Graphical abstract
Keywords: AgNPs, Clarias gariepinus, Hepatocyte, Biochemical, Histopathological, Histochemical, MMCs
Highlights
-
•
AgNPs-induced hepatotoxic effects on Clarias garepinus in a dose-dependent manner.
-
•
Some biochemical, histopathological, and histochemical changes were measured in liver tissue as biomarkers.
-
•
The results can assume liver sensitivity of C. garepinus for AgNPs and the recovery period is a must.
Abstract
The current study investigates the hepatotoxic effects of two acute doses of silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on African catfish (Clarias garepinus) using biochemical, histopathological, and histochemical changes and the determination of silver in liver tissue as biomarkers. AgNPs-induced impacts were recorded in some of these characteristics based on their size (20 and 40 nm) and their concentration (10 and 100 μg/L). Concentrations of liver enzymes (Aspartic aminotransferase; AST, Alanine aminotransferase; ALT), alkaline phosphatase (ALP), total lipids (Tl), Glucose (Glu) and Ag-concentration in liver tissue exhibited a significant increase under stress in all exposed groups compared to the control group. The total proteins (Tp), albumin (Al), and globulin (Gl) concentrations exhibited significantly decrease in all treated groups compared to the control group. At tissue and cell levels, histopathological changes were observed. These changes include proliferation of hepatocytes, infiltrations of inflammatory cells, pyknotic nuclei, cytoplasmic vaculation, melanomacrophages aggregation, dilation in the blood vessel, hepatic necrosis, rupture of the wall of the central vein, and apoptotic cells in the liver of AgNPs-exposed fish. As well as the depletion of glycogen content in the liver (feeble magenta coloration) was observed. The size and number of melanomacrophage centers (MMCs) in liver tissue showed highly significant difference in all exposed groups compared to the control group. Recovery period for 15 days led to improved most alterations in the biochemical, histopathological, and histochemical parameters induced by AgNPs and AgNO3. In conclusion, one can assume liver sensitivity of C. garepinus for AgNPs and the recovery period is a must.
1. Introduction
Nanotoxicology is a study of the impact of manufactured nanomaterials on living organisms and the environment [1]. There is an increasing demand for nanoparticles in various fields such as industrial applications, biological and biomedical science, health care technology, and household appliances [[1], [2], [3], [4]]. Increasing presence of AgNPs in commercial products increases worries about potential poisoning and its risks in the environment [5]. Although, many studies on AgNPs poisoning but toxic evidence on AgNPs is still not available, and little information is available about their accumulation and effects on the internal organs of fish [1,[6], [7], [8], [9], [10], [11], [12]].
Analysis of biochemical parameters can help to identify target organs for toxicity, the general health status of animals, and provide an early warning signal in a stressful organism [[13], [14], [15]]. Histopathology of the liver can be used as indicators for the effects of aquatic animals’ exposure to toxins in the aquatic environment [[16], [17], [18]]. The liver structure of the teleosts fish is highly sensitive to environmental changes and their exposure to nanoparticles shows a significant decrease in cell membrane integrity, a decrease in metabolic activity, and sometimes apoptosis or necrosis of hepatocytes [[19], [20], [21]].
Melanomacrophage centers (MMCs) of fish were identified as cells containing pigment and a prominent feature in hematopoietic tissue [22,23]. The main functions of MMCs in fish are the storage, destruction and detoxification of exogenous and endogenous materials, immunological protection such as phagocytosis used as biomarkers of environmental pollution [22,24]. Several studies have investigated the melanomacrophage centers (MMCs) in a wide range of fish [22,23,25,26,78].
Fish are widely used as indicators for environmental pollution and to evaluate the health of the aquatic ecosystem and physiological changes [1]. C. gariepinus is widely used because of it is able to tolerate both well and poorly oxygenated waters and survives for considerable periods out of the water [27,28]. Therefore, it is used as biological indicators of ecotoxicological studies.
According to the aforementioned findings, present work was suggested and was aimed to studying the hepatotoxicity of African catfish; C. gariepinus (Burchell, 1822) induced by AgNPs and AgNO3 in an attempt to determine silver in liver tissue, histopathological, and histochemical biomarkers in the corresponding with determination of biochemical biomarkers in the liver.
2. Materials and methods
2.1. Silver nanoparticles characterization
Silver nanoparticles with a size of 20 nm and 40 nm were purchased from Nanostructured and Amorphous Materials Inc. (Houston). The characterization of these silver nanoparticles has been studied and identified in details by Mekkawy et al. [29] and Mahmoud et al. [30] with confirmation of its crystalline nature in Assiut University Labs. X-ray diffraction (Four Bragg reflections at 38.114°, 44.298°, 64.441° and 77.395° corresponding to the combinations of 111, 200, 220 and 311 lattice planes, respectively) and the stability of AgNPs as well as their average sizes estimated by the Transmission Electron Microscope (Average particle size and SD of 11.21 ± 4.13 and 32.62 ± 13.48 for 20 nm and 40 nm of AgNPs, respectively) were considered for this concern [30]. Also, the stability of nanoparticles was measured at the beginning of the trial and before the water renewal (48 h) only [29].
2.2. Fish
Juveniles of the African catfish, C. gariepinus were collected from private fish farm in May 2015, and then transported to Fish Biology and Pollution Laboratory, Zoology Department, Faculty of Science, Assiut University. The fish were fed commercial fish food (5 % body weight) twice daily and kept at about 28 °C with 12 h: 12 h light-dark cycle in many tanks (100 L each) for three months to adapt to laboratory conditions prior to experiments. During the acclimation period about 20 % of the water (dechlorinated and aerated tap water) in each tank was replaced daily. Fish ranged between 23.5–32 cm in total length and 70–110 g in weight. Water temperature, pH and dissolved oxygen concentrations (DO) were measured daily (29.17 ± 0.27 °C, 8.5 ± 0.03 pH and 34.47 ± 11.99 mg/L DO).
2.3. Experimental design and AgNPs exposure
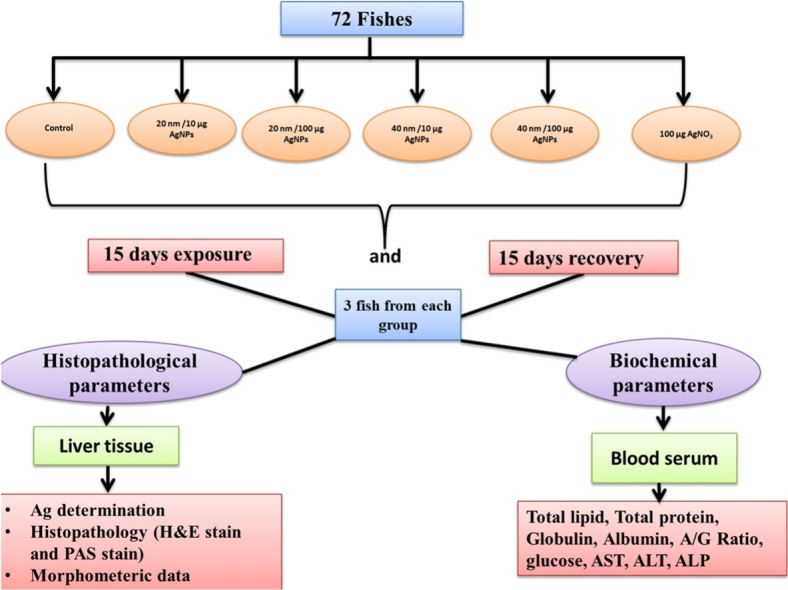
The acclimatized fish were randomly divided into 6 groups, control and five exposed ones. Each group contained 12 specimens in a glass tank measuring 100 × 35 × 50 cm (L x W x H) with a total volume of 100 L. These fish groups are control, 20 nm/10 μg/L AgNPs, 20 nm/100 μg/L AgNPs, 40 nm/10 μg/L AgNPs, 40 nm/100 μg/L AgNPs and 100 μg/L silver nitrate (AgNO3). The exposure period was 15 days followed by 15-day recovery period. To minimize the decrease of nominal concentration of AgNPs, which is potentially to adsorb onto residual food and feces in the test water, each experimental aquarium was supplied with food for 1 h prior to dosing.
Dosing stocks of silver particles were made by suspending 1.5 mg of each particle in 1 L ultrapure water and sonicating for 30 min and diluting as required. The stock of silver nitrate doses (1.5 mg/L) was also made up in ultrapure water and sonicated similarly. The experimental tanks were filled with dechlorinated water and dosed by chemicals with immediate addition of fish to minimize the reduction of nominal dosing concentrations through adhesion of the particles/chemical to the glass. Then, every 48 h, 75 % of the tank water was changed and the dosing was immediately restored.
2.4. Behavior and morphological changes
Fish behavior was observed daily during the experimental periods with naked eye and pictures were taken with a digital camera.
2.5. Biochemical analyses
At the end of the experimental periods, six fish from each group were collected and anesthetized using 200 ppm solution of clove powder [31]. Blood samples were collected from the caudal veins of fish and allowed to clot in clean and dry centrifuge tubes at room temperature, then centrifuged at 5000 rpm, at 4 °C for 20 min and the serums were separated for biochemical analysis (glucose, total lipids, AST, ALT, ALP, total protein, albumin, and Globulin). Biochemical kit of glucose was purchased from Spinreaet Co., Spanish and total lipid kit was purchased from Bio-Diagnostic Co., Cairo, Egypt. All other biochemical kits (AST, ALT, ALP, total protein, albumin, and Globulin) were purchased from Germany.
2.6. Determination of silver concentrations in liver tissue
Tissue analyses were conducted according to Shaw et al. [32] with minor differences. The samples were weighed (about 1.0 g), dried (50 ◦C for 48 h in the oven) and then digested in 5 ml concentrated nitric acid at 50 °C in the oven until nitric acid evaporated and the mixture was approximately 1 ml. The mixture was cooled, diluted to 10 mL using ultrapure deionized water and filtered. Samples were analyzed by atomic absorption spectrophotometer with Buck 210 VGP model (Buck Scientific Inc. East Norwalk,CT (USA)) in the Department of Chemistry, Faculty of Science, Assiut University. The results were expressed as μg/g wet weight of tissue.
2.7. Histological and histopathological examination
After 15 days of exposure and 15 days of recovery periods, three fishes from each group (six groups) were removed and used for biochemical assessment. The same fish were sacrificed and used for histopathologic assessment. Small pieces of liver were taken and fixed immediately in 10 % neutral buffered formalin. Fixed tissue was routinely processed for paraffin embedding technique. The embedded tissue was sectioned with a thickness of 5 μ and then stained with the following stain: Harris’s hematoxylin and eosin stain (HE) [33]. Sixty randomly selected sections of three fish (ten slides) were selected from each experimental group to refer to each histopathological parameter as (control, - no alteration (0–2); mild, + (>2–10 %) area of section; moderate, ++ (>10–40 %) area of section; and severe, +++ (>40 % area of section). Finally, tissue was examined and imaged using Omax advanced trinocularbiological microscope with a 14 M P USB Digital Camera (A35140U3; China). Measurements of MMCs were performed on liver tissue images. To calculate the MMCs, six fields were randomly selected on each slide. Thus, sixty readings per treatment were performed at 400 X magnification. After each field was imaged, the area (μm2) (Motic Images Plus2.0) was measured and the number of MMCs per field counted.
2.8. Histochemical preparation
The estimation of general carbohydrates represents important parameters between histochemical ones. For the demonstration of the polysaccharides status, Periodic acid Schiff’s (PAS) technique was applied [34]. In this regard, carbohydrates were first oxidized with 0.1℅ periodic acid; aldehyde groups (−HCO−HCO), were liberated from the glycol reagent, producing in a compound of magenta coloration.
2.9. Statistical analysis
Basic statistics, means, standard errors, and ranges of the measured parameters were estimated. Levene’s test was applied to equalize the variance of error in variables, with the rejection of the null hypothesis of raw, log-transformed and SQRT-transformed data. Therefore, the homogeneity of variance was assumed for raw data. The pattern of variations in parameters due to the size and concentration of the AgNPs and size-concentration interaction was studied by two-way ANOVA. Moreover, in the absence of interactions, the pattern of variations was recorded by one-way ANOVA in all treatments and control groups. The Tukey-HSD test was considered for multiple comparisons. The IBM-SPSS package version 21 [35] and Xls-sheets were considered at 0.05 significance level.
3. Results
3.1. Behavior, mortality and morphological changes
Fish showed abnormal behavior during the experimental period. At the start of the exposure, fish were alert, stopped swimming, and remained static in position. After some time they tried to avoid toxic water by fast swimming and jumping in tanks with silver nanoparticles, the fish swam unsteadily with more or less nervous manifestations in the form of jerky uncoordinated movement, stretching fins and irregular swimming, loss of equilibrium, and inability to remain upright. Changes in color and loss of appetite were also recorded for some fish, especially in those fish exposed only to silver 20 nm/100 μg/L AgNPs. Moreover, after 15 days of recovery period fish were noticed in better conditions and no mortality were recorded in all treatments.
3.2. Biochemical parameters
The biochemical parameters of Clarias gariepinus for 15 days of exposure and 15 days of recovery periods is shown in Table 1.
Table 1.
The basic data of biochemical parameters of Clarias gariepinus exposed to silver nanoparticles and silver nitrate for 15 days exposure and 15 days recovery periods (N = 3).
| Treatments Parameters | C | AgNO3 (100μg/L) | 20 nm/10 μg/L AgNPs | 20 nm/10 μg/L AgNPs | 40 nm/10 μg/L AgNPs | 40 nm/100 μg/L AgNPs |
|---|---|---|---|---|---|---|
| Mean±SE(Min-Max) | Mean±SE(Min-Max) | Mean±SE(Min-Max) | Mean±SE(Min-Max) | Mean±SE(Min-Max) | Mean±SE(Min-Max) | |
| Exposure | ||||||
| Glucose (mg/dl) | 57.03±4.40 a (50.5–65.4) |
62.43±1.59 ab (60.2–65.5) |
79.50±1.42 cd (77.2–82.1) |
89.07±0.90 d (87.4–90.5) |
66.97±1.13 ab (65.6–69.2) |
72.03±1.48 bc (69.3–74.4) |
| TL (g/l) | 59.47± 4.055 a (65.857–51.948) |
90.844 ± 2.771 b (95.74–86.147) |
111.59 ± 7.574 cd (120.87–96.58) |
126.84 ± 3.286 d (133.16–122.121) |
97.09 ± 4.663bc (105.857–89.948) |
121.78 ± 1.950 d (124.909–118.199) |
| TP (mg/dl) | 4.18±0.29 a (3.88–4.76) |
3.50±0.21 ab (3.27–3.92) |
2.99±0.12 bc (2.82–3.23) |
2.51±0.08 c (2.36–2.64) |
2.99±0.10 bc (2.78–3.12) |
2.89±0.16 bc (2.68–3.21) |
| Albumin (mg/dl) | 1.90±0.0504 a (1.8–1.97) |
1.64±0.0977 ab (1.5–1.83) |
1.55±0.0328 b (1.5–1.61) |
1.00±0.0153 c (0.97–1.02) |
1.38±0.1017 b (1.22–1.57) |
0.91±0.0145 c (0.88–0.93) |
| Globulin (mg/dl) | 0.88±0.021 a (0.84–0.91) |
0.79±0.020 b (0.75–0.82) |
0.80±0.006 b (0.79–0.81) |
0.69±0.012 d (0.67–0.71) |
0.76±0.006 bc (0.75–0.77) |
0.70±0.012 cd (0.68–0.72) |
| A/G Ratio | 2.16±0.08 a (2.022–2.29) |
2.09±0.14 a (1.83–2.32) |
1.93±0.05 a (1.85–2.013) |
1.46±0.03 bc (1.42–1.52) |
1.82±0.14 ab (1.58–2.066) |
1.30±0.04 c (1.22–1.37) |
| ALT (U/ml) | 13.20±1.185 a (11.1–15.2) |
17.13±1.040 a (15.3–18.9) |
23.10±1.217 b (21.1–25.3) |
27.53±0.89 c (26.5–29.3) |
22.43±0.410 b (21.8–23.2) |
24.77±0.437 bc (23.9–25.3) |
| AST (U/ ml) | 272.43±5.253 a (262.3–279.9) |
305.30±3.320 bc (299.6–311.1) |
309.07±4.103 bc (301.5–315.6) |
353.00±10.584 d (333.1–369.2) |
298.97±1.185 b (296.7–300.7) |
325.97±2.252 c (322.1–329.9) |
| ALP (U/L) | 234.83±1.041 a (233.1–236.7) |
263.80±2.854 ab (259.5–269.2) |
348.30±6.745 c (335.9–359.1) |
538.23±35.737 d (496.1–609.3) |
334.97±3.162 bc (329.2–340.1) |
368.00±5.269 c (359.3–377.5) |
| Recovery | ||||||
| Glucose (mg/dl) | 57.03±4.40 a (50.5–65.4) |
65.55±2.26 ab (61.5–69.3) |
69.50±1.25 ab (67.1–71.3) |
75.98±1.89 b (72.65–79.2) |
67.80±0.76 ab (66.6–69.2) |
70.17±4.03 ab (62.3–75.6) |
| TL (g/l) | 59.47 ± 4.055 a (65.857–51.948) |
84.08 ±4.011 b (90.0825–76.47) |
94.77 ± 2.101 bc (98.138–90.909) |
105.11 ± 3.241 c (110.31–99.16) |
90.04 ± 2.964 b (95.5–85.31) |
97.90 ± 2.363 bc (102.6–95.099) |
| TP (mg/dl) | 4.18±0.29 a (3.88–4.76) |
4.11±0.23 a (3.81–4.57) |
3.88±0.22 a (3.6–4.31) |
3.69±0.15 a (3.49–3.99) |
3.95±0.26 a (3.59–4.46) |
3.82±0.12 a (3.6–4.03) |
| Albumin (mg/dl) | 1.90±0.05 a (1.8–1.97) |
1.88±0.02 a (1.85–1.91) |
1.85±0.01 a (1.83–1.88) |
1.79±0.02 a (1.75–1.82) |
1.84±0.03 a (1.8–1.89) |
1.81±0.01 a (1.78–1.83) |
| Globulin (mg/dl) | 0.88±0.02 a (0.84–0.91) |
0.87±0.01 a (0.86-0.88) |
0.85±0.02 ab (0.82–0.88) |
0.81±0.01 b (0.79–0.82) |
0.85±0.01 ab (0.83–0.86) |
0.84±0.01 ab (0.82–0.85) |
| A/G Ratio | 2.16±0.08 a (2.02–2.29) |
2.1 7±0.03 a (2.13–2.22) |
2.17±0.03 a (2.14–2.23) |
2.22±0.03 a (2.16–2.27) |
2.17±0.05 a (2.09–2.28) |
2.16±0.03 a (2.09–2.21) |
| ALT (U/ml) | 13.20±1.18 a (11.1–15.2) |
13.43±0.68 a (12.1–14.3) |
15.47±1.27 ab (13.2–17.6) |
19.03±0.81 b (17.9–20.6) |
15.58±0.77 ab (14.25–16.9) |
18.23±1.08 b (16.2–19.9) |
| AST (U/ ml) | 272.43±5.25 a(262.3–279.9) | 276.30±5.89 a (266.3–286.7) |
278.43±4.90 a (269.2–285.9) |
298.45±8.45 a (282.6–311.45) |
275.53±5.49 a (265.2–283.9) |
292.10±8.38 a (280.3–308.3) |
| ALP (U/L) | 234.83±1.04 a (233.1–236.7) |
238.33±1.24 a (235.9–240) |
253.37±3.94 b (245.9–259.3) |
266.83±1.39 c (264.8–269.5) |
240.63±0.80 a (239.6–242.2) |
264.97±2.95 c(259.5–269.6 ) |
Different letters indicate significant difference at (P < 0.05).
C = control, AgNPs = silver nanoparticles and AgNO3= silver nitrate.
3.2.1. Glucose level
The main effects of AgNPs size and concentration on glucose level were significant with no significant size-concentration interaction. In the recovery period, such main effects were not significant. In comparison to the control and silver nitrate groups, the glucose level of the nanoparticle-exposed fish exhibited significant variability (Table 1). After 15-day recovery, the pollutant adverse effects on the glucose level compared with the silver nitrate group were eliminated, while these effects are still recorded to some extent compared to the control group (Table 1).
3.2.2. Total lipids level
The main effects of AgNPs size and concentration on total lipids level were significant with no significant size-concentration interaction. In the recovery period, the total lipids level was still impacted by the nanoparticles size and was stressed by the particle concentrations. In comparison to the control and silver nitrate groups, the total lipids level of the nanoparticle-exposed fish exhibited significant variability (Table 1). After 15-day recovery, the pollutant adverse effects on the total lipids level are still recorded to some extent compared to control and silver nitrate groups (Table 1).
3.2.3. Total proteins level
The main effects of AgNPs concentration on the total proteins level were significant, whereas the main effects of size factor were not significant. No significant size-concentration interaction. In the recovery period, these significant main effects were eliminated. In comparison to the control and silver nitrate groups, the total proteins level of the nanoparticle-exposed fish exhibited significant variability (Table 1). After 15-day recovery, no significant variation was recorded compared to the control and silver nitrate groups (Table 1).
3.2.4. Total albumin, globulin, and A/G ratio levels
The main effects of AgNPs concentration on total albumin, globulin, and A/G Ratio levels were significant, whereas the main effects of size factor were not significant except at the level of albumin level. There is no significant size-concentration interaction except at the level of globulin. In the recovery period, these parameters were not impacted by the nanoparticle size but stressed by the concentration of these particles except for A/G Ratio with no significant size-concentration interaction. In comparison to the control and silver nitrate groups, these parameters of the nanoparticle-exposed fish showed significant variation. (Table 1). On the recovery of pollutant, there is no significant variation compared to the control and silver nitrate groups (Table 1).
3.2.5. ALT, AST, and ALP activities
The main effects of AgNPs size and concentration on liver enzymes activities were significant with no significant size-concentration interaction except for ALP activities. In the recovery period, only ALP activities were impacted by the nanoparticle size, while the main effects of concentration factor were significant in such parameters. In comparison to the control and silver nitrate groups significant variation was observed (Table 1). On the recovery of pollutant, significant variation was recorded except for AST activities compared to control and silver nitrate groups (Table 1).
3.3. Determination of silver concentrations in liver tissue
Silver concentrations in liver tissue for15 days of exposure and 15 days of recovery periods were given in Table 2. The main effects of AgNPs size and concentration on silver concentrations were significant with no significant size-concentration interaction. In the recovery periods, liver tissue was still impacted by the nanoparticle size and stressed by these particle concentrations with no significant size-concentration interaction. In comparison to the control and silver nitrate groups, liver tissue of the nanoparticle-exposed fish exhibited significant variability (Table 2). After 15-day recovery, the pollutant adverse effects on the liver tissue are still recorded (Table 2).
Table 2.
Determination of silver nanoparticles in liver tissues of Clarias gariepinus for 15 days exposure and 15 days recovery periods (N = 3).
| Liver Tissue μg/L |
||
|---|---|---|
| Treatments | 15 days exposure | 15 days recovery |
| Mean ± SE (Min-Max) |
Mean ± SE (Min-Max) |
|
| C | 0.736 ± 0.02511a (0.69-0.78) |
0.7365 ± 0.025a (0 .69- 0.78) |
| AgNO3 (100 μg/L) | 4.0855 ± 0.3109b (12.36–13.47) |
1.186 ± 0.00058a (8.72–9.65) |
| 20 nm/10 μg/L AgNPs | 8.212 ± 0.36258c (3.55–4.62) |
4.6835 ± 0.27684b (1.18–1.19) |
| 20 nm/100 μg/L AgNPs | 12.914 ± 0.322d (5.54–6.37) |
9.189 ± 0.26847c (2.87–3.5) |
| 40 nm/10 μg/L AgNPs | 5.958 ± 0.2384e (9.28–10.21) |
3.1875 ± 0.181d (6.16–7.14) |
| 40 nm/100 μg/L AgNPs | 9.742 ± 0.2684f (7.58–8.84) |
6.6485 ± 0.28261e (4.2–5.16) |
Different letters indicate significant difference at (P < 0.05).
C = control, AgNPs = silver nanoparticles and AgNO3= silver nitrate.
3.4. Histopathological and histochemical studies
3.4.1. Control liver
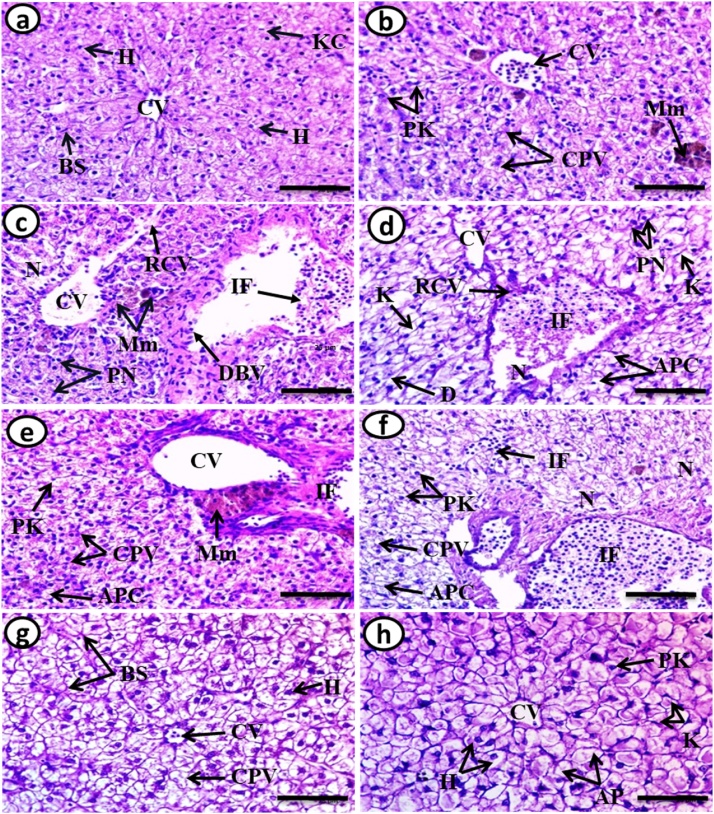
The liver of the control fish Clarias gariepinus appears as a continuous mass of hepatic cells (hepatocytes) that exhibit a cord-like pattern and is interrupted by blood vessels and sinusoids. The cords of hepatocytes are arranged around the central vein. The hepatocytes are large in size, polygonal in shape with a centrally located nucleus. The hepatocytes have homogenous eosinophilic cytoplasm. The sinusoids are seen as communication channels occupied by blood cells (Fig. 1a).
Fig. 1.
Transverse sections of control, exposed and recovery fish liver (H & E, X 400).
(a) Control fish liver showing the general structure. Blood sinusoids (BS), Central vein (CV), hepatocytes (H) and Küpffer cell (KC).
(b) Exposure to 100 μg/L of silver nitrate (AgNO3) for 15 days showing proliferation of hepatocytes (crowded of nuclei), infiltrations of inflammatory cell (IF), pyknotic nuclei (PK) and cytoplasmic vaculation (CPV) and an aggregation of melanomacrophage (Mm).
(c) Exposure to 20 nm/10 μg/L AgNPs for 15 days showing proliferation of the hepatic cells with a decrease in cell size, infiltration of the inflammatory cells (IF) inside the blood vessels, an aggregation of melanomacrophage (Mm) beside central vein. Dilation in the blood vessel (DBV) and areas of hepatic necrosis (N), rupture of the wall of the central vein (RCV) and some hepatocyte having pyknotic nuclei (PK).
(d) Exposure to 20 nm/100 μg/L AgNPs for 15days showing rupture of the cell membrane of central vein (RCV), Apoptotic cell (APC), pyknotic nuclei(PK), degeneration of the hepatocytes(D) with infiltration of inflammatory cells (IF), Küpffer cells (KC) and necrotic area (N).
(e) Exposure to 40 nm/10 μg/L AgNPs for 15 days showing infiltration of the inflammatory cells (IF), melanomacrophage (Mm) beside central vein, Apoptotic cells (APC), pyknotic nuclei (PK) with cytoplasmic vaculation (CPV) and proliferation of the hepatic cells.
(f) Exposure to 40 nm/100 μg/L AgNPs for 15 days showing infiltration of inflammatory cells (IF) inside the central vein and between hepatocyte, Apoptotic cells (APC), pyknotic nuclei (PK), cytoplasmic vaculation (CPV) and necrotic area (N).
(g&h) Recovery fish liver sections showing normal structure of hepatic tissue and arrangement of the hepatocytes (H), central vein (CV) and blood sinusoid (BS) with increased number of Küpffer cells (KC), the limits between hepatocytes become thick. Some cells still suffering from the absence of nuclei while the others were having pyknotic nuclei (PK), and Cytoplasmic vaculation (CPV).
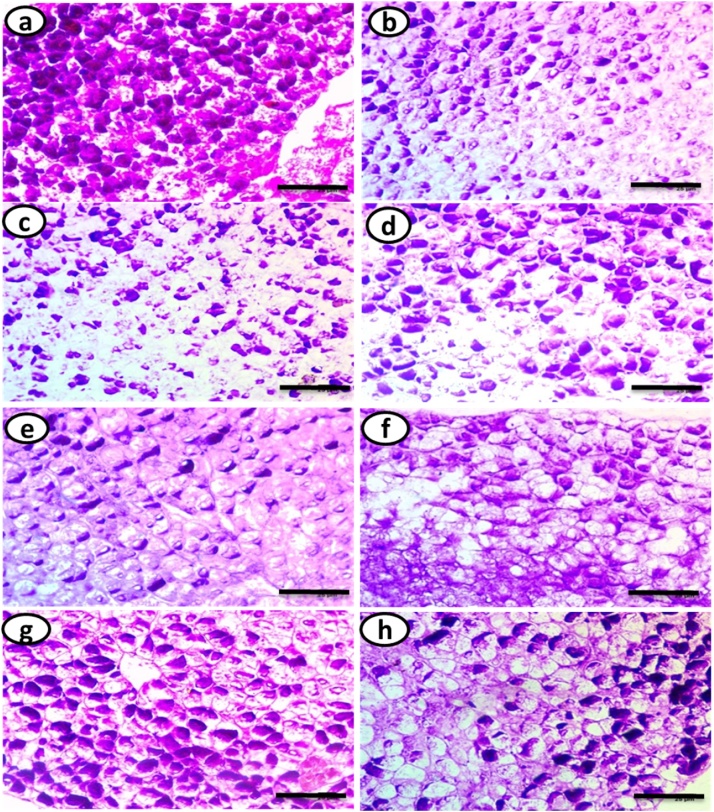
PAS-technique exhibited a distinct distribution of polysaccharides in control fish hepatocyte. The positively stained materials have been shown to be glycogen as verified by PAS-staining with and without pretreatment with diastase. This reaction appeared relatively higher in hepatocytes around the central vein areas compared to the peripheral organs (Fig. 2a).
Fig. 2.
Transverse sections of control, exposed and recovery fish liver. (PAS-reaction, X 400).
(a) Control fish liver showing the great amount of glycogen in the cytoplasm of hepatocytes.
(b) Exposure to 100 μg/L of silver nitrate (AgNO3) for 15 days showing a remarkable depletion of glycogen content in the liver cells.
(c) Exposure to 20 nm/10 μg/L AgNPs for 15 days showing a remarkable depletion in the glycogen content in hepatocyte.
(d) Exposure to 20 nm/100 μg/L AgNPs for 15days showing depletion in the glycogen content in hepatocyte.
(e) Exposure to 40 nm/10 μg/L AgNPs for 15 days showing depletion in the glycogen content in hepatocyte.
(f) Exposure to 40 nm/100 μg/L AgNPs for 15 days showing depletion in the glycogen content in hepatocyte.
(g&h) Recovery fish liver sections showing more accumulation of carbohydrate materials.
3.4.2. Exposure of Clarias gariepinus to100 μg/L of silver nitrate (AgNO3)
The silver nitrate dose used for 15 days of exposure led to adverse impacts on the liver including the hepatocytes delimited by ruptured cell membranes in some areas and dispersion of cell contents, some hepatocytes were characterized by the absence of nuclei while the others were pyknotic nuclei and cytoplasmic vacuolation. Also, infiltration of inflammatory cells inside the central vein and between the hepatocytes, proliferation of hepatocytes (crowded of nuclei), and an aggregation of melanomacrophages was observed (Fig. 1b). Such impacts are reflected in terms of morphometric data and semiquantitative evaluations of the histopathology of the AgNO3-exposed liver of C. gariepinus (Tables 3 & 4 ). After 15 days of recovery period, the fishes retained their normal appearance of hepatic tissues and blood sinusoids. The boundary between the cells becomes clear. Absence of MMCs with cytoplasmic vacuolation was observed (Fig. 1g&h).
Table 3.
Morphometric data on liver alterations in C. gariepinus for 15 days exposure and 15 days recovery periods (N = 3).
| Treatments Histopathology |
C | AgNO3 (100 μg/L) | 20 nm/10 μg/L AgNPs | 20 nm/100 μg/L AgNPs | 40 nm/10 μg/L AgNPs | 40 nm/100 μg/L AgNPs |
|---|---|---|---|---|---|---|
| Mean ± SE (Min-Max) |
Mean ± SE (Min-Max) |
Mean ± SE (Min-Max) |
Mean ± SE (Min-Max) |
Mean ± SE (Min-Max) |
Mean ± SE (Min-Max) |
|
| Exposure | ||||||
| Dilation of central vein | 1.56 ± 0.37 a (0.62–2.59) |
4.61 ± 1.04 a (0–13.48) |
6.04 ± 0.86 a (0–12.36) |
4.73 ± 1.17 a (0–14.84) |
4.91 ± 0.98 a (0–13.36) |
3.07 ± 1.075 a (0–21.34) |
| Infiltrations of inflammatory cell | 1.44 ± 1.44 a (0–7.19) |
10.51 ± 3.58 a (0–46.52) |
2.85 ± 0.83 a (0–9.72) |
10.38 ± 4.83 a (0–63.37) |
6.64 ± 2.46 a (0–28.01) |
6.74 ± 2.18 a (0–39.69) |
| Hepatic necrosis | 0 | 10.37 ± 7.08 a (0–84.4) |
7.01 ± 1.45 a (0–22.01) |
10.60 ± 5.82 a (0–92.8) |
10.71 ± 3.62 a (0–54.98) |
21.07 ± 7.97 a (0–93.48) |
| Hemorrhage | 0 | 3.05 ± 3.05 a (0–48.84) |
2.25 ± 1.04 a (0–13.37) |
6.90 ± 4.80 a (0–75.31) |
2.70 ± 0.84 a (0–9.22) |
3.05 ± 1.67 a (0–21.13) |
| Dilation of blood vessels | 0 | 0 | 3.32 ± 2.29 a (0–35.77) |
0 | 0.17 ± 0.17 a (0–2.79) |
0 |
| Recovery | ||||||
| Dilation of central vein | 0.97 ± .079 a (0.70–1.21) |
1.63 ± 0.63a (.52–4.00) |
3.44 ± .88 a (0–10.72) |
4.95 ± 1.26a (0–7.15) |
3.03 ± 1.58 a (0–9.53) |
.623 ± 0.35 a (0–2.29) |
| Infiltrations of inflammatory cell | 0.32 ± .32 a (0–2.27) |
0 | 0 | 4.1 ± 2.69 ab (0–13.39) |
8.19 ± 4.2 b (0–23.97) |
0 |
| Hepatic necrosis | 0 | 0 | 3.02 ± 3.01 a (0–39.20) |
6.41 ± 3.92 a (0–16.08) |
3.05 ± 1.96 a (0–11.02) |
0 |
| Hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 |
| Dilation of blood vessels | 0 | 0 | 1.33 ± 1.32 a (0–17.28) |
0 | 0 | 0 |
C = control, AgNPs = silver nanoparticles and AgNO3= silver nitrate.
Table 4.
Semi quantitative scoring of the histopathology in the liver of Clarias gariepinus exposed to silver nanoparticles and silver nitrate for 15 days exposure and 15 days recovery periods (N = 3).
| Histopathologic lesion | C | AgNO3 (100 μg/L) | 20 nm/10 μg/L AgNPs | 20 nm/100 μg/L AgNPs | 40 nm/10 μg/L AgNPs | 40 nm/100 μg/L AgNPs |
|---|---|---|---|---|---|---|
| Exposure | ||||||
| Infiltrations of inflammatory cell | – | ++ | + | ++ | + | + |
| Melanomacrophage aggregation | – | ++ | + | ++ | + | ++ |
| Dilation of blood vessel | – | – | + | – | – | – |
| Hepatic necrosis | – | ++ | + | ++ | ++ | ++ |
| Central vein dilation | – | + | + | + | + | + |
| Hemorrhage | – | + | + | + | + | + |
| Recovery | ||||||
| Infiltrations of inflammatory cell | – | – | – | + | + | – |
| Melanomacrophage aggregation | – | – | – | + | – | – |
| Dilation of blood vessel | – | – | – | – | – | – |
| Hepatic necrosis | – | – | + | + | + | – |
| Central vein dilation | – | – | – | + | + | – |
| Hemorrhage | – | – | – | + | – | – |
Score: (-) No alteration, (+) Mild alteration, (++) moderate alteration, (+++) severe alteration.
C = control, AgNPs = silver nanoparticles and AgNO3= silver nitrate.
PAS-technique revealed depletion of glycogen content in hepatocyte after 15 days of exposure compared to control ones (Fig. 2b). After 15 days of recovery period, more accumulation of carbohydrates was observed (Fig. 2g&h).
3.4.3. Exposure of Clarias gariepinus to 10 μg/L of silver nanoparticles (20 nm)
Liver of fish exposed to 10 μg/L of silver nanoparticles for 15 days showed severe damage represented by heterogeneous liver structure, hepatocytes lost their normal polygonal shape and boundary between cells became invisible. Accordingly, the sinusoidal lumen collapsed and a few Küpffer cells were observed. Rupture of hepatocyte membranes and proliferation of hepatic cells with a decrease in cell size were observed. Also, infiltration of inflammatory cells inside the blood vessels and between the hepatocytes and an aggregation of MMCs beside central vein were observed. The dilation of the blood vessel, the areas of hepatic necrosis began to appear in some areas and the rupture of the central vein wall were noticed, some hepatocytes were characterized by the absence of nucleus (Apoptotic cell) while others had pyknotic nuclei (Fig. 1c). In terms of morphometric data and semiquantitative evaluations of the histopathology of AgNPs-exposed liver of C. gariepinus (Tables 3 & 4) damage was recorded. After 15 days of recovery period, the fish retained their normal appearance of hepatic tissue. The number of Küpffer cells increased with a marked decrease in the number of the MMCs. The boundary between the cells becomes clear. However, some cells still suffer from lack of nuclei while others cells were suffer from pyknotic nuclei (Fig. 1g&h).
The application of the PAS reaction showed significant depletion of glycogen content in hepatocyte after 15 days of exposure. The cytoplasm of the majority of hepatocytes exhibited a faint staining with PAS reaction compared to those of the control (Fig. 2c). After 15 days of the recovery period, a significant accumulation of carbohydrates in hepatocytes was observed in comparison with fish exposed to silver nanoparticles (Fig. 2g&h).
3.4.4. Exposure of Clarias gariepinus to 100 μg/l of silver nanoparticles (20 nm)
In addition to previous changes that increased the AgNPs concentration of 20 nm led to sever alterations including advanced necrotic areas, the hepatocytes are delimited by rupture cell membranes in some areas and dispersion of cell contents, rupture of the cell membrane of the central vein and loss of stainability in others. More hydropic degeneration of hepatocytes, granulocyte cytoplasm and increased number of küpffer cells were observed (Fig. 1d). Morphometric data and semiquantitative evaluations of the histopathology of AgNPs-exposed liver of C. gariepinus were recorded (Tables 3 & 4).
After 15 days of recovery period produced normal structure of liver tissue. The boundaries between hepatocytes appear and show a few Küpffer cells. Some individual have produced histopathological alterations in liver tissue. These alterations included cytoplasmic vacuolation, some of which were characterized by the absence of nuclei (apoptotic cell) while others were suffering from pyknotic nuclei (Fig. 1g&h).
PAS-technique revealed a depletion of glycogen content in hepatocyte after 15 days of exposure compared to those of control (Fig. 2d). After 15 days of recovery period, more accumulation of carbohydrates was observed (Fig. 2g&h).
3.4.5. Exposure of Clarias gariepinus to 10 μg/L and 100 μg/L of silver nanoparticles (40 nm)
As regard the two concentrations of AgNPs of 40 nm-sizes, sever alterations in fish liver structures were recorded in terms of histopathology and PAS-reaction (Figs. 1 & 2) with variation after 15 days of exposure. These alterations were improved by 15-day recovery period. These patterns of damage (Figs.1 & 2 e&f) and improved recovery (Figs. 1 & 2 g&h) were similar to those considered in the case of two concentrations of AgNPs of 20 nm-size previously mentioned (Tables 3 & 4) with a variable percentage of damage.
3.5. Measurements of MMCs
Melanomacrophage centers of Clarias gariepinus for 15 days of exposure and 15 days of recovery periods are shown in Table 5. The main effects of AgNPs concentration on the size and number of MMCs were significant, whereas the main effects of the size factor were significant only in the size of MMCs with no significant size-concentration interaction. The main effects of these factors were observed in the recovery period. In comparison to the control group, the MMCs size and number of the nanoparticle-exposed fish exhibited significant variability (Table 5). After 15-days recovery period, the adverse effects of pollutants are still recorded to some extent compared to the control group (Table 5).
Table 5.
Changes in number and size of MMCs/μm2organ after exposure to Ag-NPs and silver nitrate in Clarias gariepinus (N = 3).
| Treatments | Size of melanomacrophage μm2 |
Number of melanomacrophage |
||
|---|---|---|---|---|
| Exposure period | Recovery period | Exposure period | Recovery period | |
| M ± SE | M ± SE | M ± SE | M ± SE | |
| (MinMax) | (MinMax) | (MinMax) | (MinMax) | |
| C | 725.76 ± 186.21 a (0–7180.9) |
718.86 ± 199.044 a (0–3617) |
3.85 ± 0.827 a (0–21) |
1.833 ± 0.57 a (0–10) |
| AgNO3 (100 μg/L) | 6253.76 ± 676.075 b (0–17982.5) |
3516.696 ± 826.83 b (0–13786) |
16.5 ± 1.918 ab (0–44) |
5.7 ± 1.14 ab (0–18) |
| 20 nm/10 μg/L AgNPs | 12335.198 ± 1842.94 c (0–71765.6) |
7615.24 ± 1109.823 bc (0–19843) |
22.1 ± 2.140 b (0–51) |
8.43 ± 1.38 bc (0–22) |
| 20 nm/100 μg/L AgNPs | 24374.955 ± 2200.80 d (0–64723.1) |
16187.56 ± 1720.707 c (0–29873.4) |
27.55 ± 2.243 d (0–57) |
10.83 ± 1.517 c (0–25) |
| 40 nm/10 μg/L AgNPs | 8206.10 ± 1149.914 bc (0–54771.9) |
5625.763 ± 1194.702 bc (0–22874.3) |
20.41 ± 2.086 b (0–49) |
7 ± 1.26 bc(0 –20) |
| 40 nm/100 μg/L AgNPs | 19952.105 ± 1922.713 d (0–54771.9) |
11786.663 ± 1322.681 bc (0–27621.3) |
23.85 ± 2.185 c (0–53) |
10 ± 1.47 bc (0–25) |
Different letters indicate significant difference at (P < 0.05).
C = control, AgNPs = silver nanoparticles and AgNO3= silver nitrate.
4. Discussion
Results in the present study indicated a significant increase in serum glucose (hyperglycaemia) level in Clarias gariepinus exposed to AgNPs for 15 days. These results were in agreement with Abdel-Khalek et al. [36] who observed a significant increase in serum glucose level of Nile Tilapia; O. niloticus after exposure to copper oxide nanoparticles. Similar results were reported in Silver carp and in juvenile Atlantic salmon (Salmo salar) exposed to Ag-NPs [37,38] respectively. The alteration in glucose level may be associated with kidney or liver damage, nutritional deficiencies, glycogenolysis, glucose synthesis, and excessive production of ROS within tissues, which may damage carbohydrates [[39], [40], [41]].
The present study reported a significant increase in serum total lipids in C. gariepinus exposed to AgNPs. These results were in agreement with Mekkawy et al. [42] and Valerio-Garcíaa et al. [43], who observed a significant increase in the total lipids level of Oreochromis niloticus and Chapalichthys pardalis exposed to cadmium and silver nanoparticles, respectively. It has been reported that, the increase in serum total lipids may be due to disturbance of lipids metabolism [44].
The results of the present study indicated a significant decrease in serum total proteins (hypoproteinemia), albumin (hypoalbuminemia), and globulin contents in C. gariepinus exposed to AgNPs. These results were in accordance with Abdel-Khalek et al. [36] and Alkaladi et al. [45], where they reported a significant decrease in total proteins, albumin, and globulin contents of O. niloticus after exposure to copper and zinc oxide nanoparticles, respectively. Thomas et al. [46] and Monfared et al. [47] observed decrease in total protein of Oreochromis mossambicus, Danio rerio, and O. mykiss exposed to magnesium oxide and silver nanoparticles, respectively. The decrease in the serum proteins level was interpreted by different authors including Hori et al. [48], Haliwell [39], Wang et al. [41], Nel et al. [49], Alkaladi et al. [45] and Kunjiappan et al. [50].
The results in the present study indicated a significant increase in serum enzyme activities (AST, ALT, and ALP) in C. gariepinus exposed to AgNPs for 15 days. These findings were in agreement with Monfared and Soltani [51], Monfared et al. [47] and Imani et al. [52] who observed a significant increase in the activities of AST, ALT, and ALP of rainbow trout (Oncorhynchus mykiss) after exposure to silver nanoparticles. These results were in agreement with Abdel-Khalek et al. [36] reported a significant increase in serum enzyme activities (AST, ALT, and ALP) of Nile Tilapia O. niloticus after exposure to copper oxide nanoparticles. High levels of AST, ALT, and ALP enzymes in the cytoplasm of liver cells may be a result of liver injury leading to increased permeability of cell membranes [[53], [54], [55]].
In the present study the results indicated a significant accumulation of silver in the liver of C. gariepinus after exposure for 15 days. These findings were in agreement with Johari et al. [56] who observed maximum amount of Ag accumulation in intestine, liver, gills, and muscles, respectively in rainbow trout after exposure to silver nanoparticles (Ag-NPs). Also, Zhao et al. [57] reported maximum amount of copper accumulation in intestine, gills, muscles, skin, scales, liver, and brain, respectively in common carp after exposure to copper oxide nanoparticles. These findings were interpreted by different authors including Best et al. [58], Handy et al. [59] and Johari et al. [56].
Histopathological studies by different authors and the present study were found to be a useful tool for assessing the damage caused by nanomaterials [1,56,60]. Liver is an important organ in active metabolism, detoxification and is extremely sensitive to pollutants [61]. In the present investigation, the impacts of AgNPs were observed in the liver tissue with different degrees of variable impacts and alterations. These impacts and alterations in agreement with those observed in Mozambique tilapia; Oreochromis mossambicus exposed to nickel nanoparticles [62], in Zebra fish Danio rerio exposed to pesticides and heavy metals [63], in Danio rerio, Drosophila melanogaste, and Oncorhynchus mykiss exposed to silver nanoparticles [19,64,56,65], respectively, in Catfish Mystus vittatus exposed to ZnS nanoparticles [66], in Mozambique tilapia, Oreochromis mossambicus exposed to nickel nanoparticles [62], in Labeo rohita exposed to silver nanoparticles [1], in rainbow trout (Oncorhynchus mykiss) and in juvenile carp (Cyprinus carpio) after exposure to titanium dioxide nanoparticles [21,67], respectively. Liver damages were interpreted by different authors including Agius and Roberts [22], Mekkawy et al. [68], Mekkawy et al. [69] Olojo et al. [70], Wassif et al. [71] and Yuness [72] these damages were also recorded by PAS reactions in terms of carbohydrates depletion under stress.
The results in the present study indicated a significant increase in the frequency and size of MMCs in the liver of C. gariepinus exposed to Ag-NPs for 15 days. These results were in agreement with some previous studies on fishes [15,22,23,25,[73], [74], [75]]. Usually, the increase in MMCs associated with histopathological alterations, suggesting oxidative stress that leads to the aggregation of lymphocytes that indicate the immune response of MMCs [76,77].
5. Conclusion
This investigation clearly demonstrated the adverse impacts of AgNPs and AgNO3 in different concentrations on the function and structure of the liver of C. gariepinus. So, we concluded that the use and application of these chemicals must be manageable and controlled to protect aquatic ecosystem. Furthermore, the recovery strategy of pollutants is essential for fish species according to their location in the food web.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Rajkumar K.S., Kanipandian N., Thirumurugan R. Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl. Nanosci. 2016;6:19–29. [Google Scholar]
- 2.Benn T.M., Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008;42:7025–7026. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- 3.Masciangioli T., Zhang W.X. Environmental technologies at the nanoscale. Environ. Sci. Tochnol. 2003;37:102–108. doi: 10.1021/es0323998. [DOI] [PubMed] [Google Scholar]
- 4.Nohynek G.J., Lademann J., Ribaud C., Roberts M.S. Grey Goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit. Rev. Toxicol. 2007;37:251–277. doi: 10.1080/10408440601177780. [DOI] [PubMed] [Google Scholar]
- 5.Colvin V. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003;21:1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- 6.Daphedar A., Taranath T.C. Characterization and cytotoxic effect of biogenic silver nanoparticles on mitotic chromosomes of Drimia polyantha (Blatt. & McCann) Stearn. Toxicol. Rep. 2018;5:910–918. doi: 10.1016/j.toxrep.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handy R.D., Al-Bairuty G., Al-Jubory A., Ramsden C.S., Boyle D., Shaw B.J., Henry T.B. Effects of manufactured nanomaterials on fishes: a target organ and body systems physiology approach. J. Fish Biol. 2011;79:821–853. doi: 10.1111/j.1095-8649.2011.03080.x. [DOI] [PubMed] [Google Scholar]
- 8.Henry S.P., Kim T.-W., Kramer-Stickland K., Zanardi T.A., Fey R.A., Levin A.A. Toxicologic properties of 2′-methoxyethyl chimeric antisense inhibitors in animals and man. In: Crooke S.T., editor. Antisense Drug Technology: Principles, Strategies and Applications. 2nd ed. CRC Press; Carlsbad, CA: 2008. pp. 327–363. [Google Scholar]
- 9.Jahan S., Yusoff I.B., Alias Y.B., Abu Bakar A.F.B. Reviews of the toxicity behavior of five potential engineered nanomaterials (ENMs) into the aquatic ecosystem. Toxicol. Rep. 2017;4:211–220. doi: 10.1016/j.toxrep.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klaine S.J., Alvarez P.J.J., Batley G.E., Fernandes T.F., Handy R.D., Lyon D.Y., Mahendra S., McLaughlin M.J., Lead J.R. Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008;27:1825–1851. doi: 10.1897/08-090.1. [DOI] [PubMed] [Google Scholar]
- 11.Moore M.N. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int. 2006;32:967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Wehmas L.C., Anders C., Chess J., Punnoose A., Pereira C.B., Greenwood J.A., Tanguay R.L. Comparative metal oxide nanoparticle toxicity using embryonic zebrafish. Toxicol. Rep. 2015;2:702–715. doi: 10.1016/j.toxrep.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dube P.N., Shwetha A., Hosetti B.B. Impact of copper cyanide on the key metabolic enzymes of freshwater fish Catla catla (Hamilton) Biotechnol. Anim. Husb. 2014;30:499–508. [Google Scholar]
- 14.Sayed A.H., Mekkawy I.A.A., Mahmoud U. Effects of 4-nonylphenol on metabolic enzymes, some ions and biochemical blood parameters of the African catfish Clarias gariepinus (Burchell, 1822) Afr. J. Biochem. Res. Acad. J. 2011;5:287–297. [Google Scholar]
- 15.Sayed A.H., Hamed H.S. Induction of apoptosis and DNA damage by 4-nonylphenol in African catfish (Clarias gariepinus) and the antioxidant role of Cydonia oblonga. Ecotoxicology and Environmental Safety. 2017;139:97–101. doi: 10.1016/j.ecoenv.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes C., Fontaínhas-Fernandes A., Rocha E., Salgado M.A. Monitoring pollution in Esmoriz-Paramos lagoon, Portugal: liver histological and biochemical effects in Liza saliens. Environ. Monit. Assess. 2008;145:315–322. doi: 10.1007/s10661-007-0041-4. [DOI] [PubMed] [Google Scholar]
- 17.Ramesh F., Nagarajan K. Histopathological changes in the muscle tissue of the fish Clarias batrachus exposed to untreated and treated sago effluent. Adv. Biosci. Bioeng. 2013;1:74–80. [Google Scholar]
- 18.Singh A., Dar M.Y., Joshi B., Sharma B., Shrivastava S., Shukla S. Phytofabrication of silver nanoparticles: novel drug to overcome hepatocellular ailments. Toxicol. Rep. 2018;5:333–342. doi: 10.1016/j.toxrep.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahamed M., Posgai R., Gorey T.J., Nielsen M., Hussain S.M., Rowe J.J. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol. Appl. Pharmacol. 2009;242:263–269. doi: 10.1016/j.taap.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Farkas J., Christian P., Urrea J.A.G., Roos N., Hassellov M. Effects of silver and gold nanoparticles on rainbow trout (Oncorhynchusmykiss) hepatocytes. Aquat. Toxicol. 2010;96:44–52. doi: 10.1016/j.aquatox.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Hao L., Wang Z., Xing B. Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in juvenile carp (Cyprinus carpio) J. Environ. Sci. 2009;21:1459–1466. doi: 10.1016/s1001-0742(08)62440-7. [DOI] [PubMed] [Google Scholar]
- 22.Agius C., Roberts R.J. Melano-macrophage centres and their role in fish pathology. J. Fish Dis. 2003;26:499–509. doi: 10.1046/j.1365-2761.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- 23.Suresh N. Effect of cadmium chloride on liver, spleen and kidneymelano macrophage centres in Tilapia mossambica. J. Environ. Biol. 2009;30:505–508. [PubMed] [Google Scholar]
- 24.Bermúdez I.C., García G.S., Piloto A.A., Pérez Y.F., Valdivieso A.G. Effect of the Cuban propolis collected in Manzanillo rea on the wounds healing in rats. Pharmacology. 2006;3:416–421. [Google Scholar]
- 25.Fafioye O.O., Adebisi A.A., Fagade S.O. Toxicity of Parkia biglobosa andRaphia vinifera extracts on Clarias gariepinus juveniles. Afr. J. Biotechnol. 2004;3:627–630. [Google Scholar]
- 26.Manrique W.G., da Silva Claudiano G., Petrillo T.R., P.d.C., M, Pereira Figueiredo M.A., de Andrade Belo M.A. Response of splenicmelanomacrophage centers of Oreochromis niloticus (Linnaeus, 1758)to inflammatory stimuli by BCG and foreign bodies. J. Appl. Ichthyol. 2014;30:1001–1006. [Google Scholar]
- 27.Hecht T., Uys W., Britz P.J. 1988. Culture of Sharptooth Catfish, Clarias gariepinus, in Southern Africa National Scientific Programmes Unit: CSIR, SANSP Report 153; p. 146. [Google Scholar]
- 28.Safriel O., Bruton M.N. CSIR; Pretoria: 1984. A Cooperative Aquaculture Research Programme for South Africa. South African National Scientific Programmes Report 89; p. 79. [Google Scholar]
- 29.Mekkawy I.A., Mahmoud U.M., Hana M.N., Sayed A.H. Cytotoxic and hemotoxic effects of silver nanoparticles on the African Catfish, Clarias gariepinus (Burchell, 1822) Ecotoxicol. Environ. Saf. 2019;171:438–446. doi: 10.1016/j.ecoenv.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Mahmoud U.M., Mekkawy I.A.A., Naguib M., Sayed A.H. Silver nanoparticle-induced nephrotoxicity in Clarias gariepinus: physio-histological biomarkers. Fish Physiol. Biochem. 2019;Dec 45(6):1895–1905. doi: 10.1007/s10695-019-00686-7. [DOI] [PubMed] [Google Scholar]
- 31.Hedayati A., Jahanbakhshi A. The effect of water-soluble fraction of diesel oil on some hematological indices in the great sturgeon Husohuso. Fish Physiol. Biochem. 2012;38:1753–1758. doi: 10.1007/s10695-012-9672-7. [DOI] [PubMed] [Google Scholar]
- 32.Shaw B.J., Al-Bairuty G., R.D., H Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout (Oncorhynchus mykiss): physiology and accumulation. Aquat. Toxicol. 2012;116:90–101. doi: 10.1016/j.aquatox.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 33.Bancroft D., Stevens A. Churchill Livingstone; Edinburgh, London, Melaborne: 1982. Theory and Practice of Histological Techniques. [Google Scholar]
- 34.Mc Manus J.P.A. Histological demonistration of mucin after periodic acid. Nature. 1946;158:2002. doi: 10.1038/158202a0. [DOI] [PubMed] [Google Scholar]
- 35.IBM-SPSS . 2012. IBM-SPSS Statistics Version 21. [Google Scholar]
- 36.Abdel-Khalek A.A., Kadry M.A.M., Badran S.R., Marie M.A.S. Comparative toxicity of copper oxide bulk and nano particles in Nile Tilapia; Oreochromis niloticus: biochemical and oxidative stress. J. Basic Appl. Zool. 2015;72:43–57. [Google Scholar]
- 37.Farmen E., Mikkelsen H.N., Evensen O., Einset J., Heier L.S., Rosseland B.O., Salbu B., Tollefsen K.E., Oughton D.H. Acute and sub-lethal effects in juvenile Atlantic salmon exposed to low mg/L concentrations of Ag nanoparticles. Aquat. Toxicol. 2012;108:78–84. doi: 10.1016/j.aquatox.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Shaluei F., Hedayati A., Jahanbakhshi A., Kolangi H., Fotovat M. Effect of subacute exposure to silver nanoparticle on hematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix) Hum. Exp. Toxicol. 2013;32:1270–1277. doi: 10.1177/0960327113485258. [DOI] [PubMed] [Google Scholar]
- 39.Haliwell B. Oxidative stress and cancer, have we moved forward? Biochem. J. 2007;401:1–10. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 40.o¨ ner M., Atli G., Canli M. Changes in serum parameters of freshwater fish Oreochromis niloticus following prolonged metal (Ag, Cd, Cr, Cu, Zn) exposures. Environ. Toxicol. Chem. 2008;2(360-):366. doi: 10.1897/07-281R.1. [DOI] [PubMed] [Google Scholar]
- 41.Wang B., Feng W.Y., Wang M., Wang T.C., Gu Y.Q., Zhu M.T., Ouyang H., Sho J.W., Zhang F., Zhao Y.L., Chai Z.F., Wang H.F., Wang J. Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. J. Nanoparticle Res. 2008;10:263–276. [Google Scholar]
- 42.Mekkawy I.A., Mahmoud U.M., Sayed A.H. Effects of 4-nonylphenol on blood cells of the African catfish Clarias gariepinus (Burchell, 1822) Tissue Cell. 2011;43:223–229. doi: 10.1016/j.tice.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Valerio-Garcíaa R.C., Carbajal-Hernándeza A.L., Martínez-Ruíza E.B., Jarquín-Díaza V.H., Haro-Pérezb C., Martínez-Jerónim F. Exposure to silver nanoparticles produces oxidative stress and affects macromolecular and metabolic biomarkers in the goodeid fish Chapalichthys pardalis. Sci. Total Environ. 2017;583:308–318. doi: 10.1016/j.scitotenv.2017.01.070. [DOI] [PubMed] [Google Scholar]
- 44.Shalaby A.M. Protective effect of ascorbic acid against mercury intoxication in Nile tilapia (Oreochromis niloticus) J. Egypt. Acad. Soc. Environ. Dev. 2001;2:79–97. [Google Scholar]
- 45.Alkaladi A., Nasr A.M., El-Deen N., Afifi M., Abu Zinadah O.A. Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles Saudi. J. Biol. Sci. 2015;22:556–563. doi: 10.1016/j.sjbs.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas J., Vijayakumar S., Thanigaivel S., Mukherjee A., Chandrasekaran N. Toxicity of magnesium oxide nano particles in two fresh water fishes tilapia (Oreochromis mossambicus) and zebra fish (Danio rerio) Int. J. Pharm. Pharm. Sci. 2014;6 [Google Scholar]
- 47.Monfared A.L., Bahrami A.M., Hosseini E., Soltani S., Shaddel M. Effects of Nano-particles on Histo-pathological changes of the fish. J. Environ. Health Sci. Eng. 2015;13:62. doi: 10.1186/s40201-015-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Hori T.S.F., Avilez I.M., Inoue L.K., Moraes G. Metabolical changes induced by chronic phenol exposure in matrinxã Brycon cephalus (teleostei: characidae) juveniles. Comp. Biochem. Physiol. 2006;143:67–72. doi: 10.1016/j.cbpc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Nel A.E., Ma¨ dler L., Velegol D., Xia T., Hoek E.M., Somasundaran P., Klaessig F., Castranova V., Thompson M. Understanding biophysicochemical interactions at the nanobio interface. Nat. Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 50.Kunjiappan S., Bhattacharjee C., Chowdhury R. Hepatoprotective and antioxidant effects of Azolla microphylla based gold nanoparticles against acetaminophen induced toxicity in a fresh water common carp fish (Cyprinus carpio L.) Nanomed. J. 2015;2:88–110. doi: 10.1007/s11626-014-9841-3. [DOI] [PubMed] [Google Scholar]
- 51.Monfared A.L., Soltani S. Effects of silver nanoparticles administration on the liver of rainbow trout (Oncorhynchus mykiss): histological and biochemical studies. Eur. J. Exp. Biol. 2013;3:285–289. [Google Scholar]
- 52.Imani M., Halimi M., Khara H. Effects of silver nanoparticles (AgNPs) on hematological parameters of rainbow trout, Oncorhynchus mykiss. Comp. Clin. Path. 2015;24:491–495. [Google Scholar]
- 53.Farkas J., Farkas P., Hyde D. Liver and gastroenterology tests. In: Lee M. 3rd, editor. Basic Skills in Interpreting Laboratory Data. 2004. [Google Scholar]
- 54.Jiraungkoorskul W., Upatham E.S., Kruatrachue M., Shaphong S., Vichasri-Grams S., Pokethitiyook P. Biochemical and histopathological effects of glyphosate herbicide on Nile tilapia (Oreochromis niloticus) Environ. Toxicol. 2003;18:260–267. doi: 10.1002/tox.10123. [DOI] [PubMed] [Google Scholar]
- 55.Kavitha C., Malarvizhi A., Senthil K.S., Ramesh M. Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla. Food Chem. Toxicol. 2010;48:2848–2854. doi: 10.1016/j.fct.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 56.Johari S.A., Kalbassi M.R., Yu I.J., Lee J.H. Chronic effect of waterborne silver nanoparticles on rainbow trout (Oncorhynchus mykiss): histopathology and bioaccumulation. Comp. Clin. Path. 2015;24:995–1007. [Google Scholar]
- 57.Zhao J., Wang Z., Liuc X., Xiea X., Zhang K., Xing B. Distribution of CuO nanoparticles in juvenile carp (Cyprinus carpio) and their potential toxicity. J. Hazard. Mater. 2011;197:304–310. doi: 10.1016/j.jhazmat.2011.09.094. [DOI] [PubMed] [Google Scholar]
- 58.Best J.H., Eddy F.B., Codd G.A. Effects of Microcystis cells, cell extracts and lipopolysaccharide on drinking and liver function in rainbow trout Oncorhynchus mykiss Walbaum. Aquat. Toxicol. 2003;64:419–426. doi: 10.1016/s0166-445x(03)00105-x. [DOI] [PubMed] [Google Scholar]
- 59.Handy R.D., Henry T.B., Scown T.M., Johnston B.D., Tyler C.R. Manufactured nanoparticles: their uptake and effects on fish: a mechanistic analysis. Ecotoxicology. 2008;17:396–409. doi: 10.1007/s10646-008-0205-1. [DOI] [PubMed] [Google Scholar]
- 60.Aghamirkarimi S.H., Mashinchian M.A., Sharifpour I., Jamili S.H.P.G.M. Sublethal effects of copper nanoparticles on the histology of gill, liver and kidney of the Caspian roach, Rutilus rutilus caspicus. Glob. J. Environ. Sci. Manage. 2017;3:323–332. [Google Scholar]
- 61.Brusle J., Anadon G.G.I. vol. 128. Science Publisher Inc.; New Hampshire: 1996. The structure and function of fish liver. (Fish Morphology, Horizon of New Research). [Google Scholar]
- 62.Jayaseelan C., Abdul Rahuman A., Ramkumar R., Perumal P., Rajakumar G., Kirthi A.V., kumar T.S., Marimuthu S. Effect of sub-acute exposure to nickel nanoparticles on oxidative stress and histopathological changes in Mozambique tilapia, Oreochromis mossambicus. Ecotoxicol. Environ. Saf. 2014;107:220–228. doi: 10.1016/j.ecoenv.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 63.Bhuvaneshwari R., Padmanaban K., BabuRajendran R. HistopathologicalAlterations in muscle, liver and gill tissues of Zebra fish Danio Rerio due to environmentally relevant concentrations of organochlo rine pesticides (OCPs) and heavy. Metals Int. J. Environ. Res. 2015;9:1365–1372. [Google Scholar]
- 64.Yazdanparast T., Sharifpour I., Soltani M., Esfahani H.K. Evaluation of silver retention in different organs of zebrafish (Danio Rerio) fed diet supplemented with silver nanoparticles. Int. J. Eng. Res. 2016;5:269–274. [Google Scholar]
- 65.Farkas J., Christian P., Tollefsen K.-E., Hylland K., Thomas K.V. Uptake and effects of manufactured nanoparticles on rainbow trout (Oncorhynchus mykiss) gill cells. Aquat. Toxicol. 2011;101(117):125. doi: 10.1016/j.aquatox.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Chatterjee N., Bhattacharjee B. Changing physicochemical properties of water due to exposure of zns nanoparticles and its detrimental effect on feeding behaviour and liver of a non-air breathing catfish mystus vittatus. Int. J. Latest Res. Sci. Technol. 2014;3:199–204. [Google Scholar]
- 67.Federici G., Shaw B.J., Handy R.D. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat. Toxicol. 2007;84:415–430. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 68.Mekkawy I.A.A., Mahmoud U.M., Wassif E.T., Mohammed R.H. Protective roles of tomato paste and vitamin E on atrazine- induced histological and histochemical changes of liver of Clarias Gariepinus (Burchell,1822) Glob. Adv. Res. J. Environ. Sci. Toxicol. 2013;2:011–021. [Google Scholar]
- 69.Mekkawy I.A.A., Mahmoud U.M., Wassif E.T., Naguib M. Protective roles of tomato paste and vitamin E on cadmium- induced histological and histochemical changes of liver of Oreochromis niloticus (Linnaeus,1758) J. Fish. Aquat. Sci. 2012;7:240–265. [Google Scholar]
- 70.Olojo E.A.A., Olurin K.B., Mbaka G., Oluwemimo A.D. Histopathology of the gill and liver tissues of the African catfish Clarias gariepinus exposed to lead. Afr. J. Biotechnol. 2005;4:117–122. [Google Scholar]
- 71.Wassif E.T., Kider B.M., Hussein S.Y., Mekkawy I.A., Hassan H.I. Effects of the herbicide Atrazine on the structure of some organs of the Nile fish Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish. 2000;4:197–234. [Google Scholar]
- 72.Yuness H.A.M. Assiut University; Egypt: 2005. Role of Melatonin in Reducing Hypoxia-Induced Oxidative Stress. M. Sc. Thesis. [Google Scholar]
- 73.De Vico G., Cataldi M., Carella F., Marino F., Passantino A. Histological, histochemical and morphometric changes of splenic melanomacrophage centers (SMMCs) in Sparicotyle-infected cultured sea breams (Sparus aurata) Immunopharmacol. Immunotoxicol. 2008;30:27–35. doi: 10.1080/08923970701812290. [DOI] [PubMed] [Google Scholar]
- 74.Kaewamatawong T., Rattanapinyopituk K., Ponpornpisit A., Pirarat N., Ruangwises S., Rungsipipat A. Short-term exposure of Nile Tilapia (Oreochromis niloticus) to mercury: histopathological changes, mercury bioaccumulation, and protective role of metallothioneins in different exposure routes. Toxicol. Pathol. 2013;41:470–479. doi: 10.1177/0192623312457269. [DOI] [PubMed] [Google Scholar]
- 75.Pronina S.V., Batueva M.-D., Pronin N.M. Characteristics of melanomacrophage centers in the liver and spleen of the roach Rutilus rutilus (Cypriniformes: cyprinidae) in Lake Kotokel during the Haff disease outbreak. J. Ichthyol. 2014;54:104–110. [Google Scholar]
- 76.Agius C. Preliminary studies on the ontogeny of the melano-macrophages of teleost haemopoietic tissues and age-relatedchanges. Dev. Comp. Immunol. 1981;5:597–606. doi: 10.1016/s0145-305x(81)80034-1. [DOI] [PubMed] [Google Scholar]
- 77.Kranz H., Gercken J. Effects of sublethal concentrations of potas-sium dichromate on the occurrence of splenic melano-macrophagecentres in juvenile plaice, Pleuronectes platessa, L. J. Fish Biol. 1987;31:75–80. [Google Scholar]
- 78.Sayed A.H., Hala A.M Y. Melanomacrophage centers in Clarias gariepinus as immunological biomarker for toxicity of silver nanoparticles. Journal of Microscopy and Ultrastructure. 2017;5(2):97–104. doi: 10.1016/j.jmau.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]