Abstract
There is significant interest in the potential utility of small-molecule activator compounds to mitigate cardiac arrhythmia caused by loss of function of hERG1a voltage-gated potassium channels. Zebrafish (Danio rerio) have been proposed as a cost-effective, high-throughput drug-screening model to identify compounds that cause hERG1a dysfunction. However, there are no reports on the effects of hERG1a activator compounds in zebrafish and consequently on the utility of the model to screen for potential gain-of-function therapeutics. Here, we examined the effects of hERG1a blocker and types 1 and 2 activator compounds on isolated zkcnh6a (zERG3) channels in the Xenopus oocyte expression system as well as action potentials recorded from ex vivo adult zebrafish whole hearts using optical mapping. Our functional data from isolated zkcnh6a channels show that under the conditions tested, these channels are blocked by hERG1a channel blockers (dofetilide and terfenadine), and activated by type 1 (RPR260243) and type 2 (NS1643, PD-118057) hERG1a activators with higher affinity than hKCNH2a channels (except NS1643), with differences accounted for by different biophysical properties in the two channels. In ex vivo zebrafish whole hearts, two of the three hERG1a activators examined caused abbreviation of the action potential duration (APD), whereas hERG1a blockers caused APD prolongation. These data represent, to our knowledge, the first pharmacological characterization of isolated zkcnh6a channels and the first assessment of hERG enhancing therapeutics in zebrafish. Our findings lead us to suggest that the zebrafish ex vivo whole heart model serves as a valuable tool in the screening of hKCNH2a blocker and activator compounds.
Keywords: activators, hERG, optical mapping, zebrafish, zERG, zkcnh6
INTRODUCTION
The human ether-à-go-go-related gene (hERG, or KCNH2) encodes the voltage-gated potassium (Kv) channel underlying the rapid cardiac delayed rectifier current, IKr (43, 44, 48). The unique properties of KCNH2 channels allow this current to play a critical role in cardiac repolarization and the termination of the ventricular action potential (43, 44, 48). Compared with other Kv channels, KCNH2 channels display unusual gating, with slow activation and deactivation and rapid inactivation and recovery from inactivation. The disruption of this current, due to inherited loss-of-function mutations in KCNH2 or pharmacological blockade of the channel, can lead to a disorder known as long QT syndrome (LQTS). LQTS is characterized by delayed cardiac repolarization and consequently prolongation of the QT interval, and this may lead to fatal cardiac arrhythmias (10, 43). As such, there is significant interest in the development of strategies to enhance KCNH2 channel function as well as the establishment of translatable screening models that account for the complexities of electrical activity in cardiomyocytes such as the presence of multiple cardiac ion currents and individual genomic variability.
There has been increasing interest in the use of zebrafish as a translatable screening model for LQTS, in part because the cardiac electrophysiology of this teleost species more closely resembles that of human than some mammals (e.g., murines). For example, the intrinsic heart rate in zebrafish, 110–130 beats/min, is similar to human (47), whereas the typical resting heart rate in the mouse is 500–700 beats/min (11, 22, 49). Morphological differences of the murine cardiac action potential, specifically the lack of a plateau phase and relatively short action potential (AP; Ref. 28), further complicate the translation of findings to the human heart, particularly for the study of phase 3 repolarization disorders, such as LQTS. In contrast, the zebrafish ventricular action potential exhibits a robust plateau phase and an action potential duration (APD) that more closely reflects that observed in human ventricular myocytes (4, 26, 34, 50). Furthermore, zebrafish ventricular myocytes possess many of the same ion currents found in the human ventricle with repolarization in the zebrafish strongly reliant on IKr (1, 3, 15, 18, 24, 34, 41, 45).
The utility of zebrafish to study repolarization disorders such as LQTS caused by hERG channel dysfunction can be well-demonstrated by previous phenotypic characterization of native or engineered mutant zebrafish strains (1, 18, 23, 24, 52). Several zebrafish kcnh genes (zERG) have been identified (zkcnh2a, zkcnh2b, zkcnh6a, and zkcnh7), with zkcnh6a, and to a lesser extent zkcnh2a, being the predominant transcript in the heart (25, 50). The breakdance mutant results from a mutation in the zkcnh6a gene and displays 2:1 atrioventricular block (8), which has been observed with LQTS (2). The reggae mutant shortens QT interval as a result of a mutation that resulted in altered gating of zkcnh6a channels (18). Other loss-of-function zkcnh6a mutations result in a silent ventricle phenotype, and one of these mutants, zkcnh6a M521K, was found to prolong the APD at 90% repolarization in a heterozygous embryo (1). In addition, zkcnh6a, and to a lesser extent zkcnh2a, knockdown using morpholinos (33) resulted in altered QT duration and bradycardia (19, 24). Similarly, application of a range of hKCNH2-blocking compounds to wild-type (WT) zebrafish embryos results in bradycardia (24, 33), and some hKCNH2 blockers have been shown to cause QT prolongation in zebrafish larvae (49a) and adult zebrafish in vivo (31). These electrophysiological characteristics of zebrafish hearts make them well-suited for the investigation of human cardiac electrical disorders and, in particular, IKr (hERG)-related phase 3 repolarization-related disorders.
Despite the prominent expression of zkcnh6a and significant interest in using zebrafish as a drug-screening platform (31, 33, 37), the pharmacological properties of zkcnh6a channels have not been examined. Furthermore, since there is significant interest in the screening of hERG activator compounds, investigation of the facilitatory effects of hERG activators on zkcnh6a channels is warranted. Here, we have characterized the effects of several hKCNH2a blocker and activator compounds to investigate the pharmacological properties of zkcnh6a channels in a heterologous expression system. We examine a type 1 activator, which increases repolarizing current primarily by slowing channel deactivation, and type 2 activators, which increase hKCNH2a tail current primarily by reducing inactivation. We then use optical mapping of whole ex vivo zebrafish hearts to demonstrate prolongation of the APD following treatment with hKCNH2a-blocking compounds and APD shortening in the presence of some hKCNH2a activator compounds. These data highlight the utility of the zebrafish whole heart model as an attractive translational model for studying hKCNH2a function and pharmacology. This model also provides insight into the complex action of hKCNH2a activator compounds on both APD and action potential morphology that are useful in validating their therapeutic potential.
MATERIALS AND METHODS
Heterologous expression of zkcnh6a in Xenopus laevis oocytes.
hKCNH2a (hERG1a) and zkcnh6a (zERG3a) channel constructs were expressed in a pBlueScript II SK(+) vector as described previously (9). zkcnh6a cDNA (acc. no. NM_212837) was synthesized commercially (GENEWIZ) and subcloned into the pBluescript vector. Complementary RNA (cRNA) was transcribed from each construct using the mMESSAGE mMACHINE T7 ULTRA cRNA transcription kit (Ambion, Austin, TX) following cDNA linearization with XbaI restriction endonuclease.
Oocytes were isolated from female Xenopus laevis frogs and hKCNH2a or zkcnh6a cRNA was injected as approved by the Simon Fraser University Animal Care Committee (protocol no. 1197K-08) and in accordance with Canadian Council on Animal Care protocols and procedures as described previously (9). Following cRNA injection, oocytes were incubated in SOS+ media (in millimolar: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, 2.5 sodium pyruvate, 100 mg/L gentamycin sulfate, and 5% horse serum, titrated to pH 7.4) for 2–7 days at 19°C before voltage-clamp experiments.
Membrane currents were recorded from hKCNH2a or zkcnh6a channels using the two-electrode voltage-clamp technique with an Axoclamp 900A amplifier (Axon Instruments, Foster City, CA) and computer-driven voltage protocols (pCLAMP 10 software and Digidata 1440A interface; Axon Instruments). Recordings were obtained in ND96 solution (in millimolar: 96 NaCl, 3 KCl, 1 MgCl2, 0.5 CaCl2, 5 HEPES, titrated to pH 7.4). Microelectrodes had a resistance of 0.2–2.0 MΩ when filled with 3 M KCl. Signals were acquired at a sampling rate of 10 kHz with a 4-kHz low-pass filter. Experiments were performed at 20–22°C.
Conductance-voltage (G-V) relations were calculated from peak tail current amplitudes. Curves were fitted with a single Boltzmann function, y = 1/{1+exp[(V1/2 − V)/k]}, where y is the conductance normalized with respect to the maximal conductance, V1/2 is the half-activation potential, V is the test voltage, and k is the slope factor. The late phase of activation, from 50% of Gmax, was fitted with a single exponential using f(t) = A·exp(t/τ) + C, where A is the amplitude of the fit, t is time, τ is the time constant of activation, and C is the residual current. Deactivating currents were fitted with a double exponential using f(t) = Aslow·exp(t/τslow) + Afast·exp(t/τfast) + C, where Afast and Aslow are the amplitudes of the two components of the fit, t is time, τfast and τslow are the time constants of the two components of deactivation, and C is the residual current. The τdeact values presented represent the sum of the two components (τfast and τslow) weighted by the relative amplitude of each component. Rectification factor was calculated as previously described (43) using R = I/Gn(V − Erev), where R is the rectification factor, I is the membrane current, G is the slope conductance calculated from the fully activated current-voltage relationship, n is the activation variable (which was set at 1 as our data were collected from fully activated channels), V is the test voltage, and Erev is the measured reversal potential. The current-voltage relationship in response to an action potential protocol was plotted to examine the voltage of peak current as previously described (5). The concentration-response relationship of drug block of hKCNH2a and zkcnh6a channels was described by the Hill equation y = 1 − xb/(cb + xb), where y is the fractional conductance, c is the IC50 value, and b is the Hill coefficient. The concentration-response relationship of drug enhancement of hKCNH2a and zkcnh6a channels was described by the Hill equation y = 1 + axb/(cb + xb), where y is the fractional conductance, a is (Imax − Imin), c is the EC50 value, and b is the Hill coefficient. Data throughout the text and figures are shown as the means ± SE.
The effects of hKCNH2 blockers dofetilide and terfenadine as well as hKCNH2 activators NS1643, PD-118057, and RPR260243 were examined. Dofetilide, PD-118057, and terfenadine were purchased from Sigma-Aldrich (Oakville, Ontario, Canada), NS1643 was purchased from Tocris Bioscience (Oakville, Ontario, Canada), and RPR260243 was purchased from AOBIOUS (Gloucester, MA). Drug solutions were prepared immediately before experiments by dilution of stock solutions: 25 mM NS1643 in DMSO; 10 mM dofetilide, PD-118057, or RPR260243 in DMSO; and 10 mM terfenadine in ethanol.
Zebrafish ex vivo whole heart optical mapping.
Zebrafish whole hearts were isolated from adult zebrafish, as approved by the Simon Fraser University Animal Care Committee (protocol no. 1156K-90) and in accordance with Canadian Council on Animal Care protocols and procedures and as described previously (27). Calcium Tyrode solution was used for all procedures. Fish were euthanized by cold shock followed by decapitation, and hearts were dissected from a tissue wedge excised from the region between the gills and pectoral fins. Isolated hearts were cannulated through the bulbus arteriosus using a 34-gauge needle. Hearts were labeled with the potentiometric dye RH 237 (20 μM; AAT Bioquest, Sunnyvale, CA) in Ca2+ Tyrode solution at room temperature for 30 min. This was followed by treatment with the myosin II inhibitor blebbistatin (20 μM; Toronto Research Chemicals, North York, Ontario, Canada) in Ca2+ Tyrode solution for 60 min. Imaging and analysis were carried out as described in Lin et al. (27). Briefly, cannulated hearts were suspended in in Ca2+ Tyrode solution supplemented with 20 μM blebbistatin in a temperature-controlled chamber. RH 237 fluorophores were excited using a 350-mW, 530-nm light-emitting diode module (Thorlabs) via a 630-nm long-pass dichroic mirror (XF2021, 630 DRLP; Omega Optical, Brattleboro, VT). A 700-nm long-pass filter (Omega 700LP) was used to isolate the fluorescence emission, and the signal was detected using a GE680 charge-coupled device camera (Allied Vision Technologies, Burnaby, British Columbia, Canada) at a rate of 205 frames/s. All hearts had an intrinsic rate >60 beats/min before the stimulation protocols. Hearts were paced at rates greater than the intrinsic heart rate (pacing rates ranging from 100 to 180 beats/min), and all experiments were conducted at 28°C. Following control recordings, hearts were superfused with a hKCNH2a-modifying compound, and recordings were taken every 20 min. Recordings represent average AP traces from a region of interest defined by the experimenter. Typically, this included the majority of the ventricle. Ventricular APs were recorded immediately before (control condition) and 60 min after superfusion with a hKCNH2-modifying compound (drug condition). The ventricular APD from 50% upstroke to either 25% downstroke (APD25) or 75% downstroke (APD75) was calculated from hearts in control and drug conditions. The ΔAPD75 represents the drug induced in measured APD75 values (ΔAPD75 = APD75 drug − APD75 control).
Statistical methods.
Statistical differences between hKCNH2a and zkcnh6a were evaluated by a two-tailed independent t test (α = 0.05) for the functional characterization, pharmacological block, and fold change in peak current in response to an action potential protocol (Figs. 1, 2, and 5). Statistical tests could not be performed to assess differences between the EC50 values for the pharmacological activators tested in hKCNH2a compared with zkcnh6a (Figs. 3 and 4) since it was not always possible to collect data at all drug concentrations in each oocyte. Concentration-response curves for activator compounds and EC50 values, therefore, reflect fits to the mean data without statistical comparison. Statistical comparisons were made between mean data describing the effect of near-saturating concentrations (i.e., the highest concentration tested that induced a near-maximal effect) of each activator using a two-tailed independent t test. Statistical differences in APD following drug treatment (compared with control) were evaluated by a two-tailed, one-way repeated-measures ANOVA (α = 0.05) for the zebrafish ex vivo whole hearts (Fig. 6).
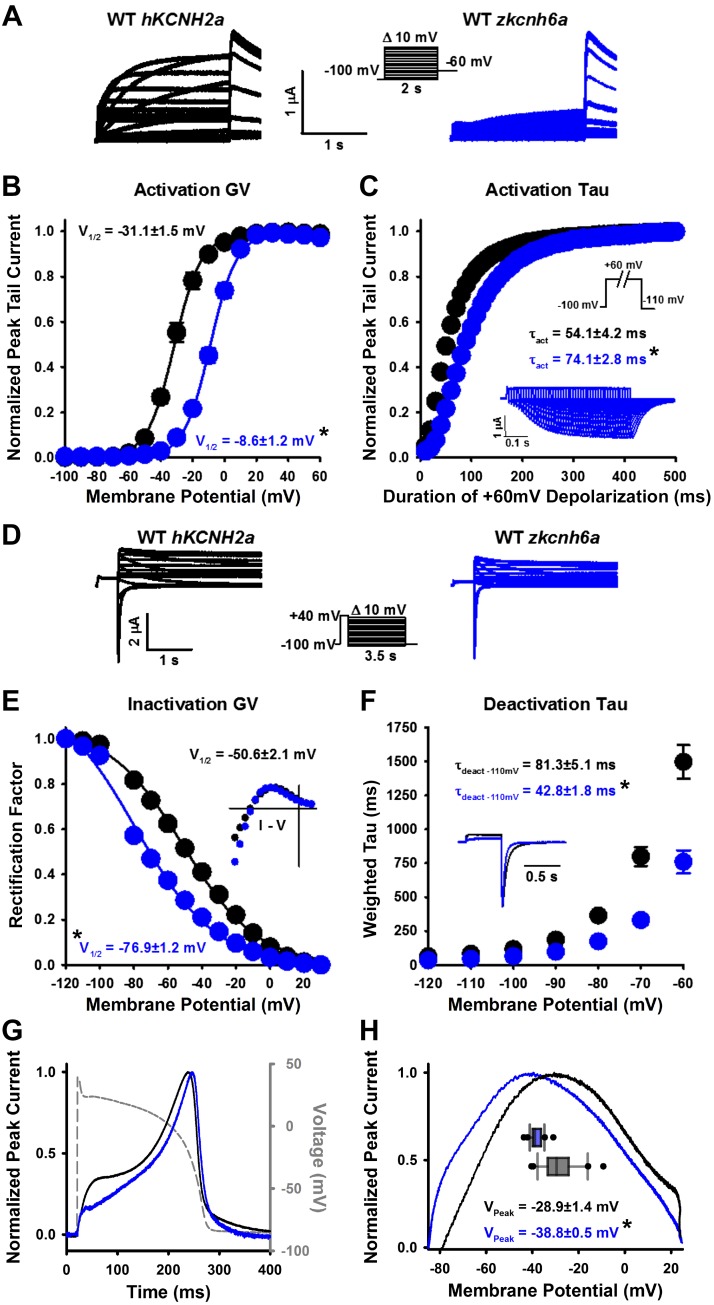
Fig. 1.
Characterization of the zkcnh6a K+ channel. A, D, and G: typical current traces recorded from hKCNH2a channels (black) and zkcnh6a channels (blue) in response to the voltage protocols shown. B: conductance-voltage (GV) relationships calculated from peak tail currents from hKCNH2a (n = 13) and zkcnh6a (n = 14) channels. C: normalized peak tail current amplitudes from hKCNH2a (n = 5) and zkcnh6a (n = 5) channels recorded during an envelope of tail voltage protocol (inset) to measure the time course of activation. Representative current traces from zkcnh6a are shown in the inset. τact, Time constant of activation. E: rectification factor for hKCNH2a (n = 13) and zkcnh6a (n = 14) calculated from the data in D as a measure of the voltage dependence of inactivation. F: weighted τ of deactivation (τdeact) following a 500-ms step to +40 mV for hKCNH2a (n = 13) and zkcnh6a (n = 14). Representative tail current traces at −110 mV are shown for hKCNH2a (black) and zkcnh6a (blue) in the inset. G: typical currents in response to an action potential protocol show that the characteristic morphology of hKCNH2a action potential current is well-preserved in the current from zkcnh6a. H: representative plot of the current-voltage relationship in response to an action potential protocol. Box and whisker plot represents the voltage of peak normalized current of hKCNH2a (n = 26) and zkcnh6a (n = 28). Asterisks denote a statistically significant difference between hKCNH2a and zkcnh6a (P < 0.05, 2-tailed independent t test), and error bars represent ± SE. I-V, current-voltage relationship; V1/2, half-activation potential; WT, wild-type.
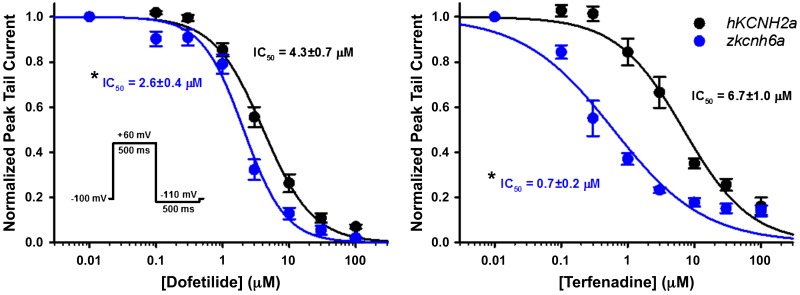
Fig. 2.
Effect of hKCNH2a channel blockers on zkcnh6a channels. Concentration-response relationships for the blocker actions of dofetilide (left) and terfenadine (right) on hKCNH2a (black) and zkcnh6a (blue) channels (n = 5–9) are shown. Concentration-response data were fitted with the Hill equation (see materials and methods). Asterisks denote a statistically significant difference in the IC50 concentration between hKCNH2a and zkcnh6a (P < 0.05, 2-tailed independent t test), and error bars represent ± SE.
Fig. 5.
zkcnh6a Current in response to a ventricular action potential voltage waveform. A: typical action potential current traces recorded before and after treatment with dofetilide (Dof; a hKCNH2a channel blocker: red) or NS1643 (NS; a hKCNH2a channel activator: green). B: plot of fold change in hKCNH2a (n = 5, 5, 4, 6, and 6) and zkcnh6a (n = 5, 5, 6, 6, and 6) peak current from control (absence of drug) following treatment with hKCNH2a channel blockers (dofetilide: 5 μM; terfenadine: 5 μM) and activators (NS6143: 30 μM; PD-118057: 10 μM; RPR260243: 10 μM). *Statistically significant difference in the fold change between hKCNH2a and zkcnh6a (P < 0.05, 2-tailed independent t test). C: zkcnh6a homology model (blue) showing from the S4 segment to the cyclic nucleotide binding homology domain (CNBHD) overlaid on the hKCNH2a cryogenic electron microscopy structure (gray; Ref. 51). D: sequence alignment of the S5 segment, pore helix, S6 segment, C-linker, and αA helix of the CNBHD (labeled as CNBHD) of hKCNH2a and zkcnh6a channels. Circles highlight residues involved in the agonist action of the hKCNH2a channel activators NS1643 (purple), PD-118057 (teal), and RPR260243 (olive; Ref. 42), and boxes highlight nonconserved residues. Ctrl, control; Terf, terfenadine.
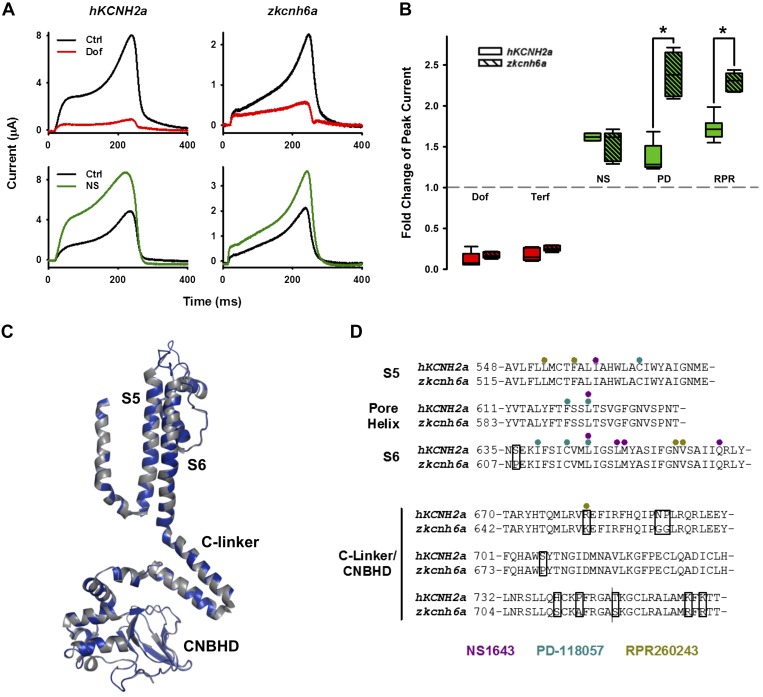
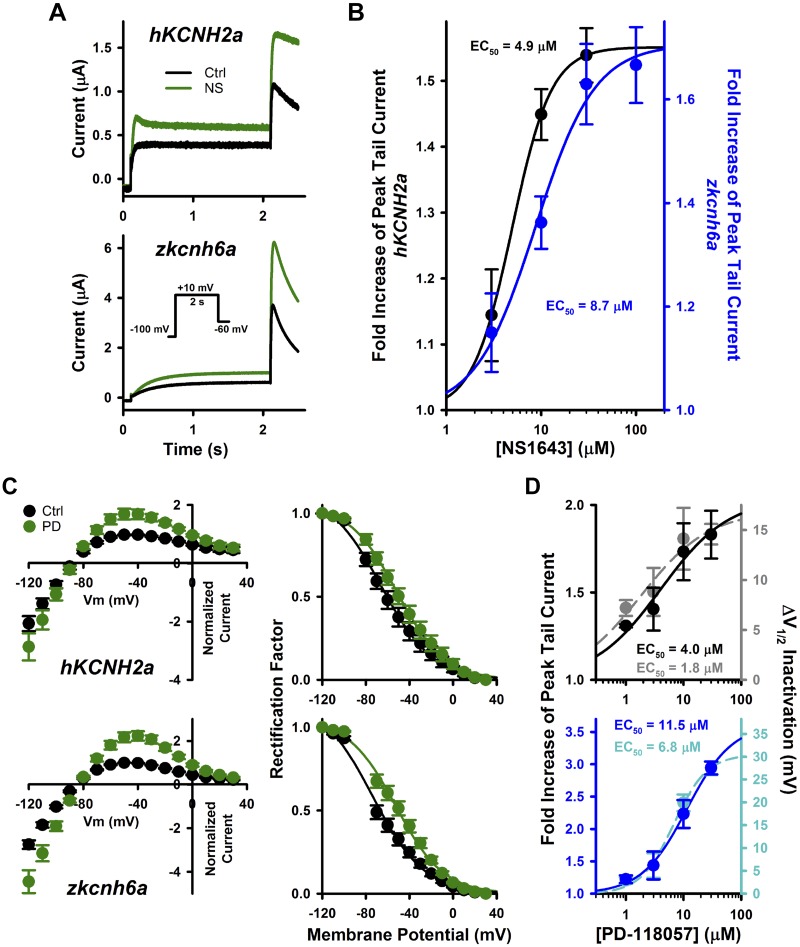
Fig. 3.
Effects of type 2 hKCNH2a channel activators on zkcnh6a channel. A: typical current traces recorded from hKCNH2a and zkcnh6a channels before and after NS1643 (NS; 30 μM) treatment. B: effect of NS1643 on peak tail current. Concentration-response data were fitted with the Hill equation (see materials and methods) for hKCNH2a (n = 5–6) and zkcnh6a (n = 5–7). C: fully activated current-voltage relationships following a 500-ms depolarizing step to +40 mV before and after treatment of hKCNH2a channels or zkcnh6a channels with 10 μM PD-118057 (PD). Fully activated I-V relationships were normalized to peak outward control tail current (left) and voltage dependence of inactivation determined from rectification of the fully activated I-V relationships (right; Ref. 43) from hKCNH2a (n = 6) and zkcnh6a (n = 6) channels before and after PD-118057 (10 μM) treatment. D: effect of PD-118057 on peak tail current (hKCNH2a: black, n = 5–6; zkcnh6a: blue, n = 5–6) and change in half-activation potential (ΔV1/2) of inactivation (hKCNH2a: gray, n = 5–6; zkcnh6a: light blue, n = 5–6). As in B, EC values were derived from the fit to the mean data. Error bars represent ± SE. Ctrl, control; Vm, membrane voltage.
Fig. 4.
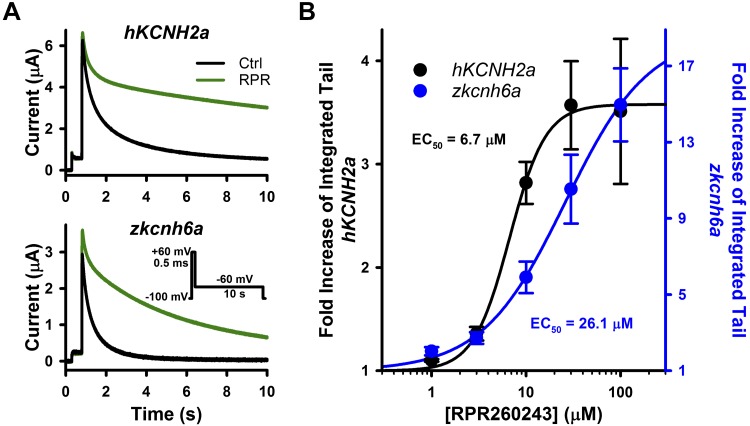
Effects of a type 1 hKCNH2a channel activator on zkcnh6a channels. A: typical current traces recorded from hKCNH2a and zkcnh6a channels before and after RPR260243 (RPR; 10 μM) treatment. A 500-ms depolarizing pulse to +60 mV was used to activate channels, and this was followed by a 10-s pulse to −60 mV to elicit tail currents. B: concentration-response relationships of RPR260243 with hKCNH2a (n = 3–6) and zkcnh6a (n = 3–5). Concentration-response data were fitted with the Hill equation (see materials and methods). EC values reflect the fit to the mean data as in Fig. 3. Effect of RPR260243 on deactivation was quantified by the integral of the tail drug and tail control currents at −60 mV during a 10-s pulse. Error bars represent ± SE. Ctrl, control.
Fig. 6.
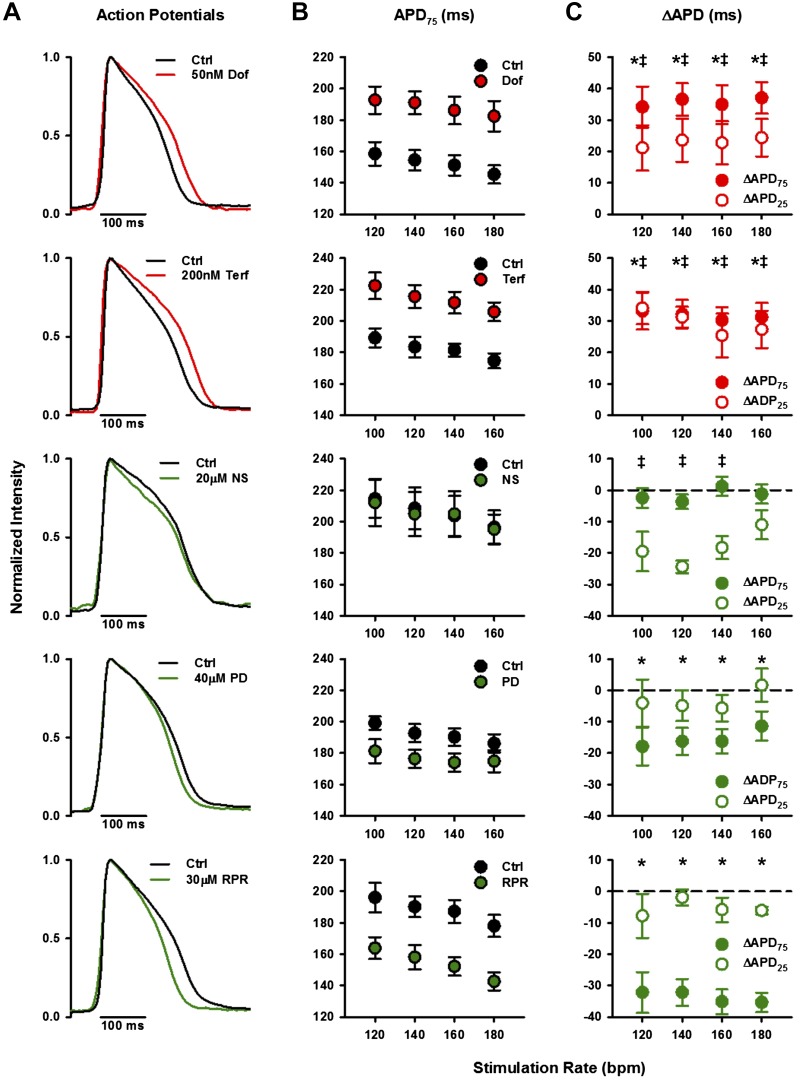
Optically recorded action potentials from paced adult zebrafish whole hearts treated with hKCNH2a channel blockers and activators. A: mean ventricular voltage responses (action potentials) of adult zebrafish whole hearts paced at 120 beats/min (bpm) before and after treatment with hKCNH2a channel blockers or activators. Control (Ctrl) averages are shown in black. hKCNH2a channel blocker averages are shown in red (top: 50 nM dofetilide, n = 6; bottom: 200 nM terfenadine, n = 5), and hKCNH2a channel activator averages are shown in green (top: 20 μM NS1643, n = 4; middle: 40 μM PD-118057, n = 6; bottom: 30 μM RPR260243, n = 5). B: mean ventricular action potential duration from 50% upstroke to 75% downstroke (APD75) before and after treatment with hKCNH2a channel blockers or activators (n = 4–6). C: change in APD (ΔAPD75, closed circles; ΔAPD25, open circles) following treatment with hKCNH2a channel blockers or activators (n = 4–6). Asterisks denote a statistically significant difference in APD75 and double-barred crosses denote a statistically significant difference in APD25 following drug treatment compared with control (P < 0.05, 2-tailed, 1-way repeated-measures ANOVA). Error bars represent ± SE. Dof, dofetilide; NS, NS1643; PD, PD-118057; RPR, RPR260243; Terf, terfenadine.
RESULTS
Functional characterization of zkcnh6a channels.
A recent study shows the presence of several zkcnh transcripts in the adult zebrafish ventricle: zkcnh2a, zkcnh2b, zkcnh6a, and zkcnh7, of which kcnh6a was by far the most predominant (50). Looking to use whole hearts to screen hERG blocker and activator compounds, we conducted a biophysical and pharmacological characterization of zkcnh6a channels expressed in a heterologous system. Our biophysical characterization of zkcnh6a (zERG3a) channels expressed in X. laevis oocytes, alongside that of hKCNH2a (hERG1a) channels, is shown in Fig. 1. The zkcnh6a channel had a right-shifted V1/2 of activation (−8.6 ± 1.2 mV) compared with that of hKCNH2a (−31.1 ± 1.5 mV; P < 0.001) with no change in the slope (8.2 ± 0.2 and 8.0 ± 0.3, respectively; P = 0.672) consistent with previous biophysical measurements (Ref. 45; Fig. 1B). The time constant of activation (τact) in response to a +60-mV depolarizing pulse was greater for zkcnh6a than hKCNH2a (Fig. 1C), with τact being 74.1 ± 2.8 and 54.1 ± 4.2 ms, respectively (P = 0.004). To assess inactivation properties, we compared the rectification of the two channels. Figure 1E shows that zkcnh6a channels display a left-shifted voltage dependence of inactivation (−76.9 ± 1.2 mV) compared with hKCNH2a channels (−50.6 ± 2.1 mV; P < 0.001). We also examined the kinetics of deactivation (τdeact) by fitting tail currents to a biexponential function. The zkcnh6a channel displayed accelerated τdeact compared with the hKCNH2a channel at all voltages examined (Fig. 1F; P < 0.001). To assess how the differences in zkcnh6a channel gating affect the morphology of the current contributing to an action potential, we recorded current traces in response to a ventricular action potential stimulus waveform (Fig. 1G). The overall morphology of the zkcnh6a channel current mimicked that of the hKCNH2a channel current, with limited outward current during the initial phases followed by a large outward current peaking during the repolarization phase of the voltage protocol. However, there was a reduction in the outward current passed during the plateau phase of the action potential in zkcnh6a channels. Quantification of the current-voltage relationship (Fig. 1H) shows that zkcnh6a channels display a negative shift in the voltage at which peak current occurred (−38.8 ± 0.5 mV) compared with hKCNH2a channels (−28.9 ± 1.4 mV; P < 0.001).
Effects of hKCNH2a channel blockers dofetilide and terfenadine on zkcnh6a channels.
Both dofetilide and terfenadine have been shown to be high-affinity hKCNH2a channel blockers. To assess the concentration dependence and efficacy of drug block by dofetilide and terfenadine, oocytes were subjected to a drug diary protocol that consisted of a repeated 500-ms depolarizing pulse to +60 mV followed by a −110-mV repolarizing pulse (pulse frequency, 0.1 Hz) to elicit tail currents. Peak tail currents in response to the −110-mV pulse were used to assess steady-state drug block of hKCNH2a channels and zkcnh6a channels (Fig. 2). Drug block of zkcnh6a channels by dofetilide was greater than the block of hKCNH2a channels with IC50 values of 4.3 ± 0.7 and 2.6 ± 0.4 μM, respectively (P = 0.045). Terfenadine showed a greater affinity for zkcnh6a channels compared with hKCNH2a channels, with IC50 values of 6.7 ± 1.0 μM for hKCNH2a and 0.7 ± 0.2 μM for zkcnh6a (P < 0.001).
Effects of a type 2 activator on zkcnh6a channels.
NS1643 is a type 2 activator compound (which primarily modifies hERG inactivation) that has been shown to increase hERG1a (hKCNH2a) channel tail currents. NS1643 leads to accelerated activation, slowed inactivation, a left-shifted voltage dependence of activation, and a right-shifted voltage dependence of inactivation, the combined influence of which leads to an increase in peak tail current amplitude (6, 17). Here, we measured the fold increase in the peak tail current (during a −60-mV pulse) following a 2-s depolarizing step to +10 mV to compare the effects of NS1643 on hKCNH2a and zkcnh6a. Both hKCNH2a channels and zkcnh6a channels showed an increase in outward current during the depolarizing +10-mV step as well as the −60-mV repolarizing step in response to treatment with 30 μM NS1643 (Fig. 3A). At this concentration, which was close to saturation of the NS1643 effect, the current was enhanced 1.5 ± 0.0-fold in hKCNH2a and 1.6 ± 0.1-fold in zkcnh6a (t test, P = 0.383), with EC50 values of 4.9 and 8.7 μM, respectively (Fig. 3B).
PD-118057 is another type 2 activator compound that has been shown to enhance hERG1a (hKCNH2a) channel function (38, 57). Application of PD-118057 leads to an increase in peak tail current as well as a right-shifted voltage dependence of inactivation (38, 57). Both hKCNH2a channels and zkcnh6a channels displayed increased tail current amplitude and a right shift in the voltage dependence of inactivation following treatment with 10 μM PD-118057 (Fig. 3C). At a near-saturating concentration (30 μM), PD-118057 increased peak tail current amplitude 1.8 ± 0.1-fold in hKCNH2a and 2.9 ± 0.1-fold in zkcnh6a (t test, P < 0.001), with EC50 values of 4.0 and 11.5 μM, respectively. The V1/2 of inactivation was shifted by +14.6 ± 1.0 mV in hKCNH2a and by +27.4 ± 1.5 mV in zkcnh6a (t test, P < 0.001), with EC50 values of 1.8 and 6.8 μM, respectively (Fig. 3D).
Effects of a type 1 activator on zkcnh6a channels.
RPR260243 is a type 1 activator compound (primarily modifies hERG deactivation) that has been shown to dramatically slow hERG1a channel (hKCNH2a) deactivation gating as well as right shift the voltage dependence of inactivation (20, 39). We observed a marked slowing of deactivation on application of 10 μM RPR260243 for both hKCNH2a and zkcnh6a channels (Fig. 4A). Since the effects of RPR260243 on deactivation kinetics are complex at higher RPR260243 concentrations, changes in deactivation were quantified by measuring the fold increase of the integrated tail current (integral Itail) as performed previously (39). At 30 μM, which was close to saturation of the effect on deactivation, RPR260243 increased the integral Itail 3.6 ± 0.4-fold in hKCNH2a and 10.5 ± 1.8-fold in zkcnh6a (t test, P = 0.016), with EC50 values of 6.7 and 26.1 μM, respectively (Fig. 4B).
Effects of hKCNH2a channel blockers and activators on zkcnh6a during an action potential.
Since many drugs that interact with hKCNH2a channels have multiple mechanisms of action, we sought to describe the composite effect of each drug tested on the current in response to an action potential voltage protocol (Fig. 5). As expected, we observed a large reduction in the current in all phases of the action potential protocol for both hKCNH2a channels and zkcnh6a channels treated with 5 μM dofetilide (Fig. 5A). Conversely, we observed enhanced current throughout the action potential protocol for both hKCNH2a channels and zkcnh6a channels treated with 30 μM NS1643 (Fig. 5A). The fold changes in zkcnh6a and hKCNH2a peak current during an action potential protocol in response to dofetilide (P = 0.221), terfenadine (P = 0.100), and NS1643 (P = 0.324) were not different, whereas fold increases in peak current in response to PD-118057 (P < 0.001) and RPR260243 (P < 0.001) were greater in zkcnh6a compared with hKCNH2a channels (Fig. 5B). A zkcnh6a homology model constructed based on the cryogenic electron microscopy structure of hERG (51) using SWISS-MODEL (Fig. 5C) and a sequence alignment of hKCNH2a and zkcnh6a with residues previously identified (14, 16, 30, 38–40, 53–55) to be involved in activator binding/coordination highlighted (Fig. 5D) suggests that the binding site for the activators examined would be expected to be conserved between zkcnh6a and hKCNH2a channels.
Evaluation of the effects of hKCNH2a-modifying compounds on zebrafish ex vivo whole hearts.
We aimed to use zebrafish whole hearts to study the potential of the model to screen for hKCNH2a blocker-induced arrhythmogenicity as well as for testing the therapeutic potential of hKCNH2a activator compounds. We examined the gross electrical activity of isolated zebrafish whole hearts by optically mapping voltage changes in excised cannulated hearts in the absence and presence of hKCNH2a blockers or activators (Fig. 6A). Superfusion of the hKCNH2a channel blockers dofetilide (50 nM) or terfenadine (200 nM) resulted in a prolongation of the APD75 (Fig. 6B). The drug-induced ΔAPD75 indicated significant prolongation (P < 0.05) at all stimulation rates tested for both dofetilide and terfenadine (Fig. 6C).
We next investigated the effects of hKCNH2a-activating compounds. Superfusion of RPR260243 (30 μM) or PD-118057 (40 μM) resulted in abbreviation of the APD75 (Fig. 6B). The RPR260243- and PD-118057-induced shortening of APD75 was significantly different (P < 0.05) at all stimulation rates tested, with the effects of RPR260243 being particularly robust, whereas there were no significant changes in APD25 (Fig. 6C). In the presence of NS1643 (20 μM), we did not observe shortening of the APD75 (Fig. 6, B and C); however, APD25 was significantly abbreviated (P < 0.05) at all but the highest stimulation rate tested (Fig. 6C).
DISCUSSION
One of the main goals of the present study was to evaluate the use of adult zebrafish hearts as a translational model for pharmacological investigation of both hKCNH2a channel blockers and activators. To do this, we first conducted a biophysical and pharmacological characterization of the zkcnh isoform, zkcnh6a, the predominant transcript expressed in adult zebrafish at 28°C. Detailed characterization showed some differences in the biophysical properties of zkcnh6a compared with hKCNH2a that manifest in a delay in repolarizing current during an action potential waveform. Our pharmacological characterization shows that the blockers tested reduce zkcnh6a and hKCNH2a current during a stylized ventricular action potential waveform to a similar degree, whereas activators increased zkcnh6a current to the same degree or greater than hKCNH2a current. Translating these findings to the zebrafish ex vivo whole heart model, we show that all hKCNH2a activator compounds tested abbreviate the ventricular action potential, although in at least one instance the mechanism by which this occurs appears complex. These findings lead us to suggest that zkcnh6a channel currents play a comparable role with hKCNH2a in the cardiac action potential, contributing primarily to phase 3 repolarization, and support the potential utility of the zebrafish whole heart model in screening for hKCNH2a channel activator compounds with some caveats discussed below.
We found the biophysical properties of zkcnh6a channels to be consistent with those described previously (45), with a right-shifted voltage dependence of activation and a left-shifted voltage dependence of inactivation compared with hKCNH2a. zkcnh6a also displayed slowed activation kinetics and accelerated deactivation kinetics compared with hKCNH2a. These differences appear to be of some potential functional significance as they produced differences in the morphology of zkcnh6a current and earlier repolarization in response to an action potential waveform voltage protocol, although the gross phenotype closely resembled that of hKCNH2a current. There are limitations to the interpretation of these observations in that our experiments, which were conducted at 20–22°C, cannot account for differential effects of temperature between hKCNH2a (37°C) and zkcnh6a (28°C) in their native in vivo environment. In addition, zkcnh6a and hKCNH2a channel currents were recorded in response to a stylized ventricular action potential, which may not fully represent zebrafish or human action potential morphology at physiological temperatures or at different rates.
Our pharmacological characterization showed that the IC50 values describing dofetilide and terfenadine drug block of zkcnh6a were reduced compared with those with hKCNH2a. The impact of these differences was assessed by measuring the effect of each compound on zkcnh6a or hKCNH2a current during an action potential waveform. We found that the blockers dofetilide and terfenadine reduced zkcnh6a and hKCNH2a current during an action potential waveform to the same extent. When examining hKCNH2a activator compounds, we found that types 1 and 2 hKCNH2a activator compounds enhanced zkcnh6a channel currents. The effect size, or potency, of an activator on the specific gating parameter measured was in some cases enhanced in zkcnh6a channels compared with hKCNH2a channels (e.g., NS1643 and RPR260243), and the EC50 values for all activators tested showed a trend to be higher in zkcnh6a compared with hKCNH2a. zkcnh6a and hKCNH2a current during an action potential waveform was again used to assess the overall significance of these gating alterations. We found that NS1643 enhanced zkcnh6a current during an action potential waveform to the same extent that hKCNH2a current was enhanced, whereas RPR260243 and PD-118057 had a greater enhancing effect on zkcnh6a than hKCNH2a currents during an action potential waveform. This may be due to a faster initial deactivation rate and a more negative initial voltage dependence of inactivation in zkcnh6a channels than hKCNH2a channels that allow for a larger effect size of the action of RPR260243 (which slows deactivation) and PD-118057 (which right shifts the voltage dependence of inactivation), respectively. Overall, these data lead us to suggest that zkcnh6a and hKCNH2a respond similarly to the blockers tested and that the effect of activators may be similar or enhanced in zkcnh6a channels compared with hKCNH2a channels depending on their mechanism of action. Given that channel biophysical properties and the action potential morphology may be different under physiological temperatures in zebrafish and human, further studies evaluating drug binding under these conditions would be valuable in assessing the utility of the zebrafish heart translational model.
With the use of optical mapping of zebrafish whole hearts, we examined the effects of hKCNH2a-modifying compounds at the adult zebrafish whole organ level at physiological temperature. Our selected hKCNH2a activator compounds have well-characterized mechanisms of action (6, 12, 13, 17, 20, 21, 29, 36, 38, 39, 55, 57) and have been shown to lead to abbreviation of the APD (12, 17, 20, 57). We found that both RPR260243 and PD-118057 caused abbreviation of the action potential duration. Both activators abbreviated APD75 (representative of the repolarization phase) without significant alteration to early phases of the action potential (assessed by changes to APD25). These abbreviations are likely to be zkcnh6a-mediated given the prominent role of IKr in zebrafish ventricular repolarization (1, 18, 24, 34). This is consistent with APD shortening observed in other models as a direct result of activator-induced gain of function of hKCNH2a channels. For example, PD-118057 and RPR260243 both shorten APD in guinea pig cardiomyocytes following dofetilide-induced APD prolongation (20, 57). Interestingly, although NS1643 shortened APD in a manner that could be reversed by E-4031 (17) and prevented triggered activity in hypokalemic Langendorff-perfused murine hearts (21), we did not observe APD shortening in the presence of NS1643. The NS1643 hKCNH2a activator did, however, alter the morphology of the plateau and reduce the APD25 (Fig. 6, A and B). In zebrafish, both L-type (ICaL) and T-type Ca2+ currents (ICaT) contribute to the plateau phase of the action potential and have a relatively larger current density compared with ICaL in the human heart (56). Evidence from canine cardiomyocytes suggests that ICaL is inhibited by NS1643 with a higher potency and a greater sensitivity (EC50 of 2.9 ± 0.4 μM; Ref. 46) than activation of IKr in the zebrafish heart. Thus a larger ICaL that is inhibited by NS1643 could account for the action potential morphology changes we observed.
The hKCNH2 blockers tested, dofetilide and terfenadine, prolonged APD as anticipated. Interestingly, both drugs prolonged APD75 and APD25, suggesting that zkcnh6a channels contribute to repolarization throughout much of the AP. Our findings of impaired repolarization are consistent with previous observations that terfenadine, cisapride, and E-4031 block caused bradycardia in 3-day-old zebrafish embryos (24), and terfenadine produced prolongation of the QTc interval, premature ventricular contractions, ventricular tachycardia, and atrioventricular block in isolated hearts (7). Terfenadine also restored WT-like function in reggae mutant embryos (18), whereas dofetilide prolonged APD in hearts isolated from WT zebrafish (15) and breakdance zebrafish (32).
Perspectives and Significance
Both electrophysiological examination of zkcnh6a channels and optical mapping of adult zebrafish whole hearts highlight the reliance on IKr current in zebrafish cardiac repolarization. Despite the differences in ion channel expression (i.e., hKCNH2a vs. zkcnh6a) in human and zebrafish cardiac tissue, our data lead us to suggest that ex vivo hearts provide an informative model for drug screening of hKCNH2a blocker compounds as well as hKCNH2a activator compounds. The apparent reliance on zERG channels for cardiac repolarization and effect of hERG-specific activators may afford a prescreening model for further evaluation in other, more sophisticated translational disease models, such as human induced pluripotent stem cell-derived cardiomyocytes. Furthermore, our findings highlight the complexity of pharmacological action in a whole organ physiologically relevant system and the importance of screening blockers and activators in a whole heart or similar complex system. Continued expansion of our understanding of zebrafish cardiac electrophysiology will enable the use of this translational model to study more complex cardiac physiology and pathology.
GRANTS
This research was supported by a Canadian Institutes of Health Research Project Grant (to T. W. Claydon) and a Natural Sciences and Engineering Research Council of Canada Discovery Grant (to T. W. Claydon). C. M. Hull was supported by a Natural Sciences and Engineering Research Council of Canada Alexander Graham Bell Canada Graduate Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M.H. and T.W.C. conceived and designed research; C.M.H., Y.H., and K.R. performed experiments; C.M.H. and Y.H. analyzed data; C.M.H. and T.W.C. interpreted results of experiments; C.M.H. prepared figures; C.M.H. and C.E.G. drafted manuscript; C.M.H., K.R., G.F.T., and T.W.C. edited and revised manuscript; C.M.H., C.E.G., Y.H., K.R., E.L., M.G., S.S., G.F.T., and T.W.C. approved final version of manuscript.
ACKNOWLEDGMENTS
Passages of this manuscript were previously presented in the PhD thesis of Dr. Hull (https://theses.lib.sfu.ca/file/thesis/5272).
REFERENCES
- 1.Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DY, Tristani-Firouzi M, Chi NC. Zebrafish model for human long QT syndrome. Proc Natl Acad Sci USA 104: 11316–11321, 2007. doi: 10.1073/pnas.0702724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz PF, Tanel RE, Zelster IJ, Pass RH, Wieand TS, Vetter VL, Vogel RL, Shah MJ. Congenital long QT syndrome and 2:1 atrioventricular block: an optimistic outcome in the current era. Heart Rhythm 7: 781–785, 2010. doi: 10.1016/j.hrthm.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 3.Baker K, Warren KS, Yellen G, Fishman MC. Defective “pacemaker” current (Ih) in a zebrafish mutant with a slow heart rate. Proc Natl Acad Sci USA 94: 4554–4559, 1997. doi: 10.1073/pnas.94.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brette F, Luxan G, Cros C, Dixey H, Wilson C, Shiels HA. Characterization of isolated ventricular myocytes from adult zebrafish (Danio rerio). Biochem Biophys Res Commun 374: 143–146, 2008. doi: 10.1016/j.bbrc.2008.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler A, Zhang Y, Stuart AG, Dempsey CE, Hancox JC. Action potential clamp characterization of the S631A hERG mutation associated with short QT syndrome. Physiol Rep 6: e13845, 2018. doi: 10.14814/phy2.13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casis O, Olesen SP, Sanguinetti MC. Mechanism of action of a novel human ether-a-go-go-related gene channel activator. Mol Pharmacol 69: 658–665, 2006. doi: 10.1124/mol.105.019943. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhari GH, Chennubhotla KS, Chatti K, Kulkarni P. Optimization of the adult zebrafish ECG method for assessment of drug-induced QTc prolongation. J Pharmacol Toxicol Methods 67: 115–120, 2013. doi: 10.1016/j.vascn.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Nüsslein-Volhard C. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development 123: 293–302, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Cheng YM, Hull CM, Niven CM, Qi J, Allard CR, Claydon TW. Functional interactions of voltage sensor charges with an S2 hydrophobic plug in hERG channels. J Gen Physiol 142: 289–303, 2013. doi: 10.1085/jgp.201310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 80: 795–803, 1995. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 11.D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW 2nd. Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci USA 94: 8121–8126, 1997. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diness TG, Hansen RS, Olesen SP, Grunnet M. Frequency-dependent modulation of KCNQ1 and HERG1 potassium channels. Biochem Biophys Res Commun 343: 1224–1233, 2006. doi: 10.1016/j.bbrc.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 13.Diness TG, Yeh YH, Qi XY, Chartier D, Tsuji Y, Hansen RS, Olesen SP, Grunnet M, Nattel S. Antiarrhythmic properties of a rapid delayed-rectifier current activator in rabbit models of acquired long QT syndrome. Cardiovasc Res 79: 61–69, 2008. doi: 10.1093/cvr/cvn075. [DOI] [PubMed] [Google Scholar]
- 14.Gardner A, Sanguinetti MC. C-linker accounts for differential sensitivity of ERG1 and ERG2 K+ channels to RPR260243-induced slow deactivation. Mol Pharmacol 88: 19–28, 2015. doi: 10.1124/mol.115.098384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genge CE, Lin E, Lee L, Sheng X, Rayani K, Gunawan M, Stevens CM, Li AY, Talab SS, Claydon TW, Hove-Madsen L, Tibbits GF. The zebrafish heart as a model of mammalian cardiac function. Rev Physiol Biochem Pharmacol 171: 99–136, 2016. doi: 10.1007/112_2016_5. [DOI] [PubMed] [Google Scholar]
- 16.Grunnet M, Abbruzzese J, Sachse FB, Sanguinetti MC. Molecular determinants of human ether-à-go-go-related gene 1 (hERG1) K+ channel activation by NS1643. Mol Pharmacol 79: 1–9, 2011. doi: 10.1124/mol.110.067728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen RS, Diness TG, Christ T, Demnitz J, Ravens U, Olesen SP, Grunnet M. Activation of human ether-a-go-go-related gene potassium channels by the diphenylurea 1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643). Mol Pharmacol 69: 266–277, 2006. doi: 10.1124/mol.105.015859. [DOI] [PubMed] [Google Scholar]
- 18.Hassel D, Scholz EP, Trano N, Friedrich O, Just S, Meder B, Weiss DL, Zitron E, Marquart S, Vogel B, Karle CA, Seemann G, Fishman MC, Katus HA, Rottbauer W. Deficient zebrafish ether-à-go-go-related gene channel gating causes short-QT syndrome in zebrafish reggae mutants. Circulation 117: 866–875, 2008. doi: 10.1161/CIRCULATIONAHA.107.752220. [DOI] [PubMed] [Google Scholar]
- 19.Jou CJ, Barnett SM, Bian JT, Weng HC, Sheng X, Tristani-Firouzi M. An in vivo cardiac assay to determine the functional consequences of putative long QT syndrome mutations. Circ Res 112: 826–830, 2013. doi: 10.1161/CIRCRESAHA.112.300664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang J, Chen XL, Wang H, Ji J, Cheng H, Incardona J, Reynolds W, Viviani F, Tabart M, Rampe D. Discovery of a small molecule activator of the human ether-a-go-go-related gene (HERG) cardiac K+ channel. Mol Pharmacol 67: 827–836, 2005. doi: 10.1124/mol.104.006577. [DOI] [PubMed] [Google Scholar]
- 21.Killeen MJ, Thomas G, Olesen SP, Demnitz J, Stokoe KS, Grace AA, Huang CL. Effects of potassium channel openers in the isolated perfused hypokalaemic murine heart. Acta Physiol (Oxf) 193: 25–36, 2008. doi: 10.1111/j.1748-1716.2007.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer K, van Acker SA, Voss HP, Grimbergen JA, van der Vijgh WJ, Bast A. Use of telemetry to record electrocardiogram and heart rate in freely moving mice. J Pharmacol Toxicol Methods 30: 209–215, 1993. doi: 10.1016/1056-8719(93)90019-B. [DOI] [PubMed] [Google Scholar]
- 23.Langenbacher AD, Dong Y, Shu X, Choi J, Nicoll DA, Goldhaber JI, Philipson KD, Chen JN. Mutation in sodium-calcium exchanger 1 (NCX1) causes cardiac fibrillation in zebrafish. Proc Natl Acad Sci USA 102: 17699–17704, 2005. doi: 10.1073/pnas.0502679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langheinrich U, Vacun G, Wagner T. Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol 193: 370–382, 2003. doi: 10.1016/j.taap.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Leong IU, Skinner JR, Shelling AN, Love DR. Identification and expression analysis of kcnh2 genes in the zebrafish. Biochem Biophys Res Commun 396: 817–824, 2010. doi: 10.1016/j.bbrc.2010.04.157. [DOI] [PubMed] [Google Scholar]
- 26.Leong IU, Skinner JR, Shelling AN, Love DR. Zebrafish as a model for long QT syndrome: the evidence and the means of manipulating zebrafish gene expression. Acta Physiol (Oxf) 199: 257–276, 2010. doi: 10.1111/j.1748-1716.2010.02111.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin E, Craig C, Lamothe M, Sarunic MV, Beg MF, Tibbits GF. Construction and use of a zebrafish heart voltage and calcium optical mapping system, with integrated electrocardiogram and programmable electrical stimulation. Am J Physiol Regul Integr Comp Physiol 308: R755–R768, 2015. doi: 10.1152/ajpregu.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.London B. Cardiac arrhythmias: from (transgenic) mice to men. J Cardiovasc Electrophysiol 12: 1089–1091, 2001. doi: 10.1046/j.1540-8167.2001.01089.x. [DOI] [PubMed] [Google Scholar]
- 29.Lu HR, Vlaminckx E, Hermans AN, Rohrbacher J, Van Ammel K, Towart R, Pugsley M, Gallacher DJ. Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B Guidelines. Br J Pharmacol 154: 1427–1438, 2008. doi: 10.1038/bjp.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao H, Lu X, Karush JM, Huang X, Yang X, Ba Y, Wang Y, Liu N, Zhou J, Lian J. Pharmacologic approach to defective protein trafficking in the E637K-hERG mutant with PD-118057 and thapsigargin. PLoS One 8: e65481, 2013. doi: 10.1371/journal.pone.0065481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milan DJ, Jones IL, Ellinor PT, MacRae CA. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am J Physiol Heart Circ Physiol 291: H269–H273, 2006. doi: 10.1152/ajpheart.00960.2005. [DOI] [PubMed] [Google Scholar]
- 32.Milan DJ, Kim AM, Winterfield JR, Jones IL, Pfeufer A, Sanna S, Arking DE, Amsterdam AH, Sabeh KM, Mably JD, Rosenbaum DS, Peterson RT, Chakravarti A, Kääb S, Roden DM, MacRae CA. Drug-sensitized zebrafish screen identifies multiple genes, including GINS3, as regulators of myocardial repolarization. Circulation 120: 553–559, 2009. doi: 10.1161/CIRCULATIONAHA.108.821082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 107: 1355–1358, 2003. doi: 10.1161/01.CIR.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- 34.Nemtsas P, Wettwer E, Christ T, Weidinger G, Ravens U. Adult zebrafish heart as a model for human heart? An electrophysiological study. J Mol Cell Cardiol 48: 161–171, 2010. doi: 10.1016/j.yjmcc.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Patel C, Antzelevitch C. Cellular basis for arrhythmogenesis in an experimental model of the SQT1 form of the short QT syndrome. Heart Rhythm 5: 585–590, 2008. doi: 10.1016/j.hrthm.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peal DS, Mills RW, Lynch SN, Mosley JM, Lim E, Ellinor PT, January CT, Peterson RT, Milan DJ. Novel chemical suppressors of long QT syndrome identified by an in vivo functional screen. Circulation 123: 23–30, 2011. doi: 10.1161/CIRCULATIONAHA.110.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry M, Sachse FB, Abbruzzese J, Sanguinetti MC. PD-118057 contacts the pore helix of hERG1 channels to attenuate inactivation and enhance K+ conductance. Proc Natl Acad Sci USA 106: 20075–20080, 2009. doi: 10.1073/pnas.0906597106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry M, Sachse FB, Sanguinetti MC. Structural basis of action for a human ether-a-go-go-related gene 1 potassium channel activator. Proc Natl Acad Sci USA 104: 13827–13832, 2007. doi: 10.1073/pnas.0703934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry M, Sanguinetti MC. A single amino acid difference between ether-a-go-go-related gene channel subtypes determines differential sensitivity to a small molecule activator. Mol Pharmacol 73: 1044–1051, 2008. doi: 10.1124/mol.107.043018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rayani K, Lin E, Craig C, Lamothe M, Shafaattalab S, Gunawan M, Li AY, Hove-Madsen L, Tibbits GF. Zebrafish as a model of mammalian cardiac function: optically mapping the interplay of temperature and rate on voltage and calcium dynamics. Prog Biophys Mol Biol 138: 69–90, 2018. doi: 10.1016/j.pbiomolbio.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Sanguinetti MC. HERG1 channel agonists and cardiac arrhythmia. Curr Opin Pharmacol 15: 22–27, 2014. doi: 10.1016/j.coph.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81: 299–307, 1995. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 44.Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol 96: 195–215, 1990. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scholz EP, Niemer N, Hassel D, Zitron E, Bürgers HF, Bloehs R, Seyler C, Scherer D, Thomas D, Kathöfer S, Katus HA, Rottbauer WA, Karle CA. Biophysical properties of zebrafish ether-à-go-go related gene potassium channels. Biochem Biophys Res Commun 381: 159–164, 2009. doi: 10.1016/j.bbrc.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 46.Szabó G, Farkas V, Grunnet M, Mohácsi A, Nánási PP. Enhanced repolarization capacity: new potential antiarrhythmic strategy based on HERG channel activation. Curr Med Chem 18: 3607–3621, 2011. doi: 10.2174/092986711796642382. [DOI] [PubMed] [Google Scholar]
- 47.Taggart P, Sutton PM, Boyett MR, Lab M, Swanton H. Human ventricular action potential duration during short and long cycles. Rapid modulation by ischemia. Circulation 94: 2526–2534, 1996. doi: 10.1161/01.CIR.94.10.2526. [DOI] [PubMed] [Google Scholar]
- 48.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269: 92–95, 1995. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 49.Uechi M, Asai K, Osaka M, Smith A, Sato N, Wagner TE, Ishikawa Y, Hayakawa H, Vatner DE, Shannon RP, Homcy CJ, Vatner SF. Depressed heart rate variability and arterial baroreflex in conscious transgenic mice with overexpression of cardiac Gsα. Circ Res 82: 416–423, 1998. doi: 10.1161/01.RES.82.4.416. [DOI] [PubMed] [Google Scholar]
- 49a.van Opbergen CJ, Koopman CD, Kok BJ, Knöpfel T, Renninger SL, Orger MB, Vos MA, van Veen TA, Bakkers J, de Boer TP. Optogenetic sensors in the zebrafish heart: a novel in vivo electrophysiological tool to study cardiac arrhythmogenesis. Theranostics 8: 4750–4764, 2018. doi: 10.7150/thno.26108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vornanen M, Hassinen M. Zebrafish heart as a model for human cardiac electrophysiology. Channels (Austin) 10: 101–110, 2016. doi: 10.1080/19336950.2015.1121335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W, MacKinnon R. Cryo-EM structure of the open human ether-à-go-go-related K+ channel hERG. Cell 169: 422–430.e10, 2017. doi: 10.1016/j.cell.2017.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warren KS, Baker K, Fishman MC. The slow mo mutation reduces pacemaker current and heart rate in adult zebrafish. Am J Physiol Heart Circ Physiol 281: H1711–H1719, 2001. doi: 10.1152/ajpheart.2001.281.4.H1711. [DOI] [PubMed] [Google Scholar]
- 53.Wu W, Gardner A, Sanguinetti MC. Concatenated hERG1 tetramers reveal stoichiometry of altered channel gating by RPR-260243. Mol Pharmacol 87: 401–409, 2015. doi: 10.1124/mol.114.096693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu W, Sachse FB, Gardner A, Sanguinetti MC. Stoichiometry of altered hERG1 channel gating by small molecule activators. J Gen Physiol 143: 499–512, 2014. doi: 10.1085/jgp.201311038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu X, Recanatini M, Roberti M, Tseng GN. Probing the binding sites and mechanisms of action of two human ether-a-go-go-related gene channel activators, 1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643) and 2-[2-(3,4-dichloro-phenyl)-2,3-dihydro-1H-isoindol-5-ylamino]-nicotinic acid (PD307243). Mol Pharmacol 73: 1709–1721, 2008. doi: 10.1124/mol.108.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang PC, Llach A, Sheng XY, Hove-Madsen L, Tibbits GF. Calcium handling in zebrafish ventricular myocytes. Am J Physiol Regul Integr Comp Physiol 300: R56–R66, 2011. doi: 10.1152/ajpregu.00377.2010. [DOI] [PubMed] [Google Scholar]
- 57.Zhou J, Augelli-Szafran CE, Bradley JA, Chen X, Koci BJ, Volberg WA, Sun Z, Cordes JS. Novel potent human ether-à-go-go-related gene (hERG) potassium channel enhancers and their in vitro antiarrhythmic activity. Mol Pharmacol 68: 876–884, 2005. doi: 10.1124/mol.105.014035. [DOI] [PubMed] [Google Scholar]