Abstract
Background
Despite the introduction of tobacco control measures, smoking remains highly prevalent in most EU countries. In Italy, one in four adults were still regular smokers in 2017. Increasing use of combustion-free delivering nicotine technologies for cigarette substitution may accelerate the current downward trends in smoking prevalence. Whether Heated Tobacco Products (HTPs) are more effective tobacco smoking substitutes that may potentially facilitate adoption and full conversion compared to e-cigarettes (ECs) is not known.
We have designed a prospective study to compare changes in cigarette consumption and adoption rates among smokers randomized to either HTPs or ECs. Product acceptability, tolerability, and their tobacco harm reduction potential will be also compared.
Methods
220 healthy smokers, not motivated to quit, will be randomized into a 12-weeks single-center, open label, non-inferiority trial comparing study outcomes from HTPs vs. ECs use. The primary outcome will be biochemically verified self-reported continuous abstinence at 12-weeks from the previous visit. Secondary outcomes will include: smoking reduction from baseline, adoption rates and product acceptability, tolerability, changes in step test values and in the level of selected biomarkers of exposure in exhaled breath (i.e. eCO) and in spot urine samples. A follow-up visit will be also included at 24-weeks to review product usage and smoking behavior under naturalistic condition of use.
Recruitment of participants started in May 2019 and enrolment is expected to be completed in November 2019.
Discussion
This will be the first study directly comparing Heated Tobacco Products with Electronic Cigarettes in term of reduction in cigarette consumption, adoption rates, product acceptability, tolerability, and tobacco harm reduction potential. This knowledge can contribute to a better understanding of the potential role of this new technology in the evolving nicotine consumer market.
Trial registration
ClinicalTrials.gov ID: NCT03569748.
Registered June 25, 2018.
https://register.clinicaltrials.gov/prs/app/action/LoginUser?ts=1&cx=-jg9qo4.
Keywords: Smoking, Effectiveness, Tolerability, Harm reduction, Acceptability, Electronic cigarettes, Just fog, Heated tobacco products, IQOS, Non-inferiority trial
1. Background
Cigarette smoke contains a mixture of over 7000 chemicals many of which harm the human body causing a broad range of diseases [1]. Smoking is the leading cause of preventable premature mortality in the world; total deaths attributable to tobacco smoking are projected to increase from approximately 5 million per year today to over 8 million annually by 2030 [2]. Death is mainly caused by ischemic heart disease, stroke, lung cancer and end-stage chronic obstructive pulmonary disease (COPD) and quitting smoking is known to reduce the risk of developing these diseases [[1], [2], [3]]. But while smoking cessation may be the most desirable final outcome from a health point of view [4], many smokers are unable or unwilling to quit. A realistic alternative is to encourage these smokers to switch to less harmful sources of nicotine and select the ones that give the greatest probability of eliminating or substantially reducing exposure to combustible toxicants from tobacco smoking [5].
Amongst the available nicotine-containing reduced risk products, the electronic cigarette (EC) has been rapidly gaining on conventional cigarettes [6]. The growing popularity of ECs appears to be associated with several factors including efficiency at reducing cigarette consumption whilst to continue having a ‘smoking’ experience, competitive prices, and the perception of a much less harmful smoking alternative [7,8] given that e-vapour is by far less problematic than cigarette smoke from a toxicological standpoint [9].
Although there is widespread demand for alternative less harmful nicotine-containing products, at present, there is low rate of conversion from trial to regular use of these products as shown in survey and clinical trials [10,11]. This is indication that - for many smokers-the current generation of ECs is not replicating a smoking experience that is of sufficient appeal to facilitate adoption and full conversion.
Consequently, better performing conventional cigarette substitutes are required to meet the demand of adult smokers for reduced risk products. Second-generation ECs (or personal vaporizers) seem to assent to a more fulfilling sensorial experience, because nicotine delivery to the bloodstream has improved compared to first-generation ECs [12,13], and is approaching that of conventional cigarettes.
More recently, products that electrically heat tobacco have been introduced as an alternative to combustible tobacco cigarettes. By heating tobacco, the temperature reached is lower than that reached in the burning cone of a conventional lit-end cigarette. These new products, known as Heated Tobacco Products (HTPs), may potentially provide a more gratifying smoking experience with reduced exposure to tobacco toxicants [14,15].
However, other studies have argued that there is a lack of evidence to show that alternative nicotine delivery products are totally safe for human health [16,17] and in their 2018 consensus study report on the public health consequences of e-cigarettes [18], the National Academies of Science, Engineering and Medicine concluded that the absolute risks of e-cigarettes cannot be unambiguously determined at this time and long-term health effects are not yet clear.
HTPs appear to replicate a smoking experience that is very close to that of conventional cigarettes thus expanding the range of tobacco smoking substitutes that may potentially facilitate adoption and full conversion. Whether HTPs may provide a more gratifying smoking experience compared to ECs is not known and a direct comparison between products has never been described. Also no comparative tolerability and harm reduction data is available for the two product categories. Therefore, we have designed a prospective study in which HTPs and ECs were compared in terms of efficacy, adoption rate and acceptability, tolerability, and tobacco harm reduction in healthy smokers, not motivated to quit.
2. Methods
2.1. Trial design
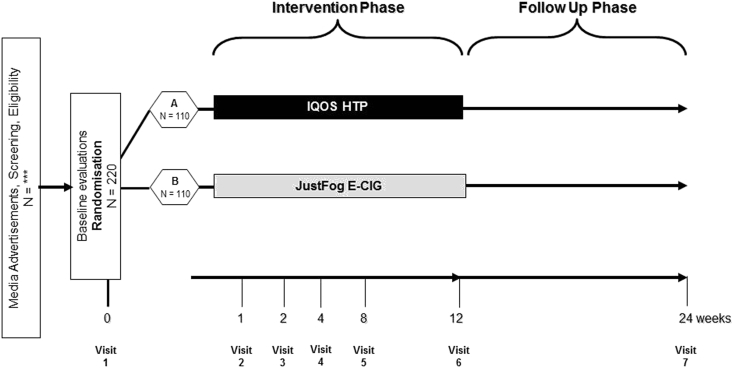
This is a 12-weeks, single-center, open label, non-inferiority trial comparing HTPs vs ECs in terms of efficacy, adoption rate and acceptability, tolerability, and tobacco harm reduction in 220 healthy smokers, not motivated to quit, randomized (1:1) to switch to one of these products. (Fig. 1). A final follow-up visit will be included at 24-weeks to review product usage and smoking behavior under naturalistic condition as well as product tolerability and long-term THR (Tobacco harm reduction) potential. The total duration of the study will be approximately 12 months. Enrolment period will last about 6 months with the support of a multi-channel strategy that includes advertisements on social networks, announcements in local news media, and distribution of flyers at the University campus.
Fig. 1.
Schematic diagram of the study design (to be inserted).
Before the baseline visit, a face to face screening (V0) for pre-eligibility checks will be conducted. Subjects will be asked to practice with the step test for 10–15 min and will be instructed on how to collect and store their morning urine before bringing it to the hospital on their Baseline visit (V1). This study will consist of a baseline visit (V1) and 6 follow-up study visits (V2-V7). At baseline (V1), participants will be randomized in two separate study groups. The randomization sequence will be computer generated by using blocks size of 4 and 6, with an allocation ratio of 1:1 for each of the study products (IQOS 2.4, Just Fog-EC).
According to the study protocol, the assignment to one of the two groups must be made on the basis of a simple list. The “dummy” randomization list will be generated by the Statistician through an SAS program (Statistical Analysis System Version 9.4). The randomization list will consist of 220 numbers of 3 digits in the form nnn (where “nnn” is a sequential number from 001 to 220). In derogation from the protocol (Study Design and Study Plan section) and in order to make it impossible to identify the subject's own arm to be randomized (before the number is assigned), the size of the blocks will not be 4 but will be variable in blocks of 4 and 6. The sequence of blocks will also be randomized and blind.
Subjects will use and familiarize with their allocated product, as per randomization. They will be trained and counseled on the use of their allocated study product; oral explanation and practical demonstration will be followed by product trial during which participants will also have the option to try and choose their preferred flavor (either from a selection of 3 e-liquids or 3 tobacco sticks, depending on the allocated arm). Participants randomized in the IQOS arm will receive one iQOS 2.4 kit and a full 1 week supply of tobacco sticks of their choice (they will receive a number of tobacco sticks/day corresponding to the number of cigarettes smoked at baseline); those randomized in the Just Fog-EC arm of the study will receive one Just Fog Starter Kit and a full 1 week supply of e-liquids of their choice (they will receive 4, 10 mls refill containers). Free products will be supplied at each subsequent visit throughout the study. No supply of tobacco stick or e-liquid will be given at week-12, but users will be offered to keep using their products to minimize the risk of relapsing back to cigarette smoking. A prospective evaluation of cigarette consumption, adoption rates, acceptability and tolerability will be recorded throughout the study (see below). A final follow-up visit will be also included at week-24 (V7) to review product usage and smoking behavior under naturalistic condition of use. Data will be recorded from every subject on an electronic CRF (Case Report Forms), provided by a CRO (Contract Research Organization), (Good Clinical Practice) GCP compliant, 21 CFR Part 11 FDA compliant, listed in AIFA list of operating CRO. Urine samples will be collected at baseline and wk 4, and sent to an external laboratory for analytical assessment of selected Biomarkers of Exposure (BoE).
The study will be conducted at University of Catania, Catania, Italy. The present protocol followed the SPIRIT guidelines and fulfilled the SPIRIT checklist (Additional file 1).
2.2. Participants
Healthy smokers, not motivated to quit, will be randomized in two intervention groups in a 1:1 ratio to compare HTPs vs ECs in terms of reductions in cigarette consumption, adoption rate and acceptability, tolerability, and tobacco harm reduction. Sample size determination (better detailed below, in the relevant section) for no-inferiority testing is based on the assumptions that 1) expected quit rates based on most recent EC literature is about 20–25% and 2) that differences in quit rates between products under investigation should not exceed 10–15% (as per non-inferiority definition). According to these hypotheses the required number of participants per study arm is 104. Hence we intend to include 220 participants, 110 per group.
Inclusion criteria: Subjects must meet all of the following inclusion criteria to be eligible for enrolment into the study:
-
1.
Able to comply with the study procedures
-
2.
Male or female healthy smokers aged ≥19
-
3.
Smoking at least 10 cigarettes a day
-
4.
Smoking for at least one year
-
5.
Not currently attempting to quit smoking or wishing to do so in the next 30 days; this will be verified at screening by the answer ‘‘NO’’ to the question ‘‘Do you intend to quit in the next 30 days?’’.
Exclusion criteria: The presence of any of the following will exclude a subject from study enrolment:
-
1.
Use of any other tobacco/nicotine products (except for own brands cigarettes) within the last 3 months.
-
2.
Use of nicotine replacement therapy or other smoking cessation therapies within the last 3 months.
-
3.
Self-reported pregnancy, planned pregnancy or breastfeeding.
2.3. Study Products tested
-
•
Heated Tobacco Product (HTP): We will use a HTP that does not involve combustion, generating a nicotine-containing aerosol. At the time of writing, IQOS 2.4 is the only HTP available on the Italian market (Fig. 2). IQOS 2.4 is composed by: 1) a tobacco stick – a novel patent-pending tobacco product with unique processed tobacco made from tobacco powder; 2) an electronic holder into which the tobacco stick is inserted and which heats the tobacco by means of an electronically controlled heating blade; 3) a charger that is used to recharge the holder after each use.
-
•
We will use the three types of tobacco sticks specifically designed for IQOS (named HEETS) (Fig. 3) that are currently for sale in the Italian market: Heets Amber, Heets Yellow, and Heets Turquoise. These products are compliant to the EU Tobacco Product Directive.
-
•
Electronic Cigarette (EC): JustFog Q16 Starter Kit and three types of e-liquid flavours, Puff Riserva Country 16 mg, Puff Riserva Toscana 16 mg, and Puff Artic 16 mg (2 tobacco-flavours and one menthol flavour), were chosen for the study (Fig. 4, Fig. 5).
-
•
Products were selected by an Italian expert panel of 3 e-liquid producers and 3 vaping product distributors, moderated by the editor of a popular e-cigarette blog. Experts convened in Verona (Italy) during a major vaping expo event. Through consensus, the expert panel provided specific recommendation on the device (JustFog Q16 Starter Kit is one of the most popular e-cigarette device in Italy due to its good performance and quality, affordable price, and user friendliness especially for beginners) and the choice of e-liquids that could best match the sensorial experience of the iQOS tobacco sticks to be used in a switching study and minimize the local irritant effects of excessive PG/VG (propylene glycol vegetable glycerin) ratio. these products are compliant to the eu tobacco product directive.
Fig. 2.
The IQOS devices (to be inserted).
Fig. 3.
The Heets of the study (to be inserted).
Fig. 4.

The JustFog devices (to be inserted).
Fig. 5.
The e-liquids of the study (to be inserted).
2.4. Study assessments
-
•
Cigarette Consumption/Smoking abstinence: This will be assessed by self-reported abstinence from tobacco smoking - not even a puff -validated by an eCO measurement ≤10 ppm since the previous study visit [11]. This will be calculated at each study visit, including at week 24. Primary endpoint is, however, fixed at week 12.
-
•
Cigarette Consumption/Smoking reduction: This will be assessed by self-reported reduction in the number of tobacco cigarettes smoked per day from baseline [11]. This will be calculated at each study visit, including at week 24.
-
•
Adoption rates and adherence to product use assessments: Adoption rates and adherence to product use will be regularly (from V2 to V6) assessed by self-reported recording in the study diary and verified by product use checks (used and unused tobacco sticks and liquid refill containers will be collected and counted). The average consumption will be calculated on a per daily basis. Final data source will be the study diary, but in order to avoid underestimation of product usage due to forgetfulness in returning products, we will evaluate also subjects' reliability comparing the data reported in the diary and the returned product counting.
-
•
Acceptability assessment: Product satisfaction, effects on craving, product preferences, risk perception, subjective and sensorial effects to compare the acceptability of HTP vs EC, will be evaluated at wk 4, wk 8, wk 12 (+follow-up at wk 24), by using: a) Modified Cigarette Evaluation Questionnaire (mCEQ) adapted for EC and HTP users; b) three of 8 items of “Smoking Cue Appeal” adapted for EC and HTP users; c) “Intent to use questionnaire” (ITUQ) – items 4–6; d) PRI-P CC Perceived Health Risk scale (for classic cigarette); e) PRI-P RRP Perceived Health Risk scale (for reduced risk products); f) percentage of choice of a particular subtype of tobacco stick or e-liquid.
-
•
Safety and tolerability assessment: Adverse and serious adverse events (AE and SAE), symptoms thought to be related to tobacco smoking, EC or HTP use, and to withdrawal from nicotine will be recorded at baseline and at each subsequent study visit in the adverse event page of the eCRF. Signs or symptoms will be elicited at each visit by open questioning, such as “How have you been feeling since your last visit?”. Participants will also be encouraged to spontaneously report AEs occurring at any other time during the study. The investigator must pursue and obtain information adequate both to determine the outcome of the AE and to assess whether it meets the criteria for classification as a SAE requiring immediate notification to the competent authority. Sufficient information should be obtained to assess causality. Follow-up of the AE, after the date of study discontinuation, is required if the AE or its sequelae persist. Vital signs (BP, HR) will be recorded at each study visit. Body weight and height will be measured at baseline and wk 12 (+follow-up at wk 24).
-
•
Tobacco harm reduction assessment: Tobacco harm reduction potential was investigated by evaluating: a) changes in step test values measured at baseline, wk 4, wk 12 (+follow-up at wk 24); b) changes in quality of life scores (EQ-VAS questionnaire) measured at baseline, wk 4, wk 12 (+follow-up at wk 24); and c) levels of selected Biomarkers of Exposure (BoE) in exhaled breath (i.e. eCO) measured at each study visit (+follow-up at wk 24) and in spot urine samples of study participants measured at baseline (V1) and wk 4 (V4). Specifically, in the urine we will measure: total NNAL (for nicotine-derived nitrosamine ketone - NNK); 2-hydroxyethylmercapturic acid (HEMA) (for ethylene oxide); 2-hydroxy-3-butenyl-mercapturic acid and isomers (MHBMA) (for 1,3-butadiene); 3-hydroxy-1-methyl propyl-mercapturic acid (HMPMA) (for crotonaldehyde); 3-hydroxypropylmercapturic acid (3-HPMA) (for acrolein); S-phenylmercapturic acid (S-PMA) (for benzene); N-acetyl-S-(2-carbamoylethyl)-l-cysteine (AAMA) and N-(R,S)-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-l-cysteine (GAMA) (respectively the mercapturic acid of acrylamide itself and the mercapturic acid of glycidamide, which is the reactive epoxide metabolite from acrylamide); 2-cyanoethylmercapturic acid (CEMA) (for acrylonitrile); 2-hydroxypropylmercapturic acid (2-HPMA) (for propylene oxide); naphthalene; fluorin; phenanthrene; pyrene (1-OHP); cotinine; creatinine.
-
•
Product failure reporting: Type and frequency of products' malfunctioning/failures will be recorded in the eCRF at each study visits from wk 1 to wk 12 (+follow-up at wk 24 if appropriate).
2.5. Study Schedule
See Table 1, Study Schedule.
Table 1.
Study schedule.
| Visit 0 (screening) V0 |
Visit 1 (baseline) V1 |
Visit 2 V2 |
Visit 3 V3 |
Visit 4 V4 |
Visit 5 V5 |
Visit 6 V6 |
Visit 7 Follow-up V7 |
|
|---|---|---|---|---|---|---|---|---|
| Assessments | ≤7 days after V0 | 1 week after V1 ± 3 days | 2 weeks after V1 ±3 days |
4 weeks after V1 ±3 days |
8 weeks after V1 ±7 days |
12 weeks after V1 ±7 days |
24 weeks after V1 ±7 days |
|
| Medical Hx | X | |||||||
| Tobacco/vaping products Hx | X | |||||||
| Eligibility criteria checks | X | |||||||
| Step Test Training (10–15 min) | X | |||||||
| Instructions for urine collection and storage; provision of containers | X | X | ||||||
| Patient Information Sheet | X | |||||||
| Informed consent | X | |||||||
| Randomization | X | |||||||
| Device Familiarization (30–60 min) | X | |||||||
| Dispense Device (plus 1 wk supply of tobacco sticks for iQOS or liquid for JustFog) | X | |||||||
| Dispense 1 wk supply of tobacco sticks for iQOS or liquid for JustFog | X | |||||||
| Dispense 2 wk supply of tobacco sticks for iQOS or liquid for JustFog | X | |||||||
| Dispense 4 wk supply of tobacco sticks for iQOS or liquid for JustFog | X | X | ||||||
| Cigarette/day | X | X | X | X | X | X | X | X |
| eCO | X | X | X | X | X | X | X | X |
| Vital Signs (BP, HR) | X | X | X | X | X | X | X | |
| Weight and height | X | X | X | |||||
| Urine sample processing | X | X | ||||||
| Product use recording | X | X | X | X | X | X | ||
| Product use checks (collect used and unused tobacco sticks and liquid refill containers) | X | X | X | X | X | Xa | ||
| mCEQ | X | X | X | Xa | ||||
| Smoking Cue Appeal | X | X | X | X | Xa | |||
| ITUQ | X | X | X | Xa | ||||
| Risk perception questionnaires (PRI-P CC; PRI-P RRP) | X | X | X | X | Xa | |||
| EQ-VAS | X | X | X | X | ||||
| Step test | X | X | X | X | ||||
| Safety reporting/AEs | X | X | X | X | X | X | Xa | |
| Product failure reporting | X | X | X | X | X | Xa |
if appropriate (i.e. only if participants were still using the same products of the study).
Screening (V0): Screening visit for eligibility criteria. Medical hx, past and current tobacco/vaping product use description, and eCO levels will be recorded. Participants will be instructed on how to collect and store their first morning urine (containers for urine collection will be handed over at V0) before bringing it to the hospital on the day established for Baseline (at V1). Participants will be asked to practice with the step test for 10–15 min.
Baseline (V1): Baseline visit will be programmed within 1 week from V0. After confirming eligibility criteria, participants will sign the Informed Consent. All baseline measurements will be then carried out (cigarette/day, eCO, AEs, BP, HR, weight and height, step test, and questionnaires). Spot urine sample will be collected, aliquotted and stored. After randomization to either IQOS or vaping product, participants will be asked to familiarize with the device and to try out the selection of study consumables/flavours for 30–60 min before choosing the one they like most. Full 1 week supply of the chosen product will be provided.
Visit 2 (V2): This will be scheduled 1 week after V1 (with a tolerance of ± 3 days). Cigarette/day, eCO, AEs, BP, HR will be measured and product use recorded. Full 1 week supply of the chosen product will be provided.
Visit 3 (V3): This will be scheduled 2 weeks after V1 (with a tolerance of ± 3 days). Cigarette/day, eCO, AEs, BP, HR will be measured and product use recorded. Participants will be instructed on how to collect and store their second urine sample before bringing it to the hospital at V4. Full 2 weeks supply of the chosen product will be provided.
Visit 4 (V4): This will be scheduled 4 weeks after V1 (with a tolerance of ± 3 days). Cigarette/day, eCO, AEs, BP, HR will be measured, questionnaires completed and product use recorded. Step test will be carried out. Second urine sample will be collected, aliquotted and stored. Full 4 weeks supply of the chosen product will be provided.
Visit 5 (V5): This will be scheduled 8 weeks after V1 (with a tolerance of ± 7 days). Cigarette/day, eCO, AEs, BP, HR will be measured, questionnaires completed and product use recorded. Full 4 weeks supply of the chosen product will be provided.
Visit 6 (V6): This will be scheduled 12 weeks after V1 (with a tolerance of ± 7 days). Cigarette/day, eCO, AEs, BP, HR, weight, height will be measured, questionnaires completed and product use recorded. Step test will be carried out. No more products will be dispensed.
Follow-up Visit (V7): This final visit will be scheduled 24 weeks after V1 (with a tolerance of ± 7 days) to review product usage and smoking behavior under naturalistic condition of use. Cigarette/day, eCO, AEs, BP, HR, weight, height will be measured, questionnaires completed and product use recorded. Step test will be carried out.
2.6. Discontinuation criteria
The reason for a subject discontinuing from the study will be recorded in the case report form. A discontinuation occurs when an enrolled subject ceases participation in the study, regardless of the circumstances, prior to completion of the protocol. The investigator must determine the primary reason for discontinuation. Withdrawal due to adverse event should be distinguished from withdrawal due to insufficient response. A discontinuation must be reported immediately to the clinical monitor or his/her designated representative if it is due to a serious adverse event. The final evaluation required by the protocol will be performed at the time of study discontinuation. The investigator will record the reason for study discontinuation, provide or arrange for appropriate follow-up (if required) for such subjects, and document the course of the subject's condition. Subjects who discontinue product under study may continue participation in the study. Subjects will be required to maintain the visit schedule and may be eligible to continue participation in the non-treatment phase of the study.
2.7. Study outcome measures
2.7.1. Primary outcome measure
Biochemically verified (validated by an exhaled breath Carbon monoxide – eCO - measurement ≤10 ppm) self-reported continuous smoking abstinence at 12-weeks from the previous visit will be used to compare effectiveness of IQOS vs. JustFog-EC.
2.7.2. Secondary outcome measures
-
•
Self-reported continuous reduction in cigarette consumption from baseline at 12-weeks will be used to compare smoking reduction of IQOS vs. JustFog-EC. A reduction in the number of conventional cigarette/day of at least 50% from baseline will be considered of importance. A ≥50% reduction in the number of cig/day since baseline, defined as self-reported reduction in the number of cig/day compared to baseline will be calculated at each study visit. eCO levels will be measured to verify smoking status and confirm a reduction compared to baseline.
-
•
Self-reported regular HTP or EC use at 12-weeks from the previous visit will be used to compare the level of adoption rate of IQOS vs. JustFog-EC. This will be also verified by product use checks (recorded number of used and unused tobacco sticks and liquid refill containers). The average number of stick/day and ml e-liquid/day compared to baseline will be calculated at each study visit.
-
•
Evaluation of product satisfaction, risk perception, effects on craving, product preferences, subjective and sensorial effects by: a) Modified Cigarette Evaluation Questionnaire (mCEQ) adapted for EC and HTP users; b) Three of 8 items of “Smoking Cue Appeal” adapted for EC and HTP users; c) Intent to use questionnaire (ITUQ) – items 4–6; d) PRI-P CC Perceived Health Risk scale (for classic cigarette); and e) PRI-P RRP Perceived Health Risk scale (for reduced risk products) will be used to compare acceptability of IQOS vs. JustFog-EC. Percentage distribution of chosen tobacco stick or e-liquid type/flavour will be tabulated.
-
•
Absolute number of AEs/SAEs as well as changes in BP and HR measurements will be considered to compare tolerability of IQOS vs. JustFog-EC.
-
•
Tobacco harm reduction potential between IQOS and JustFog-EC will be evaluated by comparing:
-
a)
changes in step test values;
-
b)
changes in quality of life scores (EQ-VAS questionnaire);
-
c)
levels of selected Biomarkers of Exposure (BoE) in exhaled breath (i.e. eCO) and in spot urine samples of study participants. Specifically, in the urine we will measure:
-
1.
total NNAL (for nicotine-derived nitrosamine ketone - NNK);
-
2.
2-hydroxyethylmercapturic acid (HEMA) (for ethylene oxide);
-
3.
2-hydroxy-3-butenyl-mercapturic acid and isomers (MHBMA) (for 1,3-butadiene);
-
4.
3-hydroxy-1-methyl propyl-mercapturic acid (HMPMA) (for crotonaldehyde);
-
5.
3-hydroxypropylmercapturic acid (3-HPMA) (for acrolein);
-
6.
S-phenylmercapturic acid (S-PMA) (for benzene);
-
7.
N-acetyl-S-(2-carbamoylethyl)-l-cysteine (AAMA) and N-(R, S)-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-l-cysteine (GAMA) (respectively the mercapturic acid of acrylamide itself and the mercapturic acid of glycidamide, which is the reactive epoxide metabolite from acrylamide);
-
8.
2-cyanoethylmercapturic acid (CEMA) (for acrylonitrile);
-
9.
2-hydroxypropylmercapturic acid (2-HPMA) (for propylene oxide);
-
10.
naphthalene;
-
11.
fluorin;
-
12.
phenanthrene;
-
13.
pyrene (1-OHP);
-
14.
cotinine;
-
15.
creatinine.
-
•
Incidence in malfunctioning events/failures will be considered to compare reliability of IQOS vs. JustFog-EC.
2.8. Statistical methods
Sample size calculation: We assume that: a) current e-vapour products provide a 25% quitting rate in clinical trials [19,20]; and b) non-inferiority in quit rates means that the proportion of subjects successfully quitting smoking using the test product is not worse than the proportion of subjects quitting smoking using the alternative or reference product, this difference is allowed a tolerance of 15% [21,22].
For sample size definition, we used the Farrington-Manning Score Test for Proportion Difference, assuming:
-
•
asymptotic normal distribution, upper one-tailed
-
•
alpha 0.05
-
•
nominal power 0.80
Thus:
-
•
Null Proportion Diff. −0.15
-
•
Ref Proportion 0.25
-
•
N per group: 104
Consequently, a total of 220 smokers not intending to quit (110 per each arm) will be recruited.
Primary study endpoint: Quit rate at 3 months in the experimental (study) group – with respect to reference group – by means a non-inferiority threshold of 15%. An alpha level of 0.05 will be used. Quit rates will be evaluated on an intention-to-treat basis (i.e. subjects known to have failed will be considered as treatment failures in the primary analysis along with subjects lost to follow-up).
Secondary study endpoints: Different urinary metabolites will be evaluated at week 4 by means of a descriptive analysis using 95% confidence intervals, adjusted for confounding variables (e.g., sex, urinary creatinine, daily use of HTP/EC) and for the baseline log-transformed level of the biomarker.
Safety assessments: The safety assessment will be conducted at each study visit. Descriptive statistics will summarize the number and percentage of subjects experiencing adverse events by study group. Continuous data will be expressed as mean (±SD) and median (and interquartile range [IQR]). Within-group (from baseline) and between-group differences will be evaluated by means of statistical tests, for paired and unpaired continuous variables, as appropriate. Significance of differences in frequency distribution of categorical variables will be tested by χ2 test, at an alpha level of 0.05.
2.9. Data Management Plan
For this study, a web-based Electronic Case Report Form (eCRF), provided by a Clinical Research Organization (CRO) with expertise in tobacco research studies, will be used for collecting the data. All the Data Management activities will be carried forward according to the CRO SOPs. Before the study start, the Investigator will be trained on the use of the eCRF functions. He/She will be provided with specific access rights, which allow him/her to enter the system, and a dedicated user's manual. Each individual data will be recorded by the Investigator or a designee into the eCRF Database. Data cleaning will be performed by CRO Data Management team, whereas validation of the inconsistencies (change or acceptance) will be made by the Investigator. All the activities related to the data managing will be described in the Data Management Plan (DMP) that will be prepared and maintained for the whole study duration by the Data Management of the CRO. The DMP will also include all the edit check implemented into the eCRF or to be run in batch on the Database. Before the data freezing, the CRO will code the medical terms. All the activities related to the Database freezing will be described in the DMP. After having frozen the Database, the CRO Data Manager will send it to the Biostatistics Unit for the Statistical Analyses. This activity will also be described in the DMP.
2.10. Results
Recruitment of participants started in May 2019 and enrolment is expected to be completed in December 2019. Results will be reported in 2020.
3. Discussion
Tobacco control measures have been implemented in Italy for the past 35 years. Inparticular, a total ban on smoking in public places came into effect from January 2005. A year later, Italy ratified the Framework Convention of the World Health Organization to control and fight tobacco smoking [23]. Despite these policy measures, one in four Italian adults (≥18 years) were still regular smokers in 2017, including 28.6% of Sicilians (who comprise 8.5% of the Italian population) [24]. Within this environment, usage of combustion-free delivering nicotine technologies for cigarette substitution in Italy is increasing. In 2014, more than 600.000 Italians regularly used vaping products [25], whereas approximately 1.4 million people used them regularly (with a market share in the whole tobacco market of 2.3%) in 2016 [26]. Of note, dual usage in the same year continues to be common with 77.6% ECs users smoking conventional cigarettes as well. Sales data of iQOS (the only currently marketed HTP in Italy) indicates that its market share in the whole tobacco market increased from 0.01% in 2015 to 0.67% in 2017 and is now approaching the market share of cigars [27].
It is not known whether HTPs provide a more gratifying smoking experience compared to ECs and a direct comparison between products in term of successful cigarette substitution has never been described. Also, no comparative tolerability and harm reduction data is available for these two products. Here we propose a prospective study to compare the level of reduction in cigarette consumption and adoption rates among smokers randomized to either HTPs or ECs. Product acceptability, tolerability, and their tobacco harm reduction potential will be also compared. However, the research condition that provides the use of three flavors of sticks and three single flavors of e-liquids and a single model of electronic cigarette and a single cigarette model of heated tobacco product represents a limitation to external validity.
Non-inferiority trials are executed when a new product it is anticipated to be at least as effective as the existing one. It could be considered an acceptable alternative if, for example, it had fewer side effects than the existing product, was improving the overall consumer experience or, if it was cheaper to buy [28]. The alternative to a non-inferiority study would be superiority, which are used to conclude that one product is better than the other. At this stage there is not any available information pointing that this could be the case and/or what product would be better than the other. Additionally, superiority testing with differences of 15%–20% between products could require thousands of volunteers, as the superiority testing is more stringent than non-inferiority. When planning this study, it could not be anticipated whether IQOS would be superior to the vaping product to successfully help cigarette substitution. Therefore, we adopted a non-inferiority trial study design. The aim was to establish whether IQOS was “as good as” - that is, not inferior to - the vaping product in term of effectiveness (i.e. quit rates). This required a pre-specified non-inferiority margin (15% between products), which was determined on the basis of quit rate data at 12-weeks follow-up from our previous clinical trials with ECs. Specifically, we considered that current vaping products provide a 20–25% quit rate [19,20] and that to prove non-inferiority, the mean difference in quit rate at 12-weeks follow-up between the 2 products should not exceed 15% [21,22].
The protocol incorporates a number of innovative approaches that contribute to the specific uniqueness and quality of the study.
In order to improve trial attendance and retention, the study protocol has adopted a flexible approach, with each participant being offered to choose from three different product flavours to best match his/her sensorial experience. An expert panel provided specific recommendation on the device and the choice of e-liquids that could best match the sensorial experience of the iQOS tobacco sticks to be used in a switching study and minimize the local irritant effects of excessive PG/VG ratio. However, we were limited in the range of nicotine concentrations we were able to offer in the study.
Trial attendance and retention is further encouraged by asking participants to bring used and unused tobacco sticks and liquid refill containers back to the clinic. At that point, investigators will be handing over enough free supply of tobacco sticks or liquid refill containers to cater for participants’ needs up until next study visit. This is also likely to stimulate adherence to product use. Of note, non-compliance to study products is in itself an interesting outcome and we will be able to assess the impact of different level of non-compliance on several biomarkers of exposure.
The study included an extension period with a final follow-up visit at 24-weeks to review product usage and smoking behavior under naturalistic condition as well as their long-term tolerability and THR potential.
Because of their distinct subjective and sensorial experience, the impact of HTPs may be substantially different from that of ECs in term of successful cigarette smoking substitution. Collecting data comparing the impact of HTPs and ECs on changes in cigarette consumption can contribute to a better understanding of the potential role of this new technology in the evolving nicotine consumer market.
Trial status
Participants recruitment started in May 2019.
Ethical approval and consent to participate
The study was reviewed by the Research Ethics Board of the Azienda Ospedaliero Universitaria “Policlinico-V. Emanuele”, Università di Catania, Catania, Italy (approval reference number: 215/2017/PO). The trial is registered with ClinicalTrials.gov (ID: NCT03569748). Informed consent will be collected in writing at the baseline session. Participants will have already received the information sheet and consent form after having had the chance to discuss any aspect of the study with the investigators. No sensitive data will be transferred electronically; it will also be anonymised beforehand (i.e. numerical codes will be used rather than names).
Consent for publication
Not applicable.
Availability of supporting data
The datasets used and/or analysed during the current study will be available from the corresponding author on reasonable request.
Funding
This research is supported by an Investigator-Initiated Study award by Philip Morris Products SA (PMI.IIS.2016.006). The study protocol was written by EM who was also the principal investigator of the study. Philip Morris Products SA had no role in the design of the study protocol and will not have any role during its execution, analysis, data interpretation or writing of the manuscript.
Authors’ contributions
PC, MC, MM, EM, RP and DS were responsible for designing the study protocol. PC, BB, RE, AP, UP, FB, CS, MG and EM drafted the manuscript. All authors critically revised and approved the final manuscript.
Declaration of competing interest
EM, DS and RP are full-time employee of the University of Catania, Italy. PC, MC and RE are fixed-term researcher at University of Catania, Italy. MM is fixed-term researcher at Centro per la Prevenzione e Cura del Tabagismo, University of Catania. BB is full-time employee of ARNAS Garibaldi, Catania, Italy. AP is full-time employee of Casa di Cura Musumeci-Gecas, Gravina di Catania, Italy. UP is full-time employee of Ospedale “San Vincenzo” - Taormina, Italy.
In relation to his work in the area of tobacco control, RP has received lecture fees and research funding from Pfizer and GlaxoSmithKline, manufacturers of stop smoking medications. He has also received support from The Consumer Advocates for Smoke-free Alternatives (CASAA) for publication and open access costs of one paper. He has also served as a consultant for Pfizer, Global Health Alliance for treatment of tobacco dependence, ECITA (Electronic Cigarette Industry Trade Association, in the UK), Arbi Group Srl., and Health Diplomats (consulting company that delivers solutions to global health problems with special emphasis on harm minimization). Lectures fees from a number of European electronic cigarette industry and trade associations (including FIVAPE in France and FIESEL in Italy) were directly donated to vapers advocacy no-profit organizations. He is also currently involved in the following pro bono activities: scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti Smoking League) and for The Consumer Advocates for Smoke-free Alternatives (CASAA); Chair of the European Technical Committee for standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4).
The other authors have no conflict of interests to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2020.100518.
ABBREVIATIONS
- AAMA
acrylamide mercapturic acid or acrylamide
- BL
baseline
- BoBE
Biomarkers of Biological Effects
- BoE
Biomarkers of Exposure
- BP
blood pressure
- CEMA
2-cyanoethylmercapturic acid or acrylonitrile
- COPD
Chronic Obstructive Pulmonary Disease
- eCO
Exhaled breath Carbon monoxide;
- ECs
electronic cigarettes
- EQ-Vas
Health Status-Visual analogue scale
- HEMA
hydroxyethylmercapturic acid
- HR
heart rate
- HMPMA
3-hydroxy-1-methyl propyl-mercapturic acid or crotonaldehyde
- 2-HPMA
2-hydroxypropylmercapturic acid or propylene oxide
- 3-HPMA
3-hydroxypropylmercapturic acid or acrolein
- HnB
Heat-not-Burn
- IQOS
I Quit Ordinary Smoking
- MA
mercapturic acid
- mCEQ
Modified Cigarette Evaluation Questionnaire
- MHBMA
2-hydroxy-3-butenyl-mercapturic acid or 1,3-butadiene
- NNK
tobacco-specific nitrosamine
- PRI-P CC
Perceived Health Risk scale (classic cigarette)
- 1-OHP
pyrene
- PRI-P RRP
Perceived Health Risk scale (reduced risk products)
- S-PMA
S-phenylmercapturic acid or benzene
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.US Department of Health and Human Services . US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. The Health Consequences of Smoking: 50 Years of Progress: a Report of the Surgeon General. [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva: 2008. WHO Report on the Global Tobacco Epidemic, 2008 – the MPOWER Package. [Google Scholar]
- 3.Doll R., Peto R., Boreham J., Sutherland I. Mortality in relation to smoking: 50 year observations on male British doctors. BMJ. 2004;328:1519–1528. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polosa R., Benowitz N.L. Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol. Sci. 2011;32(5):281–289. doi: 10.1016/j.tips.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polosa R., Rodu B., Caponnetto P., Maglia M., Raciti C. A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm Reduct. J. 2013;10 doi: 10.1186/1477-7517-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adelman D.J., Grainger M., Ayala V., Paxton K. Morgan Stanley Research North America; New York: March 7, 2013. Tobacco: new years' resolutions + E-Cigs = Weaker volumes? [Google Scholar]
- 7.Farsalinos K.E., Romagna G., Tsiapras D., Kyrzopoulos S., Voudris V. Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int. J. Environ. Res. Public Health. 2014;11(4):4356–4373. doi: 10.3390/ijerph110404356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EtterJF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 9.FarsalinosKE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarettes substitutes: a systematic review. Ther. Adv. Drug Saf. 2014;5:67–86. doi: 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dockrell M., Morrison R., Bauld L., McNeill A. E-cigarettes: prevalence and attitudes in Great Britain. Nicotine Tob. Res. 2013 Oct;15(10):1737–1744. doi: 10.1093/ntr/ntt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caponnetto P., Campagna D., Cibella F., Morjiaria J.B., Caruso M., Russo C., Polosa R. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawkins L., Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacol. (Berl) 2014;231(2):401–407. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- 13.FarsalinosKE, Spyrou A., Tsimopoulou K., Stefopoulos C., Romagna G., Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci. Rep. 2014;4:4133. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frost-Pineda K., Zedler B.K., Oliveri D., Liang Q., Feng S., Roethig H.J. 12-Week clinical exposure evaluation of a third-generation electrically heated cigarette smoking system (EHCSS) in adult smokers. Regul. Toxicol. Pharmacol. 2008;52:111–117. doi: 10.1016/j.yrtph.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Roethig H.J., Koval T., Muhammad-Kah R., Jin Y., Mendes P., Unverdorben M. Short term effects of reduced exposure to cigarette smoke on white blood cells, platelets and red blood cells in adult cigarette smokers. Regul. Toxicol. Pharmacol. 2010;57:333–337. doi: 10.1016/j.yrtph.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Pisinger C., ERS Tobacco Control Committee . European Respiratory Society; 2019. ERS Position Paper on Tobacco Harm Reduction.https://www.ersnet.org/the-society/news/ers-position-paper-on-heated-tobacco-products [Google Scholar]
- 17.World Health Organization . WHO; 2019. WHO | Heated Tobacco Products (HTPs) Information Sheet.http://www.who.int/tobacco/publications/prod_regulation/heated-tobacco-products/en/ [Google Scholar]
- 18.National Academies of Sciences . National Academies Press; Washington, D.C.: 2018. Engineering, and Medicine. Public Health Consequences of E-Cigarettes. [PubMed] [Google Scholar]
- 19.Polosa R., Caponnetto P., Maglia M., Morjaria J.B., Russo C. Success rates with nicotine personal vaporizers: a prospective a prospective 6-month pilot study of smokers not intending to quit. BMC Public Health. 2014;14:1159. doi: 10.1186/1471-2458-14-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polosa R., Caponnetto P., Morjaria J.B., Papale G., Campagna D., Russo C. Effect of an electronic nicotine delivery device (E-cigarette) on smoking cessation and reduction: a prospective pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tulloch H.E., Pipe A.L., Els C., Clyde M.J., Reid R.D. Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Med. 2016 Jun 7;14:80. doi: 10.1186/s12916-016-0626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker T.B., Piper M.E., Stein J.H., Smith S.S., Bolt D.M., Fraser D.L. Effects of Nicotine Patch vs Varenicline vs Combination Nicotine Replacement Therapy on Smoking Cessation at 26 Weeks: a randomized clinical trial. J. Am. Med. Assoc. 2016;315(4):371–379. doi: 10.1001/jama.2015.19284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO . World Health Organization; Geneva, Switzerland: 2013. Report on the Global Tobacco Epidemic.http://www.who.int/tobacco/global_report/2013/en [Google Scholar]
- 24.http://www.epicentro.iss.it/passi/dati/fumo.asp
- 25.Gallus S., Lugo A., Pacifici R., Pichini S., Colombo P., Garattini S., La Vecchia C. E-cigarette awareness, use, and harm perceptions in Italy: a national representative survey. Nicotine Tob. Res. 2014 Dec;16(12):1541–1548. doi: 10.1093/ntr/ntu124. [DOI] [PubMed] [Google Scholar]
- 26.http://www.epicentro.iss.it/temi/fumo/EpidItalia.asp
- 27.Liu X., Lugo A., Spizzichino L., Tabuchi T., Gorini G., Gallus S. Heat-not-burn tobacco products are getting hot in Italy. J. Epidemiol. 2018 May 5;28(5):274–275. doi: 10.2188/jea.JE20180040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedgwick P. What is a non-inferiority trial? BMJ. 2013;347:f6853. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study will be available from the corresponding author on reasonable request.