Abstract
In our present studies, we seek to determine whether increased osmolarity stimulates deflation-activated receptors (DARs). In anesthetized, open-chest, and mechanically ventilated rabbits, we recorded single-unit activities from typical slowly adapting receptors (SARs; responding only to lung inflation) and DAR-containing SARs (DAR-SARs; responding to both lung inflation and deflation) and identified their receptive fields in the lung. We examined responses of these two groups of pulmonary sensory units to direct injection of hypertonic saline (8.1% sodium chloride; 9-fold in tonicity) into the receptive fields. Hypertonic saline decreased the activity in most SAR units from 40.3 ± 5.4 to 34.8 ± 4.7 imp/s (P < 0.05, n = 12). In contrast, it increased the activity in DAR-SAR units quickly and significantly from 15.9 ± 2.2 to 43.4 ± 10.0 imp/s (P < 0.01, n = 10). Many units initially had increased activity, mainly in the deflation phase. DAR-SAR activities largely returned to the control level 30 s after injection. Since hypertonic saline stimulated DAR-SAR units but not SAR units, we conclude that hypertonic saline activates DARs.
Keywords: airway receptors, lung, vagus nerve
INTRODUCTION
Airway sensory receptors (sensors) provide information to the central nervous system to regulate breathing and many other very important physiological functions. Conventionally, airway receptors are divided into three types: C-fiber receptors (CFRs; bronchial and pulmonary CFRs), rapidly adapting receptors (RARs), and slowly adapting receptors (SARs) (2). Hypertonic saline administered by intravenous or right atrial injection, aerosol inhalation, and direct intrabronchiolar delivery are known to stimulate airway sensors and cause a reflex effect (3, 7, 8). Alterations of osmolarity are believed to stimulate CFRs and RARs, but not SARs (7, 8). However, in those reports, some SAR units actually are also stimulated by hypertonic saline. This raises the question: why? It is a conventional belief that each pulmonary vagal afferent fiber connects to only one mechano-sensor (one-sensor theory). Recently, a multiple-sensor theory has been proposed, in which one fiber connects to multiple sensors, forming a sensory unit (11). In a unit, the composition of sensors can be homogeneous or heterogeneous. If all of the sensors are SARs, the sensory unit behaves as a typical SAR. If a unit also contains deflation-activated receptors (DARs) (11), the unit can also respond to lung deflation, behaving as RAR-like SARs (10). Similarly, a typical RAR unit contains RARs and DARs to respond to both lung inflation (activity adapts rapidly) and deflation. DARs are a group of sensors that respond to lung deflation. They include pure DARs (respond only to lung deflation) and DARs associated with RARs and/or SARs (in these cases, they also respond to lung inflation with rapidly and/or slowly adapting features). DARs can be rapidly adapting or slowly adapting during constant pressure deflation. Accepting the multiple-sensor theory, it is likely that, in the dog, the SAR units stimulated by hypertonic saline (7, 8) are DAR containing SAR (DAR-SAR) units. In the present studies, we tested the hypothesis that hypertonic saline can stimulate DARs by comparing responses of DAR-SAR (deflation activated) and typical SAR (deflation not activated) units to hypertonic saline. We found that DAR-SAR units, but not SAR units, responded and conclude that hypertonic saline stimulates DARs.
METHODS
Current studies conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No.85-53). The Institutional Animal Care and Use Committee at University of Louisville and the Robley Rex Veterans Affairs Medical Center approved the use of animals and the study protocol. Rabbits were anesthetized with pentobarbital sodium (30 mg/kg iv). Then doses of anesthetics (6 mg iv) were given hourly. The trachea was cannulated low in the neck, and the chest was opened widely in the midline. The lungs were ventilated by a Harvard ventilator (model 683); 3- to 4-cmH2O PEEP (positive end-expiratory pressure) was applied. Afferent activities were recorded according to the conventional method (7, 8, 10). Briefly, the vagus nerve (either right or left) was separated from the carotid sheath, placed on a dissecting platform, and covered by mineral oil. A small slip was cut from the vagus nerve, and the peripheral end was placed on recording electrodes; the main trunk of the vagus nerve was left intact. The electrodes were connected to a high-impedance probe (Grass HIP5) from which the output was led to an amplifier (Grass P511). After suitable amplification, action potentials from single fiber strands of the vagus nerve were displayed on an oscilloscope and monitored by a loudspeaker. In addition, a voltage analog of impulse frequency was produced by a ratemeter (Frederick Haer, Brunswick, ME) at a bin width of 0.1 s. The single unit is ensured by a uniformity in amplitude and contour of action potentials displayed in the oscilloscope. Action potentials and its analog frequency, along with airway pressure, were recorded by a thermorecorder (Astro-Med; Dash IV). SAR units were identified according to their adaptation rate (<45%). These units were further divided into SAR units (responding only to lung inflation) and DAR-SAR units (responding to both lung inflation and deflation). Then receptive fields were determined by identifying the most sensitive point on the lung surface to the gentle touching with a cotton tip. Responses of these two groups of units to direct injection of hypertonic saline (8.1% sodium chloride, 0.02–0.1 ml) into the receptive fields were examined. Afferent activity was reported as impulses per second (imp/s), calculated from whole respiratory cycles. A statistical program (GB-Stat v9.0 Dynamic Microsystems, Silver Spring, MD) was used for data analysis. Data were expressed as means ± SE. Group comparisons involved the use of repeated-measures analysis of variance (ANOVA), followed by Bonferroni post hoc analysis. Differences were considered statistically significant at P < 0.05.
RESULTS
In 16 anesthetized, open-chest, and mechanically ventilated male rabbits, we recorded single-unit activities from typical SAR units (n = 12) and DAR-SAR units (n = 10). One to two units were examined in each rabbit. We examined responses of these two groups to direct local injection of hypertonic saline (8.1% sodium chloride, 0.02–0.1 ml) into receptive fields. Hypertonic saline decreased the activity in most SAR units, although not substantially (Fig. 1). A few SAR units had a slight increase in activity. All of the responses were slow and gradual. At baseline, activity of typical SAR units was 40.3 ± 5.4 imp/s (n = 12). It decreased to 34.8 ± 4.7 imp/s (P < 0.05) at 17.5 s after injection of hypertonic saline (Fig. 2). On the other hand, hypertonic saline stimulated activity in DAR-SAR units immediately (within a few seconds after injection) and significantly (Fig. 3). Their averaged activity increased from the baseline (15.9 ± 2.2 imp/s) to a peak (43.4 ± 10.0 imp/s) at 10.5 s after injection (n = 10, P < 0.01; Fig. 2). Most units initially had their activities confined to the deflation phase (Figs. 3 and 4). This ventilatory modulation of the unit activities gradually subsided. In three units, the increased activities did not have a clear correlation to ventilatory cycles. The increased activity returned to the control level at ~30 s (Fig. 2).
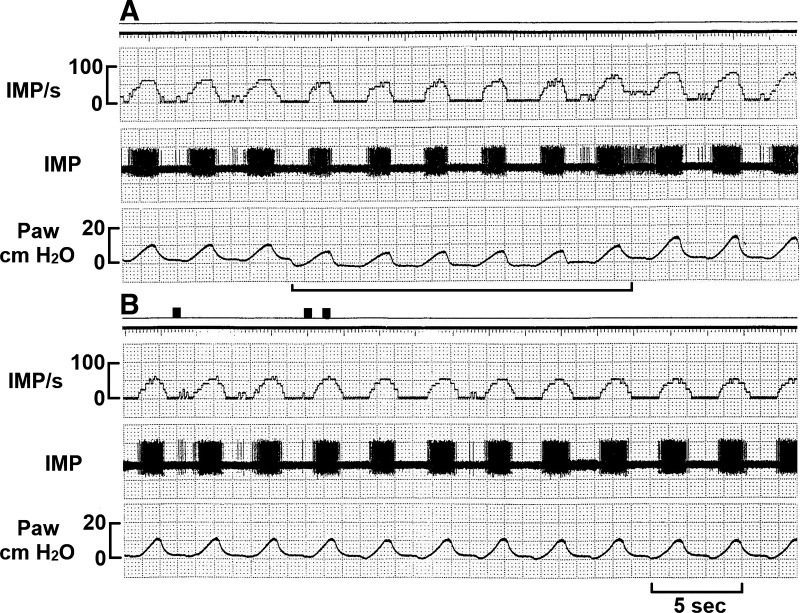
Fig. 1.
Response of a typical slowly adapting receptor unit to hypertonic saline. The unit was not stimulated by positive end-expiratory pressure removal, which was denoted by a solid line (A), or by local injection of hypertonic saline (8.1% NaCl; B). Three black marks at the top of B indicate insertion of the needle and start and end of the injection, respectively. IMP/s, impulses per second; Paw, airway pressure.
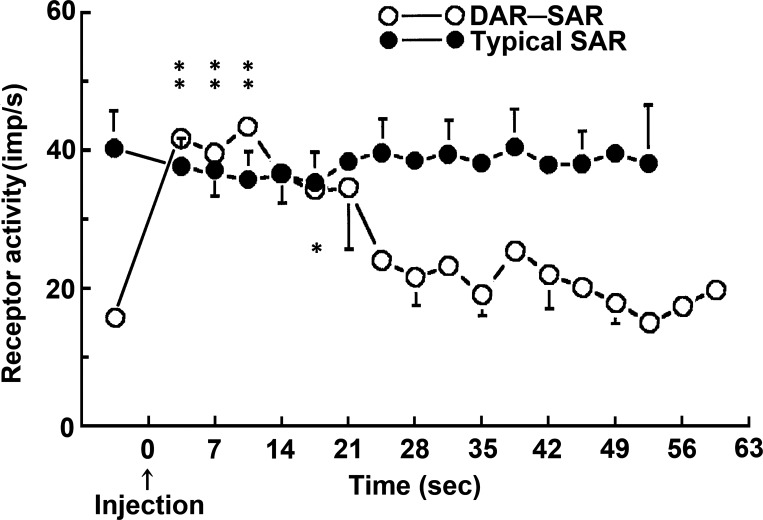
Fig. 2.
Mean responses of slowly adapting receptor (SAR) units and deflation-activated receptor (DAR)-SAR units to injection of 8.1% NaCl into receptive fields. The data points before time 0 are baseline activities. Hypertonic saline was injected at time 0. Please note that SAR units decreased activity slightly (ANOVA test, P < 0.05), whereas the DAR-SAR units were stimulated by hypertonic saline (ANOVA test, P < 0.0001), and peaked 5–10 s after injection. The nerve activity returned to its control level ~30 s after the injection. *P < 0.05 and **P < 0.01 indicate a significant difference between the group activity and the baseline by Bonferroni post hoc analysis (3 groups in DAR-SAR unit indicated by **, and one group in SAR unit indicated by *). imp/s, Impulses per second.
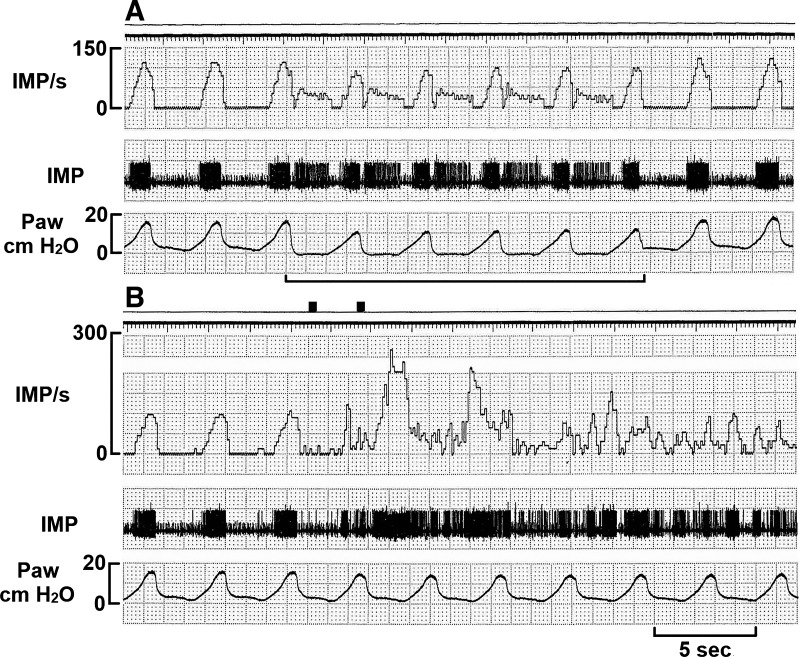
Fig. 3.
A deflation-activated receptor-slowly adapting receptor unit stimulated by local injection of hypertonic saline. The unit was stimulated by positive end-expiratory pressure removal (see solid line in A) and by local injection of hypertonic saline (B). Please also note that the sensory unit was stimulated mainly during the deflation phase. Two black marks at the top of B indicate insertion of the needle and end of the injection, respectively. IMP/s, impulses per second; Paw, airway pressure.
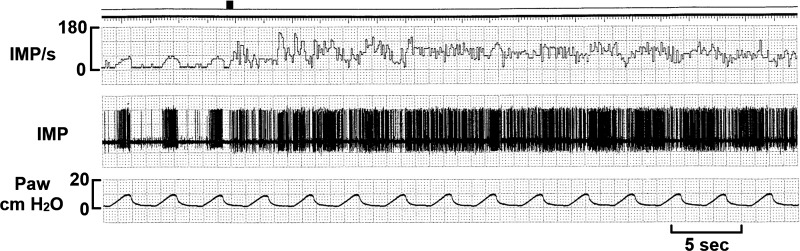
Fig. 4.
Another deflation-activated receptor-slowly adapting receptor unit in response to hypertonic saline injection. Initially, the unit had a clear ventilator modulation with activities increased more during deflation phase. This was followed by a segment, in which the unit shows no clear ventilatory modulation. Then the deflation modulation resumes. The black mark at the top denotes injection of hypertonic saline. IMP/s, impulses per second; Paw, airway pressure.
DISCUSSION
Airway sensors are mainly innervated by vagal afferents. Certain types of these sensors can be activated by hypertonic saline. These sensors often can also be activated by inflammatory mediators and may play a significant role in the pathophysiology of a variety of pulmonary diseases (5). Thus it is important to know which types of sensors respond to the stimulus. In the conventionally accepted three types of airway sensors, CFRs and RARs, but not SARs, were believed to respond to hypertonic saline. Recently, two additional airway sensors with myelinated afferents, high-threshold Aδ receptors, and DARs were identified (5). High-threshold Aδ receptors, like CFRs, are stimulated by hypertonic saline (5). However, DARs have never been investigated. DARs are airway mechanosensors that respond to lung deflation. The present study is to determine whether hypertonic saline stimulates DARs. Our results show injection of hypertonic saline increases activities in DAR-SAR units but not SAR units. In fact, SAR units slightly decreased their activities following injection of hypertonic saline (Fig. 2). This is probably due to partial blockade of the airway that supplies the tissue that houses the sensory unit. These results strongly support our hypothesis that hypertonic saline stimulates DARs.
In the present rabbit study, responses of the DAR-SAR units to hypertonic saline are the same as those of the stimulated SAR units observed in the dog (8). It was reported that: “Water stimulated only 4 of 23 receptors, which began to discharge throughout deflation within 1–9 s of the injection, thereby increasing mean activity (from 22 ± 5 to 57 ± 10 impulses/s) while reducing the ventilatory modulation … the control pattern of discharge returned in 22–96 s” (8). SARs in both species have similar levels of stimulation (2.7-fold in the rabbit vs. 2.6-fold in the dog) and time course of the response (including peak response and recovery times). Furthermore, their responses in discharge patterns were similar: both had initial increased activities, mainly during the deflation phase [see Figs. 3 and 4 in this paper and Fig. 8A in the dog paper (8)]. In the legend of Fig. 8A in Pisarri et al. (8), it states that injection of water evoked firing during deflation and slightly reduced firing at the peak of inflation. The increased activities in the deflation phase strongly support a stimulation of DARs. Careful review of the literature shows that altered osmolarity stimulated a significant percentage of total SAR units [4/23 SARs, 17.4% via bronchiolar delivery (8) and 6/21 SARs, 28.6% via intravenous injection (7)]. This fact should not be dismissed. It is very possible that the SAR units activated in dogs are DAR-SAR units.1
If we accept that hypertonic saline stimulates DARs, we can explain that the stimulated SARs observed in dogs are DAR-SAR units. This also explains the behavior of RAR units in response to intravenous injection of hypertonic saline. As reported: “Whereas control firing was usually confined to the inflation phase of the ventilator cycle, the evoked response included a conspicuous component during deflation in the majority of the receptors….” (7). Thus those activated RAR units observed in dogs are probably due to activation of DARs but not activation of RARs in the units. Therefore, whether RARs can be stimulated by hypertonic saline is debatable. This issue requires further experiments to solve.
If DARs are responsible for the stimulation by hypertonic saline, we expect that the respond rate will be 33% for SARs and 67% for RARs, because 1/3 SARs and 2/3 RARs contain DARs (stimulated by lung deflation) (4). In the above studies, only 17–29% of SARs were stimulated; in contrast, 18/19 (95%) of RARs were stimulated by alteration of osmolarity. The low and high incidences for SARs and RARs can be explained by receptor selection. In practice, usually typical SARs and RARs were selected for studies; the percentage of units stimulated by hypertonic saline could be overestimated for RARs and underestimated for SARs. This is because “typical RARs” include response to PEEP removal (selectively including DARs), whereas “typical SARs” do not respond to PEEP removal (selectively excluding DARs) [see note 3 in a review article (11)].
The mechanism by which hypertonic saline stimulates DARs is unknown. Further experimentation for clarification is worthwhile. However, hypertonic saline may directly activate sensory terminals by acting on bioactive proteins or channels. For example, hypertonic saline can stimulate pulmonary nociceptors by activating transient receptor potential vanilloid 4 (TRPV4) receptors (6, 9). So far, there are no studies that examine whether TRPV4 receptors are expressed on DAR terminals or not. Hypertonic saline may indirectly activate sensory terminals via fluid movement from capillaries or interstitial space into the airway due to osmotic difference. We can speculate that, if DARs are present inside the interstitial space, depletion of fluid there may enhance the negative pressure during lung deflation and, therefore, stimulate the receptors.
In summary, we tested the hypothesis that hypertonic saline can stimulate DARs by examining responses of SAR units and DAR-SAR units to hypertonic saline injection and found that only DAR-SAR units responded and, therefore, conclude that hypertonic saline stimulates DARs.
Perspectives and Significance
By comparing responses of DAR-SAR and typical SAR units to hypertonic saline, we conclude that hypertonic saline stimulates DARs. Our results support a multiple-sensor theory that one afferent fiber connects to multiple heterogeneous sensors, forming a sensory unit. Therefore, the sensory unit is not merely a transducer but also an integrator that processes a significant amount of information. Thus, the unit can provide the brain with different types of information. This new insight challenges our current view on how the central nervous system deciphers the incoming signals.
GRANTS
This study was supported by a Veterans Affairs Merit Review Award (PULM-024-17S).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.Y. conceived and designed research; J.G. and J.Y. performed experiments; J.G. and J.Y. analyzed data; J.G., M.S., and J.Y. interpreted results of experiments; J.G. and J.Y. prepared figures; J.G., M.S., and J.Y. drafted manuscript; J.G., M.S., and J.Y. edited and revised manuscript; J.G., M.S., and J.Y. approved final version of manuscript.
Footnotes
In dogs, some SAR units are found to be stimulated by lung deflation (12). In one-sensor theory, these units are considered to be atypical, because lung deflation is believed to be a major character of RARs. For this reason, we exclude those units that responded to lung deflation to differentiate SARs from RARs. This leads to a study on RAR-like SARs in rabbits (10). There are reports regarding SAR unit in the large airways, such as trachea. These SARs could be activated by both positive and negative transmural pressure (i.e., during both expiratory and inspiratory phases, respectively) (1).
REFERENCES
- 1.Bartlett D Jr, Sant’ambrogio G, Wise JC. Transduction properties of tracheal stretch receptors. J Physiol 258: 421–432, 1976. doi: 10.1113/jphysiol.1976.sp011428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleridge HM, Coleridge JCG. Reflexes evoked from tracheobronchial tree and lungs. : Handbook of Physiology, The Respiratory System, Control of Breathing, edited by Cherniack NS and Widdicombe JG. Bethesda, MD: American Physiological Society, 1986, p. 395–429. doi: 10.1002/cphy.cp030212. [DOI] [Google Scholar]
- 3.Eschenbacher WL, Boushey HA, Sheppard D. Alteration in osmolarity of inhaled aerosols cause bronchoconstriction and cough, but absence of a permeant anion causes cough alone. Am Rev Respir Dis 129: 211–215, 1984. [PubMed] [Google Scholar]
- 4.Knowlton GC, Larrabee MG. A unitary analysis of pulmonary volume receptors. Am J Physiol 147: 100–114, 1946. doi: 10.1152/ajplegacy.1946.147.1.100. [DOI] [PubMed] [Google Scholar]
- 5.Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol 4: 287–324, 2014. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- 6.Ni D, Gu Q, Hu HZ, Gao N, Zhu MX, Lee LY. Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol 291: R541–R550, 2006. doi: 10.1152/ajpregu.00016.2006. [DOI] [PubMed] [Google Scholar]
- 7.Pisarri TE, Jonzon A, Coleridge HM, Coleridge JC. Intravenous injection of hypertonic NaCl solution stimulates pulmonary C-fibers in dogs. Am J Physiol Heart Circ Physiol 260: H1522–H1530, 1991. doi: 10.1152/ajpheart.1991.260.5.H1522. [DOI] [PubMed] [Google Scholar]
- 8.Pisarri TE, Jonzon A, Coleridge HM, Coleridge JC. Vagal afferent and reflex responses to changes in surface osmolarity in lower airways of dogs. J Appl Physiol (1985) 73: 2305–2313, 1992. doi: 10.1152/jappl.1992.73.6.2305. [DOI] [PubMed] [Google Scholar]
- 9.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2: 695–702, 2000. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 10.Yu J. Spectrum of myelinated pulmonary afferents. Am J Physiol Regul Integr Comp Physiol 279: R2142–R2148, 2000. doi: 10.1152/ajpregu.2000.279.6.R2142. [DOI] [PubMed] [Google Scholar]
- 11.Yu J. Deflation-activated receptors, not classical inflation-activated receptors, mediate the Hering-Breuer deflation reflex. J Appl Physiol (1985) 121: 1041–1046, 2016. doi: 10.1152/japplphysiol.00903.2015. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Schultz HD, Goodman J, Coleridge JC, Coleridge HM, Davis B. Pulmonary rapidly adapting receptors reflexly increase airway secretion in dogs. J Appl Physiol (1985) 67: 682–687, 1989. doi: 10.1152/jappl.1989.67.2.682. [DOI] [PubMed] [Google Scholar]