Abstract
Tissue engineering is promising in realizing successful treatments of human body tissue loss that current methods cannot treat well or achieve satisfactory clinical outcomes. In scaffold-based bone tissue engineering, a high performance scaffold underpins the success of a bone tissue engineering strategy and a major direction in the field is to produce bone tissue engineering scaffolds with desirable shape, structural, physical, chemical and biological features for enhanced biological performance and for regenerating complex bone tissues. Three-dimensional (3D) printing can produce customized scaffolds that are highly desirable for bone tissue engineering. The enormous interest in 3D printing and 3D printed objects by the science, engineering and medical communities has led to various developments of the 3D printing technology and wide investigations of 3D printed products in many industries, including biomedical engineering, over the past decade. It is now possible to create novel bone tissue engineering scaffolds with customized shape, architecture, favorable macro-micro structure, wettability, mechanical strength and cellular responses. This article provides a concise review of recent advances in the R & D of 3D printing of bone tissue engineering scaffolds. It also presents our philosophy and research in the designing and fabrication of bone tissue engineering scaffolds through 3D printing.
Keywords: 3D printing, Bone tissue engineering, Scaffold, Bioceramic, Polyester, Hydrogel, Biomolecule, Controlled release
Highlights
-
•
3D printing of bioceramic pastes followed by sintering improves bone formation.
-
•
Cryogenic 3D printing of drug loaded polymer solution supports cell differentiation.
-
•
Surface modification of 3D printed scaffolds improves the cellular responses.
1. Introduction
Three-dimensional (3D) printing, also known as additive manufacturing (AM) and rapid prototyping, is a process of joining materials to make objects from 3D model data, usually layer-by-layer, as opposed to subtractive manufacturing methodologies [1]. 3D printing is a versatile technique to fabricate a variety types of materials including polymers, ceramics, metals and composites, with customized shapes and dense or macro/micro porous architecture [[2], [3], [4], [5], [6], [7]]. 3D printed objects can be used in many industries for applications such as manufacturing of turbine blade, jewelry designing, mould making, building, tissue engineering, etc [[8], [9], [10], [11], [12]]. The origin of 3D printing dates back to late 19th century, when photosculpture and geomorphology technologies were developed. Between 1980s and 2010s, a number of 3D printing techniques including fused deposition modeling (FDM), selective laser sintering (SLS), stereolithography, selective laser melting, ink jet 3D printing, adhesive droplet and powder bed-based AM, digital laser processing, continuous liquid interface production, etc., have been developed based on different working principles [[13], [14], [15], [16], [17], [18]]. The 3D printed materials and products are advantageous in following aspects: customized shape, tailored pore size/porosity, tuned mechanical properties, etc. The number of publications on 3D printing, its application in different industries and 3D printing products has sky-rocketed over the past decades and in 2018–2019 alone there were about 15,726 journal publications on 3D printing and associated topics.
3D printing has attracted great attention in the biomedical field for two main reasons: the technology itself - its versatility, ease of use and precise control of the fabrication process, and the products it produces - customized shape and structure possessing unique architecture and properties that are highly sought after for biomedical applications. In the biomedical field, the number of peer-reviewed articles on 3D printing, 3D printed structures and their potential clinical applications has increased exponentially from 8 publications in 2002–2906 publications in 2018. Good review articles on 3D printing and associated topics, which are relevant to biomedical engineering, have been written by leading or established researchers [[19], [20], [21], [22], [23]] and the 3D printing technology and 3D printing products have been constantly investigated worldwide by an increasing number of researchers, established or new in the biomedical field. For human bone tissue regeneration, a few strategies hold great promises. Among these strategies, scaffold-based tissue engineering has been widely and intensively investigated [24]. A tissue engineering scaffold should provide a conductive microenvironment for the selected cells and recent trend is to create customized scaffolds with pre-designed shapes, structure and functions for enhanced tissue regeneration [25]. Among numerous methods, 3D printing has been considered as an advantageous technique in fabricating tissue engineering scaffolds, as the 3D printed macro-micro structure could morphologically mimic the multi-scale structure of human body tissues [26]. Apart from this, scaffolds produced through certain 3D printing technique are excellent delivery vehicles to provide local, sustained release of drugs and/or biomolecules [27]. Many investigations have been and are being conducted to impart 3D printed scaffolds with new functions [28]. By appropriate surface modification, biologically active molecules and cell recognizable ligands can be physically or chemically linked to scaffold surface. Functional nanoparticles and drugs can also be directly mixed with a synthetic/natural polymer solution which is then 3D printed to form functional tissue engineering scaffolds. This article provides a concise review of recent advances in the R & D of 3D printing of bone tissue engineering scaffolds. It also gives our philosophy in designing and making advanced bone tissue engineering scaffolds with appropriate functions and presents some of our recent research in this area.
2. Requirements of 3D printed bone tissue engineering scaffolds
The regeneration of bone is complex because it involves a number of molecular, cellular, biochemical and mechanical cues. Therefore, porous bone tissue engineering scaffolds with appropriate shape, pore size, porosity, degradability, biocompatibility, mechanical properties and desirable cellular responses are required to induce bone regeneration.
2.1. Composition
Bioceramic powders, natural/synthetic hydrogels, non-hydrogel-based polymers and their composites have been adopted as raw materials to formulate printing inks for 3D printing. Biodegradable and biocompatible polyesters such as poly(l-lactic acid) (PLLA), poly(vinyl alcohol) (PVA), poly-β-hydroxybutyrate (PHB), polyurethane elastomers, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), poly(D,l-lactic acid) (PDLLA), poly(lactic-co-lgycolic acid) (PLGA) and polycaprolactone (PCL) and polyurethanes can be processed into wires, pellets and even powders to enable 3D printing of polymer scaffolds with the assistance of high temperature melting-extrusion and sintering, or dissolved in organic/aqueous solvents to allow micro extrusion-based 3D printing at room/low temperature [[29], [30], [31], [32], [33], [34]]. Likewise, hydrogel precursors made of aqueous solutions of natural polymers including chitosan, collagen, gelatin, gelatin methacryloyl, hyaluronic acid, sodium alginate and polyethelyene glycol diacrylate (PEGDA) could be 3D printed into scaffolds at room- or low temperature and further stabilized via UV-, ion- or temperature-assisted crosslinking. Bioceramic precursors or bioceramic powders including micro- or nanosized calcium phosphates (Ca–P), hydroxyapatite (HA), β-tricalcium phosphate (β-TCP), carbonated calcium deficient hydroxyapatite (CDHA), bioactive glasses, etc., can be blended with binders or photopolymers to form printing inks to make 3D printed “green body”, which are further sintered to remove the organic phase, leaving only hard bioceramic scaffolds. Bioceramic micro/nanoparticles can be further blended with biodegradable synthetic/natural polymers to make 3D scaffolds through micro-extrusion without post-sintering. The use of composites as printing inks not only provides scaffolds with osteoconductivity but also improves the degradability of the inorganic particulate fillers and alters physical, mechanical and biological properties of scaffolds. In addition, functional agents such as drugs, biomolecules could also be incorporated to provide scaffolds with capabilities of anti-bacteria, osteogenesis, angiogenesis, etc.
2.2. Structure
Appropriate macro- and microstructures are key features in bone tissue engineering scaffolds. On one hand, patterned macropores in scaffolds could influence the cell penetration and cell distribution, and most importantly, enables the transportation of gases and nutrients into the deeper layer of scaffolds, hence maintaining the cell viability at a high level [35]. On the other hand, micropores on scaffold struts are crucial in determining the initial cellular responses such as cell attachment and cell spreading [36]. Moreover, adjusting the size of micropores could tune the release behaviour of drugs/biomolecules/agents loaded in the scaffold matrix or adsorbed/absorbed/conjugated on the scaffold surface.
2.3. Mechanical properties
Human bone tissue comprise of cortical bone and cancellous bone. Cortical bone is dense, showing a porosity of only 5–10%, while cancellous bone, which has a spongy-like structure, has a porosity of 50–90%. Cortical bone accounts for up to 80% of the weight of human skeleton while cancellous bone accounts for the rest 20%. Cortical bone has significantly higher compressive strength and Young's modulus than cancellous bone [37]. Therefore, when repairing load bearing and non-load bearing bone tissues, the 3D printed bone tissue engineering scaffolds should have match mechanical properties in order to provide sufficient mechanical support and avoid stress shielding.
2.4. Cellular responses
Whether 3D printed bone tissue engineering scaffolds could induce favorable cellular responses is also of great importance in scaffold designing. First and foremost, the scaffold should be biocompatible, showing no acute or long-term toxicity in vitro and in vivo. Secondly, as the initial cell attachment, spreading and proliferation closely relate to the scaffold surface properties such as wettability and surface chemistry, providing scaffolds with suitable surface hydrophilicity is desirable and this can be achieved by coating hydrophilic polymers and bio-agents such as peptides and biomolecules on scaffold surface. Besides, to improve bone regeneration, the controlled delivery of osteoconductive and osteoinductive agents in scaffolds is strongly recommended to induce sufficient osteogenesis in vitro and in vivo. Last but not the least, growth factors to enhance vascular formation and angiogenesis can also be delivered to improve the scaffold vascularization during bone regeneration process.
3. Ceramic-based bone tissue engineering scaffolds
3.1. 3D printing of ceramic scaffolds followed by sintering
To date, 3D printed bioceramic scaffolds have been widely used as bone tissue engineering scaffolds, as bioceramic scaffolds are mechanically, structurally and compositionally similar to bone apatite in native bone tissue. The most employed strategy to produce ceramic-based bone scaffolds is to print “green body” scaffolds with customized shape and pore size/porosity, followed by high temperature sintering, which burns out all organic phases, forming pure ceramic scaffolds. Size shrinkage may occur after sintering but the mechanical strength and Young's modulus could be greatly increased. Seidenstuecker et al. produced bioglass/β-TCP green body scaffolds via micro extrusion-based 3D printing at room temperature, using dextrin as binder to bind bioglass and β-TCP powders, followed by high temperature sintering [38]. Although the mechanical strengths of the scaffolds were 0.17–0.64 MPa, making them unsuitable for load bearing applications, these scaffolds can be used as bone fillers due to the improved cellular responses. Song et al. used both cryogenic 3D printing and post-sintering to produce bone tissue engineering scaffolds with both hierarchically porous structure (interconnective macro pores and microholes on strut surface) and superior compressive strength [39]. Chen et al. constructed a lithium-calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction through 3D printing, followed by post-sintering [40]. The scaffolds were mechanically strong and supported both osteogenic differentiation of mesenchymal stem cells (MSCs) and chondrogenic differentiation of chondrocytes in vitro and in vivo. The advantages and disadvantages of scaffolds made through major 3D printing techniques are listed in Table 1.
Table 1.
Comparison of bone tissue engineering scaffolds made through different 3D printing techniques.
| Technique | Illustrative schematic | Advantage | Challenge | Ref |
|---|---|---|---|---|
| 3D printing of ceramic based bone tissue engineering scaffolds | 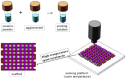 |
Scaffolds are mechanically, structurally and compositionally similar to native bone; scaffolds have higher porosity, controlled swelling profile, enhanced biomineralization capacity and osteogenic property; polymer coating on scaffolds promotes bone ingrowth with improved osteoblast cell viability and proliferation under hypoxic conditions, | Unsuitable for load bearing applications; relatively low compressive strength and modulus; brittleness | [[38], [39], [40]], [[42], [43], [44]] |
| 3D printing of hydrogel based bone tissue engineering scaffolds | 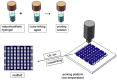 |
High water content; relatively high tensile strength; large stretchability; excellent protein/cell loading ability; controlled release of biomolecules/drugs; micro/nanoporous structure for up-regulated cell attachment, proliferation and osteogenic differentiation. | PVA resist protein absorption and cell adhesion; much lowered compressive strength than that of the human cancellous bone tissue; quick degradation | [[45], [46], [47], [48], [49], [50], [51], [52], [53]] |
| 3D printing of polyester scaffolds at high temperature & post-treatment | 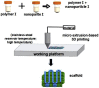 |
Simplified operation process; greater convenience and flexibility; excellent reproducibility; eco-friendly; high printing resolution; better cell colonization and proliferation; incorporate bioceramic particles to improve mechanical properties and wettability; coating of natural polymers on scaffold surface improves cell attachment; delivery of biomolecules improve osteogenesis and angiogenesis | Uneven distribution of bioceramic particles; defects between bioceramics and polyester due to bad interaction; scaffolds with a higher porosity have lower mechanical strength; quick release of biomolecules | [[54], [55], [56], [57], [58], [59], [60], [61], [62],[70], [71], [72], [73], [74], [75], [76], [77], [78]] |
| Cryogenic 3D printing of bone tissue engineering scaffolds | 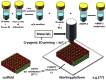 |
Excellent compressive strength; in situ delivery of biomolecules with preserved biological activity; good biodegradability and biocompatibility; macro/micro porous structure for improved cell adhesion and infiltration; controlled release of biomolecules; incorporation of bioceramics for better osteogenesis; incorproation of graphene oxide enhances the osteogenesis | – | [[63], [64], [65]] |
| 3D printing of synthetic/natural polymer bone tissue engineering scaffolds via electrostatic field-assisted micro extrusion |  |
Electrospun fibers can be printed into regular pattern with varied layers; incorporation of nHA in polyesters provides scaffolds with good biocompatibility and facilitated cellular alignment and proliferation in vitro. | Limited by the maximum number of layers that can be produced before control over fibre placement is lost due to the accumulation of instabilities | [[66], [67], [68], [69],[80], [81], [82], [83]] |
3.2. 3D printing of ceramic scaffolds at room/low temperature
Apart from high temperature sintering, in recent years, an increasing number of studies adopted non-sintered ceramic scaffolds to induce bone tissue regeneration. On this condition, a small portion of organic/natural polymer binders are used to bind ceramic powders and not removed after 3D printing. Song et al. reported the fabrication of nano-biphasic calcium phosphate (nBCP)/PVA (mass ratio of nBCP: PVA = 84: 16) composites incorporated with platelet-rich fibrin using an extrusion-based low temperature 3D printing [41]. Improved in vitro biocompatibility and biological activity toward bone marrow-derived MSCs (BMSCs) such as BMSC adhesion, proliferation, and osteogenic differentiation were obtained and a greater extent of appropriate bone formation in a critical-size segmental bone defect model in rabbits was also observed. However, the scaffolds had a relatively low compressive strength and modulus, showing only 1/10 of that of human cancellous bone. To mimic the natural architecture of hollow bones, which allow nutrient exchange and bone neovascularization, Boga et al. fabricated cylindrical graphene oxide/TCP/alginic acid (GO/TCP/AA) scaffolds using a Fab@home 3D printer [42]. The GO containing scaffolds showed higher porosity, without impairing their mechanical properties. These scaffolds also presented a controlled swelling profile, enhanced biomineralization capacity and increased alkaline phosphatase (ALP) activity.
3.3. Post-functionalization of 3D printed ceramic scaffolds
To further provide bioceramic scaffolds with additional functions, post-functionalization including coating and post-adsorption of functional agents has been widely adopted. Touri et al. fabricated latticed scaffolds from the robocasting paste containing 60 vol% HA and 40 vol% β-TCP through 3D printing, followed by sintering [43]. The sintered 3D scaffolds were further coated with calcium peroxide (CPO)/PCL composite through dip-coating for in situ production of oxygen in the implanted sites. The oxygen released in a sustained manner and was dependant on the concentration of CPO encapsulated in the PCL coating matrix. The coated scaffolds could provide a great potential for promoting bone ingrowth with improved osteoblast cell viability and proliferation under hypoxic conditions. Kim et al. incorporated bone morphogenetic protein-2 (BMP-2)-loaded PLGA nanoparticles on the surface of a 3D printed HA scaffold using an PCL emulsion coating method [44]. BMP-2/PLGA nanoparticles were uniformly distributed on the scaffold surface and BMP-2 was released gradually. Moreover, PCL coating improved the compressive strength of the scaffold. The in vitro cell proliferation, adhesion, and osteogenic differentiation and in vivo new bone formation were improved with PCL-BMP-2/PLGA nanoparticle coated scaffold.
4. Hydrogel bone tissue engineering scaffolds
4.1. 3D printing of hydrogel scaffolds
In recent years, due to the capability of encapsulating biomolecules and cells in situ, hydrogels have been used to make bone tissue engineering scaffolds via extrusion-based 3D printing. By tuning the hydrogel composition, scaffolds with varied mechanical properties and cellular responses can be obtained, and some of them showed potential in bone regeneration. Kim et al. employed gelatin/PVA solution as printing inks to produce tissue engineering scaffolds for hard tissue repair on a low temperature plate [45]. The addition of PVA was to provide biocompatible gelatin scaffolds with improved mechanical strength. Through composition optimization, scaffolds with excellent printability and porous structure can be produced. The gelatin/PVA scaffolds with a gelatin: PVA ratio of 1:1 showed both improved mechanical properties and enhanced cell activities such as improved cell seeding efficiency, proliferation and osteogenic differentiation. However, the compressive strength was still much lower than that of the human cancellous bone tissue. Sithole et al. produced a polymeric scaffold by employing sodium alginate as a bio-ink which interacted with a poly (ethyleneimine) solution during 3D bioprinting to form a polyelectrolyte complex through ionic bond formation. The silica gel was included in the bio-ink as temporal inorganic support component and for ultimate enhancement of osteoinduction [46]. The fabricated scaffolds had relatively high tensile strength, suggesting this scaffold could be used for bone tissue engineering. Lee et al. fabricated micro/nanoporous collagen/decellularized extracellular matrix (ECM)/silk-fibroin biocomposite scaffolds using a low temperature 3D printing [47]. The micro/nanoporous structure and the ECM-like composition were favorable for inducing up-regulated cell attachment, proliferation and osteogenic differentiation in vitro. However, the compressive strength and modulus were significantly lower than that of human cancellous bone, making them a bit difficult to fill the requirements for bone tissue repair. Gao et al. produced hydrogel scaffolds with gradient composition/structure and excellent mechanical strength through direct 3D printing [48]. The cartilage layer and the subchondral layer in the gradient scaffolds had distinct microscopic structures, compositions and anisotropic mechanical strengths. The gradient scaffolds could induce in vitro chondrogenic and osteogenic differentiation of MSCs in a spatial-tempro manner. Moreover, the in vivo study also confirmed their effectiveness in simultaneously achieving new bone formation and cartilage repair, which is highly desirable for the osteochondral tissue regeneration. Luo et al. used concentrated alginate/gelatin solutions as printing inks to make scaffolds via direct 3D printing, followed by homogeneous precipitation of nanoapatite coating on scaffold surface [49]. The biomimetic scaffolds had excellent mechanical properties which were higher than that of human cancellous bone and had excellent capability of adsorbing proteins. The precipitated apatite stimulated the proliferation and osteogenic differentiation of rat BMSCs on scaffolds. They also produced 3D bioprinted scaffolds using alginate/PVA as inks [50]. By adjusting the mass ratio between alginate and PVA, scaffolds without collapse can be constructed, followed by immersion in CaCl2 solution for fully crosslinking. Moreover, bovine serum albumin (BSA) and recombinant human BMP-2 (rhBMP-2) were encapsulated in alginate hydrogel microparticles and loaded in printing inks to produce BSA or rhBMP-2 loaded alginate/PVA scaffolds, in which the release behaviour of BSA and rhBMP-2 can be controlled by adjusting the pore size of microparticles. Heo et al. incorporated bone forming peptide into alginate through EDC/NHS coupling to formulate bioinks and printed them into hybrid bone tissue engineering scaffolds [51]. In vitro and in vivo results demonstrated that the alginate-based scaffolds provided a stable environment for the growth of human adipose-derived stem cells and induced synergistic effect to enhance bone regeneration. However, the mechanical properties of the scaffolds were not studied.
4.2. 3D printing of cell-laden hydrogel scaffolds
Zhai et al. 3D printed osteoblast-laden nanocomposite constructs based on PEGDA/nanoclay/hyaluronic acid sodium salt hydrogel bio-inks, with a two-channel 3D bioprinting method [52]. The cell-laden PEG/clay constructs not only encapsulated osteoblasts with higher than 95% viability in the short-term but also exhibited excellent osteogenic ability in the long-term due to the release of bioactive ions such as magnesium ions and silicon ions, which induced the suitable microenvironment to promote the differentiation of the loaded exogenous cells in vitro and in vivo, suggesting a promising way for bone tissue regeneration in terms of cell engraftment, survival, and ultimately long-term function. Demirtas et al. reported MC3T3-E1 pre-osteoblastic cell-laden chitosan and chitosan-HA hydrogels were produced via extrusion-based 3D printing [53]. Cells printed within chitosan/HA composite hydrogel had peak expression levels for early- and late stage osteogenic markers. Compared to alginate-based hydrogels, chitosan-based hydrogels showed superior printability and potential to act as suitable bio-inks for making cell-laden hydrogel scaffolds for bone tissue engineering. However, the elastic modulus (15 kPa) is significantly lower than that of human cancellous bone.
5. Non-hydrogel-based polymer bone tissue engineering scaffolds
5.1. 3D printing of polyester scaffolds at high temperature
Within the employment of extrusion-based 3D printing, apart from ceramics and hydrogels, polyesters can be used as the main component of printing inks to make bone tissue engineering scaffolds. Due to the advantages such as simplified and eco-friendly operation process and low cost, FDM which uses wires or pellets made from PLA, PLGA, PCL and other biodegradable and biocompatible polyesters are often adopted to produce bone tissue engineering scaffolds. Upon continuous transport of raw materials into the heated chamber connected with a metal nozzle, polyester wires and pellets are melted and extruded from the nozzle to draw pre-designed pattern in a programmed way, following the CAD model. The exuded microfluidic patterns solidify quickly due to the heat dissipation at the room temperature, hence enabling the layer-by-layer building of the polyester scaffolds for bone tissue engineering. Gremare et al. produced PLLA scaffolds via FDM and evaluated the physical, chemical and biological performance. The pore size (0–250 μm) was identical to that in the CAD file, whereas the thread diameter (120 μm) was smaller than that designed in CAD file [54]. Similar tensile strength was obtained for different scaffolds and all scaffolds showed supportive performance when seeding osteoblastic cells on them. Liu et al. produced PLGA scaffolds with varied macroporous architecture through FDM [55]. By tuning the nozzle inner diameter, extrusion temperature, pressure, layer thickness and printing angles, PLGA scaffolds with pre-designed architecture, height, pore size and porosity were obtained. The scaffolds with varied macroscopic structure had a compressive strength of 15–23 MPa, which decreased with increasing scaffold porosity. As PLGA is biodegradable, the pH, weight remaining and compressive strength of 3D printed PLGA scaffolds showed varying degrees of decrease with increasing incubation time. The printed PLGA scaffolds showed favorable cell responses, however, due to the lack of micropore on scaffold struts, only limited cells can be observed on scaffold surface after a period of culture. Mohseni et al. conducted independent evaluation of medical-grade bioresorbable filaments for making tissue engineered constructs via FDM [56]. Raw materials including Dioxaprene® 100 M (DIO), CapropreneTM 100 M (CAP), Lactoprene® 100 M (LAC) and Max-Prene®955 (MAX) were printed into scaffolds with varied pore size/porosity and showed distinct mechanical properties according to varied porosity and matrix type. As these scaffolds had varied hydrophilicity and degradability, accelerated degradation and eroded morphology were observed for MAX and DIO scaffolds, whereas LAC and CAP scaffolds showed a medium and slow degradation with a more contact morphology, respectively. Although customized scaffolds can be produced via FDM to repair bone defects, these scaffolds lack of bioactivity to elicit favorable cell responses, hence delaying the bone regeneration.
5.2. Post-treatment of polyester scaffolds 3D printed at high temperature
Although biodegradable polymeric scaffolds fabricated through FDM can be used as bone tissue engineering scaffolds, shortcoming such as smooth strut surface which hinders effective cell attachment still exists and improvement should be done. By blending graphene with PCL at a high temperature, Wang et al. fabricated graphene/PCL scaffolds via FDM, in order to enhance the surface hydrophilicity and cell attachment [57]. The addition of pristine graphene was found to have a positive impact on cell viability and proliferation. Kosik-Koziol et al. conducted surface modification on 3D printed PCL constructs by dipping 3D printed PCL scaffolds into acetone and/or NaOH with the assistance of ultra-sonication [58]. Much rougher strut surface with a lowered water contact angle could be obtained. The adsorption rate of osteogenic biomolecules such as BMP-2 was significantly improved, although the mechanical properties were slightly deceased. The density of human MSCs as well as improved osteogenic differentiation was significantly up-regulated on solvent-treated scaffolds. Other methods are also developed to improve the cell attachment by increasing the wettability of scaffolds produced through FDM. Park et al. fabricated PCL/PEG scaffolds through FDM, followed by washing out of water soluble PEG to form micropores on thread surface [59]. The wettability of PCL scaffolds was significantly improved and improved cell proliferation was also observed on PCL/PEG scaffolds. These findings indicate that 3D printing of scaffolds with partially sacrificial features is a promising way to endow scaffold with advanced cellular responses. In addition to coating hydrophilic molecules on thread surface of scaffolds, many biomolecules which could induce desirable cellular responses have also been coated on the scaffold surface. Teixeira et al. coated mussel-inspired polydopamine (PDA) and type I collagen onto the surface of PLA scaffolds fabricated through FDM by directly immersing PLA scaffolds into dopamine solution for 24 h within a shaking water bath [60]. The type I collagen solution was then conjugated to the PDA-coated PLA scaffolds with the assistance of EDC/NHS for 48 h. The coating of PDA increased the coupling amount of type I collagen on the scaffolds and cells showed the highest cell density on the PLA scaffolds coated with PDA/collagen and showed significantly up-regulated expression level of vinculin adhesive plaques and F-actin cytoskeleton. In addition, greatly up-regulated cell ingrowth and osteogenic differentiation was obtained for PDA/collagen/PLA scaffolds. Jang et al. fabricated PCL scaffolds via FDM and further adsorbed with BMP-2 and umbilical cord serum, which were finally blocked by alginate layer crosslinked by CaCl2 [61]. The dual delivery scaffolds induced significantly higher level of osteogenic differentiation and cell mineralization in vitro and new bone formation in vivo. However, whether the crosslinked alginate layer could reduce the burst release of bio-agents into the outer environment was not discussed, which is highly important to provide sustained induction for osteogenesis. Ritz et al. developed PLA scaffolds through FDM and they were further loaded with collagen I and stromal-derived factor-1 (SDF-1) [62]. Both cage- and disc-like scaffolds were produced and PLA cages loaded with SDF-1 collagen displayed a steady SDF-1 release, supported cell growth of endothelial cells and induced neo-vessel formation in vivo, demonstrating the potential for PLA scaffolds in bone tissue engineering.
5.3. 3D printing of synthetic/natural polymer bone tissue engineering scaffolds at room/low temperature
Apart from FDM, SLS, etc., extrusion-based 3D printing at room temperature or in a cryogenic environment is considered an advanced 3D printing technique to produce synthetic/natural polymer bone tissue engineering scaffolds with capabilities of in situ delivery of biomolecules. By dissolving synthetic polyesters in a variety of organic solvents such as 1,4-dioxane, dimethyl sulfoxide (DMSO), etc., printing inks can be formed and they are capable of loading a large amount of bio-agents including bioceramic particles, drugs and biomolecules. By tuning the processing parameters, multi-delivery bone tissue engineering scaffolds could be printed and stabilized. Kim et al. formulated alendronate (ALN) incorporated PCL/DMSO solution and produced ALN/PCL scaffolds through an extrusion-based 3D printing [63]. Sustained ALN release over 4 weeks can be obtained from the ALN/PCL scaffolds which showed no toxicity against MG63 cells. The ALN/PCL scaffolds enhanced the osteoblast activity and mineralization. Moreover, the implantation of ALN/PCL scaffolds in a rat tibial defect model greatly promoted bone formation compared to pure PCL scaffold. In our study, we employed P(DLLA-TMC), a thermo-responsive shape memory polymer, as polymer matrix to print rhBMP-2 loaded P(DLLA-TMC) scaffolds [64] in which rhBMP-2 containing water phase was blended with P(DLLA-TMC)/DCM to form water-in-oil emulsion inks. The scaffold had excellent compressive strength at room temperature and became soft at 37 °C, showing great potential to induce bone regeneration through minimally invasive implantation. Yang et al. further employed polyester-based water-in-oil Pickering emulsions (silicon dioxide nanoparticles as emulsifiers) as inks to produce hierarchical macroporous scaffolds using 3D printing at room temperature [65]. The volume ratio between PLLA/PCL/DCM and water phase was very low (30: 70), enabling the production of scaffolds with homogeneous micropores (20 μm) on struts and very high overall porosity (98.3%). The scaffolds also showed favorable capability for improved cell adhesion and proliferation, indicating great potential for inducing bone tissue regeneration.
5.4. 3D printing of synthetic/natural polymer bone tissue engineering scaffolds via electrostatic field-assisted micro -extrusion
Electrostatic field-assisted micro -extrusion, also known as near-field electrospinning direct-write is another important 3D printing technique to produce tissue engineering scaffolds with micro- or nanofeatures. Ristovski et al. employed direct write near-field melt electrospinning to produce ordered scaffolds with 200 layers [66]. Patterned microstructure was obtained (fiber spacing 1 mm and diameter 40 μm) and successful cell attachment and homogenenous cell distribution in scaffolds was observed, demonstrating the feasibility of using electrostatic control for fabrication of scaffolds with regular microstructure, which was desirable for tissue engineering. He et al. also produced a layer-structured HA/PCL scaffold with uniform pore sizes which was suitable for 3D cell culture by near-field electrospinning [67]. The results showed that the scaffold had an average pore size of 167 μm, which can be tuned based on the required application; the degradation rate was controllable depending on the ratio of PCL to HA. The scaffolds showed no toxicity and MC3T3-E1 cells could effectively attach, proliferate, and differentiate in the 3D skeleton of the scaffolds. Qu et al. constructed microscale biomimetic nano HA/PCL composite scaffolds for bone tissue engineering via near-field electrospinning as well [68]. The scaffolds had a mostly regular lattice structure with a pore size of 50–100 μm and the strands had a diameter of 10 μm MC3T3-E1 cells could attach to the fiber surface and proliferate along with culture time. These scaffolds could potentially be used to regulate cellular microenvironment in multi-scale and multi-material levels for improved bone tissue regeneration. Kim et al. employed electrohydrodynamic printing, which is principally the same to the near-field direct write electrospinning, to produce 3D microfibrous scaffolds with very high loading level of TCP [69]. The fabricated ceramic structure consisted of layer-by-layered struts entangled with PCL microfibers and the bioceramic phase. Different from other near-field electrospinning-based scaffolds which had a regular latticed microfibrous structure (strut fiber: 1–20 μm, strut fiber distance: 20–100 μm), the struts in this study had a diameter of 500 μm, showing a rough microporous structure in which numerous curly PCL microfiber and TCP particles can be observed. Various processing conditions (such as applied electric field, flow rate, nozzle size, and weight fraction of the bioceramic) were manipulated to obtain an optimal processing window. Several physical and cellular activities using preosteoblasts helped confirm that the newly designed bioceramic scaffold demonstrated significantly high metabolic activity and mineralization.
6. 3D printing of inorganic agent/polymer composite as bone tissue engineering scaffolds
6.1. 3D printing of bioceramic/polymer composite as bone tissue engineering scaffolds
In order to improve the osteoconductivity of printed scaffolds, additives such as bioceramics have been directly blended with polyesters to form composites to produce bioactive scaffolds through FDM. Davila et al. produced β-TCP/PCL composite scaffolds through a mini-screw extrusion-based 3D printing [70]. The β-TCP acted as nucleating agents, allowing a better organization of polymer chains, hence increased the crystallinity of the polymer matrix. Although some agglomerations of β-TCP were observed, the β-TCP reinforcement improved mechanical and hydrophilic behavior in comparison with PCL scaffolds. Goncalves et al. reported the construction of composite scaffolds comprising of nano HA, carbon nanotubes and PCL matrix via FDM, aiming at bringing together the properties of all components into a unique material for bone tissue engineering [71]. The 3D printed composite scaffolds with an interconnected network of square pores (450–700 μm) and scaffolds containing 2 wt% carbon nanotubes exhibited the best balance between mechanical strength and electrical conductivity, showing a compressive strength of 4 MPa, which is comparable with the cancellous bone. The composites induced typical HA deposition, showing excellent bioactivity and capability for cell adhesion and spreading. Nyberg et al. fabricated TCP/PCL, HA/PCL, Bio-Oss/PCL and decellularized bone matrix (dBM)/PCL scaffolds through FDM [72]. The incorporation of mineral particles did not significantly decrease the compressive modulus of the graft, which was on the order of 260 MPa for solid blocks and 32–83 MPa for porous scaffolds. Gene expression of collagen I and osteocalcin was 10-fold greater than PCL in Bio-Oss/PCL and dBM/PCL when adipose-derived stromal/stem cells were cultured on scaffolds in vitro, suggesting that Bio-Oss/PCL and dBM/PCL hybrid materials were advantageous for bone healing applications over HA/PCL or TCP/PCL blends. Park et al. further investigated the use of β-TCP/PCL composites with applied mechanical stimulation as scaffold for bone tissue engineering [73]. After blending ball-milled PCL with β-TCP particles, β-TCP/PCL composite powders were subjected to FDM, forming bone tissue engineering scaffolds with a regular latticed macroscopic morphology. The scaffolds were mechanically comparable to human trabecular bone and desirable cell responses were obtained by seeding MSCs on PCL/TCP scaffolds with a TCP content of 30%. With mechanical stimulation, expression of osteogenic markers was lower on samples with a TCP content of 10 wt% than without TCP, whereas scaffolds with a TCP content of 30 wt% exhibited significantly higher expression of those markers than the other samples, suggesting that mechanical stimulation interacted closely with the addition of TCP, in which TCP with a mass ratio of 30% was particularly useful as a bone tissue scaffold when accompanied by mechanical stimulation. Oladapo et al. used carbonatite HA/PLA composites as raw materials to produced biomimetic composite bone tissue engineering scaffolds through FDM [74]. The printed scaffolds had a concentric cylinder structure which could closely mimic the macroscopic architecture of the segmented long bone. The incorporation of carbonatite HA in PLA matrix improved the bioactivity of the whole scaffolds, hence improving the bone regeneration ability. Similarly, Neumann et al. 3D printed CaCO3/PCL biocomposite scaffolds for hard tissue regeneration through FDM [75]. CaCO3 crystals were found to be uniformly distributed in the PCL matrix. Compared to the CaCO3/PCL composite materials with a dense structure, which were produced through mould casting, the 3D printed scaffolds had a lowered compressive strength (16 vs. 35 MPa) and Young's modulus (160 vs. 600 MPa), which was still 5-fold higher than that of human cancellous bone. As CaCO3 had a very quick dissociation rate in aqueous environments, 75–95% weight decrease was obtained in PBS-lipase solution, whereas obvious mineral deposition on the scaffold surface could be observed. Neufurth et al. blended Ca–P microparticles with PCL microparticles with a ratio of 2:1 and transferred the mixed powders into the printing head heated to 100 °C to conduct 3D printing using a FDM-like 3D printer [76]. The addition of Ca–P microparticles into PCL matrix not only significantly improved the mechanical properties but also enhanced the biological performance by showing much more live osteoblastic cells on the surface of scaffolds. Shim et al. immobilized BCP nanoparticles on the surface of 3D printed scaffolds [77]. After treating PCL scaffolds with l-lysine, aminated-PCL scaffolds were obtained. BCP nanoparticles were modified with heparin-dopamine (Hep-DOPA) to get Hep-BCP nanoparticles.Then, BCP-immobilized PCL (BCP-PCL) scaffolds were prepared by immobilization of Hep-BCP nanoparticles on the surface of aminated-PCL scaffolds. In vitro and in vivo results showed that BCP-PCL scaffolds significantly enhanced osteogenic markers such as ALP activity, mineralization and osteogenic gene expression and markedly increased new bone formation and mineralized bone tissues in tibial defects, compared to unmodified PCL and BCP-mixed PCL scaffolds, demonstrating that the immoblization of BCP nanoparticles to PCL scaffold surface are promising templates for bone tissue regeneration.
6.2. Functionalization of 3D printed bioceramic/polymer composite scaffolds
Surface functionalization of composite bone tissue engineering scaffolds is an useful route to improve the biological performance of bioceramic/polymer composite scaffolds. Saska et al. produced PHB bone tissue engineering scaffolds through SLS, followed by post-adsorption of osteogenic growth peptide [78]. The scaffolds had a regular latticed 3D pattern, in which the struts had a rough surface which was favorable for peptide adsorption. Duan et al. also employed SLS to produce Ca–P/PHBV scaffolds and further grafted scaffold surface with heparin for the conjugation of rhBMP-2, in order to realize a sustained rhBMP-2 release. Both in vitro and in vivo results demonstrated that the rhBMP-2 conjugated Ca–P/PHBV scaffolds could greatly improve osteogenesis [79]. However, post-functionlization only allows limited loading amount of agents and relatively fast release rate, resulting in insufficient bone forming activity. To increase the loading content of biologically active agents (i.e., osteogenic agents), elongate the release duration and preserve the biological activity during 3D printing process, advanced 3D printing techniques such as cryogenic 3D printing has been used, to produce inorganic/polymer composite bone tissue engineering scaffolds loaded with biomolecules. Lee and Kim mixed α-TCP (80 v/v%) with collagen solution (4 wt%) and plate-rich plasma (PRP, 1 mg/mL) to form PRP/collagen/TCP printing inks, which were then subjected to extrusion-based 3D printing under a cryogenic environment at a temperature of −16 to −18 °C [80]. A multi-layered mesh structure was printed, which was then freeze dried for 12 h, followed by immersion in tannic acid solution to allow the crosslinking of collagen and immersion in PBS for the hydrolysis of α-TCP, respectively. The printed scaffolds had a macro-micro multiscale hierarchically porous structure. The crosslinking of collagen with tannic acid could increase the mechanical properties, but still showing a limited compressive strength and modulus, which were only 1/10 to 1/5 of that of human cancellous bone. The PRP/collagen/TCP scaffolds up-regulated the viability, proliferation as well as osteogenic differentiation of cells by showing increased expression level of osteopontin and cell mineralization. Lai et al. utilized TCP/PLGA/1,4-dioxane composite solution containing icariin as printing inks to produce bone tissue engineering scaffolds in a low temperature environment [81]. The printed scaffolds had a hierarchically porous structure and were mechanically similar to human cancellous bone. The scaffolds degraded gradually with increasing incubation time and the compressive strength as well as Young's modulus were also decreased. With the incorporation of traditional Chinese herbal medicine, icariin, improved cell proliferation and osteogenic differentiation were achieved in vitro while the new bone formation and vascular vessel formation was also up-regulated. In our investigation, water-in-oil emulsion printing inks (water/PLLA/DCM) containing rhBMP-2 or Ca–P nanoparticles were formulated [82]. By alternatingly printing rhBMP-2/water/PLLA/DCM and water/Ca–P/PLLA/DCM inks, bicomponent bone tissue engineering scaffolds with spatially balanced osteoconductivity/osteoinductivity were constructed in a cryogenic environment at a temperature of −30 °C and DCM were removed via cryo-drying. The fabricated scaffolds had a hierarchically porous structure, showing latticed scaffold pattern and adjustable micropores on struts. Scaffolds were mechanically comparable to human cancellous bone and provided sustained release of rhBMP-2 and calcium ions. The scaffolds improved the human MSC attachment, spreading and proliferation and further enhanced the osteogenic differentiation in vitro. Moreover, the biological activity of rhBMP-2 loaded in emulsion inks and scaffolds was well-preserved. We further incorporated graphene oxide (GO) and osteogenic peptide into water/TCP/PLGA/DCM inks and printed peptide/GO/TCP/PLGA scaffolds [83]. Likewise, scaffolds had a hierarchically porous structure and were mechanically stronger than human cancellous bone. The addition of GO made the release of peptide more sustained and improved cellular responses. Both greatly improved in vitro osteogenic differentiation of rat MSCs and in vivo cranial bone regeneration in rats were achieved, suggesting our cryogenic 3D printed scaffolds were suitable for inducing customized bone tissue regeneration. Moreover, emulsion can be considered as a promising template to tune the microstructure and protect biomolecules.
7. Combinatory use of 3D printing and other fabrication techniques to make bone tissue engineering scaffolds
To date, to design and fabricate bone tissue engineering scaffolds with multiple components and functions, the use of a single 3D printing technique might be insufficient. Therefore, combining 3D printing and other fabrication techniques in a rational way could lead to successful production of bone tissue engineering scaffolds with a sophisticated composition and structure, in order to better mimic native bone tissue for inducing improved bone regeneration. Kankala et al. produced microfibrous porous scaffold through 3D printing, assisted by a hybrid approach, in which PLGA scaffolds were first 3D printed via FDM, followed by the immersion of PLGA scaffolds in gelatin and nano-HA solutions sequentially, hence forming gelatin/nano-HA/PLGA scaffolds for bone tissue engineering [84]. These scaffolds with proper degradation, excellent mechanical properties and good biocompatibility enabled improved attachment, proliferation and osteogenic differentiation of MC3T3-E1 cells. So far, the main limitation of cell seeding in conventional tissue engineering is inhomogenous cell distribution in scaffolds and poor cell viability in the middle of the scaffold due to limited diffusion of oxygen and nutrients and insufficient vascularization. Therefore, the uniform loading of cells in scaffold with high cell viability is highly favorable. Guduric et al. used PLA/chlorofrom solution as printing inks to produce PLA membranes using an extrusion-based 3D printer [85]. Afterwards, layer-by-layer bioassembly of cellularized (endothelial progenitor cell, human bone marrow stromal cell or cocultures) porous PLA membranes were achieved for bone tissue engineering. In the 3D assembled scaffolds, cell migration between layers of layer-by-layer constructs and osteogenic differentiation were observed, indicating that layer-by-layer assembly of PLA layers was suitable for bone tissue engineering to promote homogenous cell distribution inside the scaffold. Based on FDM, Duan et al. fabricated composite scaffolds consisting of 3D printed PCL/HA and bioactive hydrogels for the prevascularization of engineered bone tissue constructs [86]. The co-culture of MSCs and human umbilical vein endothelial cells (HUVEC) promoted in vitro vascularization, cell migration, and sprouting, but did not affect osteogenesis. The photocurable hydrogels provide an engineered vascular bed and facilitated the formation of microvessels and vasculature. This strategy provide an alternative for prevascularization of patient specific constructs in vitro to achieve rapid anastomosis in vivo and enhance bone repair. Ahlfeld et al. designed complex bone tissue engineering scaffolds by combined 3D plotting of a calcium phosphate cement (CPC) and a growth factor-loaded hydrogel [87]. After 3D plotting, two-step post-processing including alignate-gellan gum hydrogel crosslinking and CPC setting was performed. The optimization of CPC plotting enabled the fabrication of highly resolved structures with a strand diameter of 200 μm. Micro-computed tomography revealed a precise strand arrangement and an interconnected pore space within the biphasic scaffold even in swollen state of the hydrogel strands.
8. Outlook
Over the past twenty years, which are very short time for R & D in science and technology, 3D printing has advanced at a phenomenal pace due to its advantages in producing products with customized shape, tailored structure, adjustable composition, etc., and wide applications of the products by many industries, especially the biomedical industry. The initial exploration on 3D printed scaffolds soon yielded great excitement in bone tissue engineering fields. The limitations of conventional 3D printing on making advanced bone tissue engineering scaffolds have been continuously tackled by inventions and innovations in 3D printing, which have made it possible to develop complex bone tissue engineering scaffolds. However, some problems are still challenging, which are expected to be solved in future: (1) as natural bone tissue has a multi-scale hierarchical structure, 3D printed scaffolds are expected to precisely mimic the structure of the native bone tissue. However, most extrusion-based 3D printed scaffolds have limited printing resolution and could only mimic the hierarchical structure at a relatively low level. Therefore, advanced micro-extrusion nozzle should be designed to enable the production of bone tissue engineering scaffolds with a significantly higher resolution (i.e., the printed struts have a significantly smaller diameter) while not causing nozzle clotting; (2) defected bone tissue often contains both cortical bone and cancellous bone, showing a heterogeneous structure with gradient mechanical properties. However, integrated bone tissue engineering scaffolds with greatly varied mechanical properties are difficult to produce, and hence better 3D printing strategies should be adopted to enable the production of customized scaffolds with complex features; (3) it is highly important to provide scaffolds with excellent vascularization to enable sufficient oxygen/nutrient transportation during bone regeneration, but few scaffolds are specifically designed to achieve bone regeneration with required vascularization. Thus appropriate strategies including the controlled release of angiogenic agents and formation of vascular-like channel in scaffolds are needed to provide scaffolds with both improved bone regeneration capability and enhanced vascularization; (4) compared to 3D printed scaffolds which could recruit host cells in vivo, loading cells into 3D printed scaffolds are considered to be more effective in treating bone defects, especially the defects with a critical size. However, post-seeding of cells on scaffolds often results in uneven cell distribution and limited cell density, whereas in situ incorporation of cells during 3D printing process would be desirable. Among current 3D printing techniques, apart from 3D bioprinting which produces cell-laden hydrogel structure, no existing 3D printing technique can enable cell incorporation during the printing process. Therefore, superior 3D printing techniques should be invented to achieve simultaneous scaffold fabrication and cell incorporation; (5) scaffolds with excellent capability for anti-bacteria or anti-cancer are increasingly needed to treat infection/bone tumor resection-induced defects. One needs to carefully design 3D printed scaffolds in order to best regenerate bone tissues under the optimum conditions.
Author contribution statement
Conceptualization: Chong Wang, Wei Huang and Min Wang.
Investigation: Chong Wang, Libing He, Zhi He, Ziling Chen, Xiao He, Shuo Tian and Jiaming Liao.
Writing - Original Draft: Chong Wang, Wei Huang and Yu Zhou.
Writing - Review & Editing: Chong Wang, Bingheng Lu, Yen Wei and Min Wang.
Supervision: Chong Wang and Min Wang.
Funding acquisition: Chong Wang and Bingheng Lu.
Declaration of competing interest
We declare no conflict of interest.
Acknowledgement
This work was supported by Dongguan University of Technology High-level Talents (Innovation Team) Research Project (KCYCXPT201603), Youth Innovative Talent Project from the Department of Education of Guangdong Province, China (2016KQNCX168), Natural Science Foundation of Guangdong Province, China (2018A0303130019).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Chong Wang, Email: wangchong@dgut.edu.cn.
Min Wang, Email: memwang@hku.hk.
References
- 1.Ngo T.D., Kashani A., Imbalzano G., Nguyen K.T.Q., Hui D. Additive manufacturing (3D printing): a review of materials, methods,applications and challenges. Compos. B Eng. 2018;143:172–196. [Google Scholar]
- 2.Ligon S.D., Liska R., Stampfl J., Gurr M., Mülhaupt R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017;117(15):10212–10290. doi: 10.1021/acs.chemrev.7b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun K., Wei T.S., Ahn B.Y., Seo J.Y., Dillon S.J., Lewis J.A. 3D printing of interdigitated Li-ion microbattery architectures. Adv. Mater. 2013;25(33):4539–4543. doi: 10.1002/adma.201301036. [DOI] [PubMed] [Google Scholar]
- 4.Ladd C So, Muth J.H., J Dickey M.D. 3D printing of free standing liquid metal microstructures. Adv. Mater. 2013;25(36):5081–5085. doi: 10.1002/adma.201301400. [DOI] [PubMed] [Google Scholar]
- 5.Scheithauer U., Slawik T., Schwarzer E., Richter H.J., Moritz T., Michaelis A. Additive manufacturing of metal-ceramic-composites by Thermoplastic3D-printing (3DTP) Journal of Ceramic Science and Technology. 2015;6(2):125–131. [Google Scholar]
- 6.Hwang S., Reyes E.I., Moon K.S., Rumpf R.C., Kim N.S. Thermo-mechanical characterization of metal/polymer composite filaments and printing parameter study for fused deposition modeling in the 3D printing process. J. Electron. Mater. 2015;44(3):771–777. [Google Scholar]
- 7.Carrico J.D., Traeden N.W., Aureli M., Leang K.K. Fused filament 3D printing of ionic polymer-metal composites (IPMCs) Smart Mater. Struct. 2015;24(12):125021. [Google Scholar]
- 8.Michal L., Michal K., Malgorzata S. Fast track integration of computational methods with experiments in small wind turbine development. Energies. 2019;12(9):1625. [Google Scholar]
- 9.Anupama P., Rachel G. Exploration of 3D printing to create zerowaste sustainable fashion notions and jewelry. Fashion and Textiles. 2018;5(1):1–18. [Google Scholar]
- 10.Altaf K., Qayyum J.A., Rani A.M.A., Ahmad F., Megat-Yusoff P.S.M., Baharom M., Aziz A.R.A., Jahanzaib M., German R.M. Performance analysis of enhanced 3D printed polymer molds for metal injection molding process. Metals. 2018;8(6):433. [Google Scholar]
- 11.Kellu C.N., Miller A.T., Hollister S.J., Guldberg R.E., Gall K. Design and structure-function characterization of 3D printed synthetic porous biomaterials for tissue engineering. Adv. Healthc. Mater. 2018;7(7):1701095. doi: 10.1002/adhm.201701095. [DOI] [PubMed] [Google Scholar]
- 12.Tay Y.W.D., Panda B., Paul S.C., Mohamed N.A.N., Tan M.J., Leong K.F. 3D printing trends in building and construction industry: a review. Virtual Phys. Prototyp. 2017;12(3):261–276. [Google Scholar]
- 13.Chia H.N., Wu B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fina F., Goyanes A., Gaisford S., Basit A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017;529(1–2):285–293. doi: 10.1016/j.ijpharm.2017.06.082. [DOI] [PubMed] [Google Scholar]
- 15.Lopes A.J., MacDonald E., Wicker R.B. Integrating stereolithography and direct print technologies for 3D structural electronics fabrication. Rapid Prototyp. J. 2012;18(2):129–143. [Google Scholar]
- 16.Yap C.Y., Chua C.K., Dong Z.L., Liu Z.H., Zhang D.Q., Loh L.E., Sing S.L. Review of selective laser melting: materials and applications. Appl. Phys. Rev. 2015;2(4) [Google Scholar]
- 17.Frazier W.E. Metal additive manufacturing: a review. J. Mater. Eng. Perform. 2014;23(6):1917–1928. [Google Scholar]
- 18.Janusziewicz R., Rolland J., Tumbleston J., Ermoshkin N., Kelly D., Johnson A., Pinschmidt R., Shirvanyants D., Ermoshkin A., DeSimone J., Chen K., Samulski E. Continuous liquid interface production of 3D objects. Science. 2015;347(6228):1349–1352. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 19.Murphy S.V., Atala A. 3D bioprinting of tissues and organ. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 20.Chia H.N., Wu B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho C.M.B., Sum H.N., Li K.H.H. 3D printed microfluidics for biological applications. Lab Chip. 2015;15(18):3627–3637. doi: 10.1039/c5lc00685f. [DOI] [PubMed] [Google Scholar]
- 22.Xing J., Zheng M., Duan X. Two-photon polymerization microfabrication of hydrogels: an advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015;44(15):5031–5039. doi: 10.1039/c5cs00278h. [DOI] [PubMed] [Google Scholar]
- 23.Hoelzl K., Lin S., Tytgat L. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016;8(3) doi: 10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- 24.Hutmacher D.W., Schantz J.T., Lam C.X.F., Tan K.C., Lim T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regenerat. Med. 2007;1(4):245–260. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 25.Denry I., Kuhn L.T. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent. Mater. 2016;32(1):43–53. doi: 10.1016/j.dental.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A., Mandal S., Barui S., Vasireddi R., Gbureck U., Gelinsky M., Basu B. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: processing related challenges and property assessment. Mater. Sci. Eng. R Rep. 2016;103:III. [Google Scholar]
- 27.Martinez-Vazquez F.J., Cabanas M.V., Paris J.L., Lozano D., Vallet-Regi M. Fabrication of novel Si-doped hydroxyapatite/gelatine scaffolds by rapid prototyping for drug delivery and bone regeneration. Acta Biomater. 2015;15:200–209. doi: 10.1016/j.actbio.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J.H., Zhao S.C., Zhu M., Zhu Y.F., Zhang Y.D., Liu Z.T., Zhang C.Q. 3D-printed magnetic Fe3O4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B. 2014;2(43):7583–7595. doi: 10.1039/c4tb01063a. [DOI] [PubMed] [Google Scholar]
- 29.Chen S.T., Zhu L., Wen W., Lu L., Zhou C.R., Luo B.H. Fabrication and evaluation of 3D printed poly(L-lactide) scaffold functionalized with quercetin-polydopamine for bone tissue engineering. ACS Biomater. Sci. Eng. 2019;5(5):2506–2518. doi: 10.1021/acsbiomaterials.9b00254. [DOI] [PubMed] [Google Scholar]
- 30.Ye X.L., Li L.H., Lin Z.F., Yang W.L., Duan M.Y., Chen L.L., Xia Y.J., Chen Z.P., Lu Y., Zhang Y. Integrating 3D-printed PHBV/Calcium sulfate hemihydrate scaffold and chitosan hydrogel for enhanced osteogenic property. Carbohydr. Polym. 2018;202:106–114. doi: 10.1016/j.carbpol.2018.08.117. [DOI] [PubMed] [Google Scholar]
- 31.Saska S., Pires L.C., Cominotte M.A.>, Mendes L.S., de Oliveira M.F., Maia I.A., da Silva J.V.L., Ribeiro S.J.L., Cirelli J.A. Three-dimensional printing and in vitro evaluation of poly(3-hydroxybutyrate) scaffolds functionalized with osteogenic growth peptide for tissue engineering. Materials Science and Engineering,C:Materials for Biological Applications. 2018;89:265–273. doi: 10.1016/j.msec.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Hung K., Tseng C., Hsu S. Synthesis and 3D printing of biodegradable polyurethane elastomer by a water-based process for cartilage tissue engineering applications. Adv. Healthc. Mater. 2014;3(10):1578–1587. doi: 10.1002/adhm.201400018. [DOI] [PubMed] [Google Scholar]
- 33.Yong L.C., Malek N.F.A., Yong E.N.S., Yap W.H., Nobuyuki M., Yoshitaka N. Fabrication of hydroxyapatite blended cyclic type polylactic acid and poly (epsilon-caprolactone) tissue engineering scaffold. Int. J. Appl. Ceram. Technol. 2019;16(2):455–461. [Google Scholar]
- 34.Temple J.P., Hutton D.L., Hung B.P., Huri P.Y., Cook C.A., Kongdragunta R., Jia X.F., Grayson W.L. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J. Biomed. Mater. Res. A. 2014;102(12):4317–4325. doi: 10.1002/jbm.a.35107. [DOI] [PubMed] [Google Scholar]
- 35.Billiet t., Gevaert E., De Schryver T., Cornelissen M., Dubruel P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35(1):49–62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 36.Borowiec J., Hampl J., Signh S., Haefner S., Friedel K., Mai P., Brauer D., Ruther F., Liverani L., Boccaccini A.R., Schober A. 3D microcontact printing for combined chemical and topographical patterning on porous cell culture membrane. ACS Appl. Mater. Interfaces. 2018;10(26):22857–22865. doi: 10.1021/acsami.8b06585. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J.T., Liu W.Z., Schnitzler V., Tancret F., Bouler J.M. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater. 2014;10(3):1035–1049. doi: 10.1016/j.actbio.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Seidenstuecker M., Kerr L., Bernstein A., Mayr H.O., Suedkamp N.P., Gadow R., Krieg P., Latorre S.H., Thomann R., Syrowatka F., Esslinger S. Materials. 2018;11:13. doi: 10.3390/ma11010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song X.L., Tetik H., Jirakittsonthon T., Parandoush P., Yang G., Lee D., Ryu S., Lei S.T., Weiss M.L., Lin D. Biomimetic 3D printing of hierarchical and interconnected porous hydroxyapatite structures with high mechanical strength for bone cell culture. Adv. Eng. Mater. 2019;21(1):1800678. [Google Scholar]
- 40.Chen L., Deng C.J., Li J.Y., Yao Q.Q., Chang J., Wang L.M., Wu C.T. 3D printing of a lithium-calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction. Biomaterials. 2019;196 doi: 10.1016/j.biomaterials.2018.04.005. SI 138-150. [DOI] [PubMed] [Google Scholar]
- 41.Song Y., Lin K.F., He S., Wang C.M., Zhang S.S., Li D.L., Wang J.M., Cao T.Q., Bi L., Pei G.X. Nano-biphasic calcium phosphate/polyvinyl alcohol composites with enhanced bioactivity for bone repair via low-temperature three-dimensional printing and loading with platelet-rich fibrinInternationa. l Journal of Nanomedicine. 2018;13:505–523. doi: 10.2147/IJN.S152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boga J.C., Miguel S.P., de Melo-Diogo D., Mendonca A.G., Louro R.O., Correia I.J. In vitro characterization of 3D printed scaffolds aimed at bone tissue regeneration. Colloids Surfaces B Biointerfaces. 2018;165:207–218. doi: 10.1016/j.colsurfb.2018.02.038. [DOI] [PubMed] [Google Scholar]
- 43.Touri M., Mortarzadeh F., Abu Osman N.A., Dehghan M.M., Mozafari M. 3D-printed biphasic calcium phosphate scaffolds coated with an oxygen generating system for enhancing engineered tissue survival. Materials Science & Engineering C-Materials For Biological Applications. 2018;84:236–242. doi: 10.1016/j.msec.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 44.Kim B.S., Yang S.S., Kim C.S. Incorporation of BMP-2 nanoparticles on the surface of a 3D-printed hydroxyapatite scaffold using an ε-polycaprolactone polymer emulsion coating method for bone tissue engineering. Colloids Surfaces B Biointerfaces. 2018;170:421–429. doi: 10.1016/j.colsurfb.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 45.Kim H., Yang G.H., Choi C.H., Cho Y.S., Kim G. Gelatin/PVA scaffolds fabricated using a 3D printing process employed with a low-temperature plate for hard tissue regeneration: fabrication and characterizations. Int. J. Biol. Macromol. 2018;120(A):119–127. doi: 10.1016/j.ijbiomac.2018.07.159. [DOI] [PubMed] [Google Scholar]
- 46.Sithole M.N., Kumar P., du Toit L.C., Marimuthu T., Choonara Y.E., Pillay V. A 3D bioprinted in situ conjugated-co-fabricated scaffold for potential bone tissue engineering applications. J. Biomed. Mater. Res. 2018;106(5):1311–1321. doi: 10.1002/jbm.a.36333. [DOI] [PubMed] [Google Scholar]
- 47.Lee H., Yang G.H., Kim M., Lee J.Y., Huh J.T., Kim G.H. Fabrication of micro/nanoporous collagen/dECM/silk-fibroin biocomposite scaffolds using a low temperature 3D printing process for bone tissue regeneration. Mater. Sci. Eng. C. 2018;84:140–147. doi: 10.1016/j.msec.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Gao F., Xu Z., Liang Q., Liu B., Li H., Wu Y., Zhang Y., Lin Z., Wu M., Ruan C., Liu W. Direct 3D printing of high strength biohybrid gradient hydrogel scaffolds for efficient repair of osteochondral defect. Adv. Funct. Mater. 2018;28(13):1706644. [Google Scholar]
- 49.Luo Y., Li Y., Qin X., Wa Q. 3D printing of concentrated alginate/gelatin scaffolds with homogeneous nano apatite coating for bone tissue engineering. Mater. Des. 2018;146:12–19. [Google Scholar]
- 50.Luo Y., Luo G., Gelinsky M., Huang P., Ruan C. 3D bioprinting scaffold using alginate/polyvinyl alcohol bioinks. Mater. Lett. 2017;189:295–298. [Google Scholar]
- 51.Heo E.Y., Ko N.R., Bae M.S., Lee S.J., Choi B., Kim J.H., Kim H.K., Park S.A., Kwon I.K. Novel 3D printed alginate–BFP1 hybrid scaffolds for enhanced bone regeneration. J. Ind. Eng. Chem. 2017;45:61–67. [Google Scholar]
- 52.Zhai X., Ruan C., Ma Y., Cheng D., Wu M., Liu W., Zhao X., Pan H., Lu W.W. 3D-Bioprinted osteoblast-laden nanocomposite hydrogel constructs with induced microenvironments promote cell viability, differentiation, and osteogenesis both in vitro and in vivo. Advanced Science. 2018;5(3):1700550. doi: 10.1002/advs.201700550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demirtaş T.T., Irmak G., Gümüşderelioğlu M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication. 2017;9(3) doi: 10.1088/1758-5090/aa7b1d. [DOI] [PubMed] [Google Scholar]
- 54.Grémare A., Guduric V., Bareille R., Heroguez V., Latour S., Fricain N.L.J., Catros S., Le Nihouannen D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A. 2018;106(4):887–894. doi: 10.1002/jbm.a.36289. [DOI] [PubMed] [Google Scholar]
- 55.Liu C., Zeng Y., Kankala R.K., Zhang S., Chen A., Wang S.B. Characterization and preliminary biological evaluation of 3D-printed porous scaffolds for engineering bone tissues. Materials. 2018;11(10):1832. doi: 10.3390/ma11101832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohseni M., Hutmacher D.W., Castro N.J. Independent evaluation of medical-grade bioresorbable filaments for fused deposition modelling/fused filament fabrication of tissue engineered constructs. Polymers. 2018;10(1):40. doi: 10.3390/polym10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang W., Caetano G., Ambler W.S., Blaker J.J., Frade M.A., Mandal P., Diver C., Bártolo P. Enhancing the hydrophilicity and cell attachment of 3D printed PCL/graphene scaffolds for bone tissue engineering. Materials. 2016;9(12):992. doi: 10.3390/ma9120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kosik-Kozio A., Graham E., Jaroszewicz J., Kumar A.C.P.T.S., Ivanovski S., Vaquette W.S.C. Surface modification of 3D printed polycaprolactone constructs via a solvent treatment: impact on physical and osteogenic properties. ACS Biomater. Sci. Eng. 2019;5(1):318–328. doi: 10.1021/acsbiomaterials.8b01018. [DOI] [PubMed] [Google Scholar]
- 59.Park S.A., Lee S.J., Seok J.M., Lee J.H., Kim W.D., Kwon I.K. Fabrication of 3D printed PCL/PEG polyblend scaffold using rapid prototyping system for bone tissue engineering application. J. Bionics Eng. 2018;15(3):435–442. [Google Scholar]
- 60.Teixeira B.N., Aprile P., Mendonça R.H., Kelly D.J., da Silva Moreira Thiré R.M. Evaluation of bone marrow stem cell response to PLA scaffolds manufactured by 3D printing and coated with polydopamine and type I collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107(1):37–49. doi: 10.1002/jbm.b.34093. [DOI] [PubMed] [Google Scholar]
- 61.Jang C.H., Lee J.U., Kim G.H. Synergistic effect of alginate/BMP-2/Umbilical cord serum-coated on 3D-printed PCL biocomposite for mastoid obliteration model. J. Ind. Eng. Chem. 2019;75:432–441. [Google Scholar]
- 62.Ritz U., Gerke R., Götz H., Stein S., Rommens P.M. A new bone substitute developed from 3D-prints of polylactide (PLA) loaded with collagen I: an in vitro study. Int. J. Mol. Sci. 2017;18(12):2569. doi: 10.3390/ijms18122569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J.I., Kim C.S. Nanoscale resolution 3D printing with pin-modified electrified inkjets for tailorable nano/macrohybrid constructs for tissue engineering. ACS Appl. Mater. Interfaces. 2018;10(15):12390–12405. doi: 10.1021/acsami.7b19182. [DOI] [PubMed] [Google Scholar]
- 64.Wang C., Zhou Y., Wang M. In situ delivery of rhBMP-2 in surface porous shape memory scaffolds developed through cryogenic 3D plotting. Mater. Lett. 2017;189:140–143. [Google Scholar]
- 65.Yang T., Hu Y., Wang C., Binks B.P. Fabrication of hierarchical macroporous biocompatible scaffolds by combining pickering high internal phase emulsion templates with three-dimensional printing. ACS Appl. Mater. Interfaces. 2017;9(27):22950–22958. doi: 10.1021/acsami.7b05012. [DOI] [PubMed] [Google Scholar]
- 66.Ristovski N., Bock N., Liao S., Powell S.K., Ren J., Kirby G.T., Blackwood K.A., Woodruff M.A. Improved fabrication of melt electrospun tissue engineering scaffolds using direct writing and advanced electric field control. Biointerphases. 2015;10 doi: 10.1116/1.4914380. [DOI] [PubMed] [Google Scholar]
- 67.He F., Li D., He J., Liu Y., Ahmad F., Liu Y., Deng X., Ye Y., Yin D. A novel layer-structured scaffold with large pore sizes suitable for 3D cell culture prepared by near-field electrospinning. Mater. Sci. Eng. C. 2018;86:18–27. doi: 10.1016/j.msec.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 68.Qu X., Xia P., He J., Li D. Microscale electrohydrodynamic printing of biomimetic PCL/nHA composite scaffolds for bone tissue engineering. Mater. Lett. 2016;185:554–557. [Google Scholar]
- 69.Kim M., Yun H., Kim G.H. Electric-field assisted 3D-fibrous bioceramic-based scaffolds for bone tissue regeneration: fabrication, characterization, and in vitro cellular activities. Sci. Rep. 2017;7:3166. doi: 10.1038/s41598-017-03461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dávila J.L., Freitas M.S., Inforçatti Neto P., Silveira Z.C., Silva J.V.L., d’Ávila M.A. Fabrication of PCL/β-TCP scaffolds by 3D mini-screw extrusion printing. J. Appl. Polym. Sci. 2016;133(15) [Google Scholar]
- 71.Gonçalves E.M., Oliveira F.J., Silva R.F., Neto M.A., Fernandes M.H., Amaral M., Vallet-Regí M., Vila M. Three-dimensional printed PCL‐hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. A B. 2016;104(6):1210–1219. doi: 10.1002/jbm.b.33432. [DOI] [PubMed] [Google Scholar]
- 72.Nyberg E., Rindone A., Dorafshar A., Grayson W.L. Comparison of 3D-printed poly-e-caprolactone scaffolds functionalized with tricalcium phosphate, hydroxyapatite, bio-oss, or decellularized bone matrix. Tissue Eng. A. 2017;23(11–12) doi: 10.1089/ten.TEA.2016.0418. [DOI] [PubMed] [Google Scholar]
- 73.ParkSu S.H., Park A., Kang Y.G., Shin J.W., Park Y.S., Gu S.R., Wu Y.R., Wei J., Shin J. PCL/β-TCP composite scaffolds exhibit positive osteogenic differentiation with mechanical stimulation. Tissue Engineering and Regenerative Medicine. 2017;14(4):349–358. doi: 10.1007/s13770-017-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oladapoa B.I., Zahedia S.A., Adeoyeb A.O.M. 3D printing of bone scaffolds with hybrid biomaterials. Compos. B Eng. 2019;158:428–436. [Google Scholar]
- 75.Neumann R., Neunzehn J., Hinüber C., Flathm T., Schulze P., Wiesmann H.P. 3d-printed poly-e-caprolactone-caco3-biocomposite-scaffolds for hard tissue regeneration. Express Polym. Lett. 2019;13(1):2–17. [Google Scholar]
- 76.Neufurth M., Wang X., Wang S., Steffena R., Ackermann M., Haep N.D., Schröder H.C., Müllera W.E.G. 3D printing of hybrid biomaterials for bone tissue engineering: calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017;64:377–388. doi: 10.1016/j.actbio.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 77.Shim K., Kim S.E., Yun Y., Jeon D.I., Kim H., Park K., Song H. Surface immobilization of biphasic calcium phosphate nanoparticles on 3D printed poly(caprolactone) scaffolds enhances osteogenesis and bone tissue regeneration. J. Ind. Eng. Chem. 2017;55:101–109. [Google Scholar]
- 78.Saska S., Pires L.C., Cominotte M.A., Mendes L.S., de Oliveira M.F., Maia I.A., da Silva J.V.L., Ribeiro S.J.L., Cirelli J.A. Three-dimensional printing and in vitro evaluation of poly(3-hydroxybutyrate) scaffolds functionalized with osteogenic growth peptide for tissue engineering. Mater. Sci. Eng. C. 2018;89:265–273. doi: 10.1016/j.msec.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 79.Duan B., Wang M. Customized Ca–P/PHBV nanocomposite scaffolds for bone tissue engineering: design, fabrication, surface modification and sustained release of growth factor. J. R. Soc. Interface. 2010;7(5):S619–S625. doi: 10.1098/rsif.2010.0127.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee J.U., Kim G.H. Calcium-deficient hydroxyapatite/collagen/platelet-rich plasma scaffold with controlled release function for hard tissue regeneration. ACS Biomater. Sci. Eng. 2017;4(1):278–289. doi: 10.1021/acsbiomaterials.7b00640. [DOI] [PubMed] [Google Scholar]
- 81.Lai Y., Cao H., Wang X., Chen S., Zhang M., Wang N., Yao Z., Dai Y., Xie X., Zhang P., Yao X., Qin L. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials. 2018;153:1–13. doi: 10.1016/j.biomaterials.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 82.Wang C., Zhao Q., Wang M. Cryogenic 3D printing for producing hierarchical porous and rhBMP-2 loaded Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering. Biofabrication. 2017;9(2) doi: 10.1088/1758-5090/aa71c9. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y., Wang C., Fu L., Ye S., Wang M., Zhou Y. Fabrication and application of novel porous scaffold in situ-loaded graphene oxide and osteogenic peptide by cryogenic 3D printing for repairing critical-sized bone defect. Molecules. 2019;24(9):1669. doi: 10.3390/molecules24091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kankala R.K., Xu X.M., Liu C.G., Chen A.Z., Wang S.B. 3D-Printing of microfibrous porous scaffolds based on hybrid approaches for bone tissue engineering. Polymers. 2018;10(7):807. doi: 10.3390/polym10070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guduric V., Siadous C.M., Bareille R., Levato R., Engel E., Fricain J., Devillard R., Luzanin O., Catros S. Layer-by-layer bioassembly of cellularized polylactic acid porous membranes for bone tissue engineering. J. Mater. Sci. Mater. Med. 2017;28:78. doi: 10.1007/s10856-017-5887-6. [DOI] [PubMed] [Google Scholar]
- 86.Kuss M.A., Wu S., Wang Y., Untrauer J.B., Li W., Lim J.Y., Duan B. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106B:1788–1798. doi: 10.1002/jbm.b.33994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahlfeld T., Akkineni A.R., Förster Y., Köhler T., Knaack S., Gelinsky M., Lode A. Design and fabrication of complex scaffolds for bone defect healing: combined 3D plotting of a calcium phosphate cement and a growth factor-loaded hydrogel. Ann. Biomed. Eng. 2017;45(1):224–236. doi: 10.1007/s10439-016-1685-4. [DOI] [PubMed] [Google Scholar]


