013. doi: 10.1016/j.neulet.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 83.Wang H, Zhou XF. Injection of brain-derived neurotrophic factor in the rostral ventrolateral medulla increases arterial blood pressure in anaesthetized rats. Neuroscience 112: 967–975, 2002. doi: 10.1016/S0306-4522(02)00085-4. [DOI] [PubMed] [Google Scholar]
- 84.Whelton PK. Epidemiology of hypertension. Lancet 344: 101–106, 1994. doi: 10.1016/S0140-6736(94)91285-8. [DOI] [PubMed] [Google Scholar]
- 85.Yang Z, Coote JH. The influence of the paraventricular nucleus on baroreceptor dependent caudal ventrolateral medullary neurones of the rat. Pflugers Arch 438: 47–52, 1999. doi: 10.1007/s004240050878. [DOI] [PubMed] [Google Scholar]
- 86.Zhang R, Jankord R, Flak JN, Solomon MB, D’Alessio DA, Herman JP. Role of glucocorticoids in tuning hindbrain stress integration. J Neurosci 30: 14907–14914, 2010. doi: 10.1523/JNEUROSCI.0522-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou J, Bradford HF, Stern GM. The stimulatory effect of brain-derived neurotrophic factor on dopaminergic phenotype expression of embryonic rat cortical neurons in vitro. Brain Res Dev Brain Res 81: 318–324, 1994. doi: 10.1016/0165-3806(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 88.Zucker IH, Schultz HD, Patel KP, Wang W, Gao L. Regulation of central angiotensin type 1 receptors and sympathetic outflow in heart failure. Am J Physiol Heart Circ Physiol 297: H1557–H1566, 2009. doi: 10.1152/ajpheart.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zucker IH, Xiao L, Haack KKV. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci (Lond) 126: 695–706, 2014. doi: 10.1042/CS20130294. [DOI] [PMC free article] [PubMed] [Google Scholar]