ABSTRACT
Background
Levodopa and dopamine agonists (dopamine replacement therapy [DRT]) are implicated in Parkinson's disease psychosis (PDP), but the relationship between DRT and neurotransmitter dysfunction inherent to PD remains unclear.
Objectives
To examine the relationship between baseline striatal dopamine transporter (DAT) binding in drug‐naïve idiopathic PD, introduction of DRT, or dose change and incident early‐onset PDP.
Methods
Baseline DAT binding was compared between patients with and without incident psychosis (defined here as hallucinations or delusions), controlling for age, sex, baseline cognition, and prospective DRT in the Parkinson's Progression Markers Initiative cohort. Incident illusions were not considered psychosis symptoms.
Results
Of 386 patients, 30 (8%) developed PDP (predominantly hallucinations, mean onset 42 months) and 355 (92%) had either no PDP symptoms (mean follow‐up 64 months) or reported illusions only (111/355, 31%). Incident PDP was associated with reduced baseline striatal DAT binding, controlling for confounders (F 1,377 = 10.9; P = 0.001), but not with a specific DRT regime. A total of 6 patients developed PDP when DRT free. There was no suggestion that PDP onset was coincident with starting levodopa or levodopa dose increase. Incident illusions were not associated with reduced DAT binding.
Conclusion
The findings highlight the role of disease‐related dopamine mechanisms in the pathophysiology of hallucinations in Parkinson's disease alongside medication. It remains to be determined how dopamine mechanisms, medication, and other neurotransmitter systems implicated in PDP interact.
Keywords: Hallucination, illusion, DAT scan, PPMI
Visual hallucinations, illusions and delusions occur at some point in most patients with Parkinson's disease (PD)1—a spectrum referred to collectively as PD psychosis (PDP).2 PDP reduces the quality of life3 while increasing carer distress4 and the risk of care home placement.5 Treatment has proved challenging as the underlying cause remains unclear. Although recent research has focussed on cholinergic6 and serotonergic7 mechanisms, clinical experience suggests that PDP is associated with the start or dose change of levodopa or dopamine agonist medication (dopamine replacement therapy [DRT]), with the first line of treatment a reduction in medication. Studies of dopamine transporter (DAT) striatal binding have found that patients susceptible to PDP have lower binding when compared with those who are not,8, 9, 10 implicating dopamine in the PDP mechanism. However, the studies have not addressed the possibility that a striatal dopamine deficit may lead to differences in subsequent DRT prescribing, leaving open the possibility that the DRT regime causes PDP, not the dopamine deficit itself. Alternatively, the greater dopamine deficit may mark patients with a receptor upregulation and dopamine hypersensitivity, with PDP triggered by first DRT exposure or a specific DRT dose threshold.
Here we set out to address these issues by examining DAT striatal binding and PDP using the fine‐grain clinical detail of the Parkinson's Progression Markers initiative (PPMI).11 Excluding patients with idiopathic PD who had already developed PDP at the time of PPMI entry, we compared DAT striatal binding in patients who went on to develop PDP with those who did not, taking into account their prospective DRT prescribing history and examining drug regime and dose changes with respect to PDP onset at a level of detail not possible in previous studies. As patients are recruited to PPMI when drug naïve, we avoided the possible influence of DRT on striatal DAT binding by focusing our analysis on the baseline study entry scans.
Methods
Data from the PPMI database (http://www.ppmi-info.org) to August 2018 were downloaded for analysis. Participants who met clinical and baseline DAT scan criteria for idiopathic PD were selected and divided into 2 groups based on the Unified Parkinson's Disease Rating Scale (UPDRS) part 1 hallucinations/psychosis item score:
PDP+: Patients with hallucinations or delusions (score of 2, 3 or 4 on the UPDRS part1 hallucinations/psychosis item) at one or more follow‐up visits but not the baseline or screening visit.
PDP−: Patients without hallucinations or delusions at baseline or any subsequent visit for a minimum follow‐up duration of 30 months (score of 0 or 1 on the UPDRS part1 hallucinations/psychosis item).
Full details of the PPMI DAT scanning, binding ratio calculation, and region‐of‐interest extraction are described at http://www.ppmi-info.org/study-design/research-documents-and-sops. Mean total striatal binding was calculated as the average of left and right caudate and putamen regions. DAT binding in the 2 groups was examined in an analysis of covariance controlling for baseline total Montreal Cognitive Assessment score, UPDRS part 3 score, age, sex, and prospective DRT, the latter defined in 1 of the following 2 ways: (1) levodopa or dopamine agonist use at any prospective visit coded as binary variables or (2) levodopa equivalent daily dose at time of hallucination onset in PDP+ and most recent follow‐up in PDP−. Detailed DRT prescription history was further examined in the PDP+ group, focusing on medication history and dose changes prior to PDP onset.
Results
A total of 386 patients with DAT‐confirmed idiopathic PD were identified for further analysis. Of these, 30 patients experienced formed hallucinations or delusions at 1 or more time points (PDP+) following the baseline visit. One patient reported hallucinations at baseline and was excluded from the DAT scan analysis. The mean onset of PDP was 42 ± 20 months (range 4–85) after baseline. None of the PDP+ group had significant eye disease (n = 20 refractive error or presbyopia; n = 2 cataracts). The most common PDP symptoms were the following: formed hallucinations with insight (n = 19), formed hallucinations without insight (n = 8), and delusions (n = 3). Illusions were reported in 87% (26/30) of the PDP+ group at 1 or more visits. A total of 355 patients had no reports of formed hallucinations or delusions (PDP−) during a mean follow‐up duration of 64 ± 11 months (range 31–88 months); 31% of the PDP− group (111/355) reported illusions at 1 or more visits. Table 1 shows the baseline characteristics of PDP+ and PDP− groups.
Table 1.
Demographic, clinical, and DAT binding data in the 2 groups at baseline and follow‐up
| Baseline | PDP−, n = 355 | PDP+, n = 30 | Sig. (2‐tailed) |
|---|---|---|---|
| Age, years (SD) | 61.35 (9.9) | 64.07 (8.7) | 0.15 |
| UPDRS part III score (SD) | 20.05 (8.9) | 22.83 (9.6) | 0.10 |
| MoCA score (SD) | 27.12 (2.3) | 26.70 (2.9) | 0.34 |
| Sex, male, % | 65.9 | 70 | 0.65 |
| Mean striatal DAT binding | 1.42 (0.4) | 1.15 (0.4) | <0.001 |
| Follow‐up | |||
| LEDD,* mg (SD) | 631.34 (629.6) | 526.92 (379.1) | 0.37 |
| Prospective levodopa use, % | 84.8 | 96.7 | 0.07 |
| Prospective dopamine agonist use, % | 60.8 | 53.3 | 0.42 |
t tests for continuous data and chi‐square tests for categorical data.
LEDD at time of PDP onset for PDP+ and last follow‐up visit for PDP−.
DAT, dopamine transporter; PDP, Parkinson's disease psychosis; SD, standard deviation; UPDRS, Unified Parkinson's Disease Rating Scale; MoCA, Montreal Cognitive Assessment; LEDD, levodopa equivalent daily dose.
Baseline mean total striatal DAT binding was reduced in the PDP+ group when compared with the PDP− group in both the model using levodopa equivalent daily dose as an index of prospective DRT (F 1,377 = 10.9; P = 0.001) and the model using binary dopamine agonist and levodopa variables (F 1,375 = 10.06; P = 0.002). The results remained significant when repeated with the 3 patients who developed delusions excluded. The effect size was greater for the caudate subregion than the putamen subregion (mean caudate β = 0.333; mean putamen β = 0.139), and greater in the right caudate nucleus than the left (right caudate β = 0.363; left caudate β = 0.303). There was no significant difference in DAT binding between patients who did and did not develop illusions in the PDP− group (F 1,339 = 0.007; P = 0.93).
DRT Medication History
At the time of data download, almost all patients (98%) were prescribed DRT, but only 8% had developed PDP. Figure 1 shows the DRT history of the PDP group, categorized by regimen at the time of PDP onset. A total of 5 patients (6 including the patient with hallucinations at baseline, 19%) developed symptoms when not on DRT. The relationship of PDP onset to DRT in 2 further patients (subject H02 and H11 in Figure 1) is unclear, as both occurred in the same month and the exact event sequence is not recorded in the database. A total of 8 patients developed PDP in the context of treatment with levodopa only (mean exposure 32 ± 19 months at PDP onset), and 4 patients developed PDP during treatment with a dopamine agonist only (mean exposure 25 ± 10 months at PDP onset). Seven patients developed PDP on combined dopamine agonist and levodopa (mean exposure for combined medications 25 ± 13 months; mean exposure of first medication 40 ± 10 months at PDP onset), and 4 patients had complex prior medication histories (mean exposure 54 ± 16 months at PDP onset).
Figure 1.
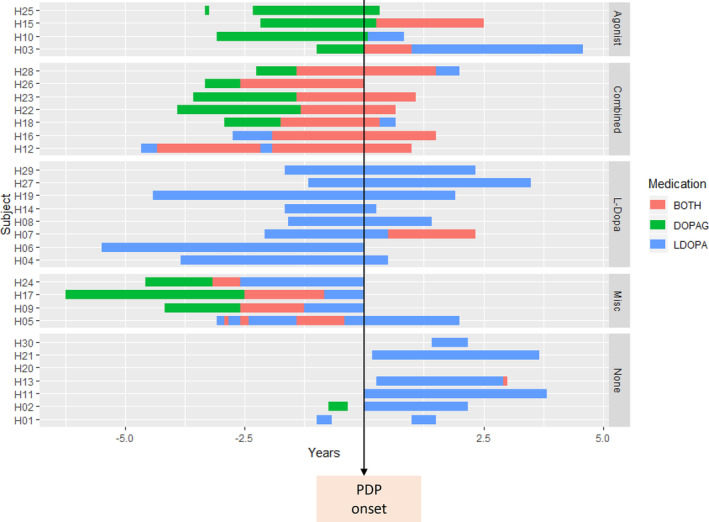
DRT medication history in the PDP+ group. The timing of dopamine agonists (green bars), levodopa (blue bars), or combined medication (red bars) is shown with respect to the onset of hallucinations (‐ years = prior to PDP onset). Patients have been categorized into subgroups based on DRT regime at time of hallucination onset: agonist = only dopamine agonist exposure, combined = both dopamine agonist and levodopa exposure, L‐Dopa = only levodopa exposure, Misc = complex DRT exposure, none = not on DRT. DOPAG, dopamine agonist; DRT, dopamine replacement therapy; LDOPA, levodopa; PDP, Parkinson's disease psychosis.
The temporal relationship between PDP onset and medication dose change was examined in the levodopa group. Of the 8 patients in the levodopa group, 5 were on a stable regime for at least 4 months before hallucination onset (range 4–20 months). One patient had a reduction in levodopa in the same month as PDP onset, likely to have been instituted as a management strategy for PDP. Levodopa dose increase may have coincided with PDP onset in 2 patients, although the sequence of events is not recorded in the database.
Discussion
Most patients in the PPMI cohort (92%) have not developed PDP despite DRT exposure at higher doses and for longer duration than the minority of patients (8%) who have developed them. Mean striatal DAT binding in patients who go on to develop PDP is reduced at baseline when compared with patients who do not, controlling for global cognition, motor severity, sex, and subsequent DRT exposure. In what follows, we explore the implications of the findings for the respective contribution of disease effects and DRT in the mechanism of PDP.
Striatal DAT Binding and Parkinson's Psychosis: Disease Effect
In previous studies, lowest quartile range mean striatal binding has been found to predict several clinical milestones at 5 years, including PDP and cognitive impairment.10 Furthermore, reduced DAT binding in the right caudate nucleus8 and ventral striatum9 may predispose patients to visual hallucinations. We add to these findings by showing reduced striatal DAT binding is independent of a range of clinical and medication‐related confounds and add further evidence for a role of the right caudate nucleus in PDP. A similar association has been reported in the PPMI dataset for incident impulse control disorder symptoms.12 The suggestion of greater DAT binding reduction in the right caudate nucleus helps account for the inhibitory executive function deficits found in previous studies.13
Dopamine Medication and Parkinson's Psychosis: Drug Effect
If dopamine medication was the sole cause of PDP, one would not expect incident cases without it. Of the PDP group, 19% had onset of PDP while not on a dopamine agonist or levodopa. This rate is similar to the 13% of incident impulse control disorder behaviors prior to DRT12 and highlights the importance of factors other than medication in the development of neuropsychiatric symptoms in PD. Although the majority of patients who developed PDP were prescribed DRT at the time of onset (81%), there was no clear predominance of exposure history for dopamine agonists, levodopa alone, or combinations of both. Patients had typically been prescribed DRT for a year or more at PDP onset and had been on a stable regime for 4 months. This suggests that PDP is not caused by striatal receptor upregulation and DRT hypersensitivity, as one would expect such effects to coincide with medication onset or a dose increase.
Limitations
The pathophysiological mechanism underlying hallucinations and delusions may differ in early‐stage and late‐stage PD,14 so the findings described here may not apply in more advanced disease. Furthermore, the relationship between dopaminergic mechanisms and serotonergic or cholinergic mechanisms has not been assessed. There are also limitations in using the Movement Disorder Society–UPDRS psychosis item to assess PDP, as it does not allow detailed analysis of symptom subtypes and sampling is limited to the week before assessment. Patients in the PDP− group may thus have PDP symptoms outside the sampling period and PDP− and PDP+ groups may therefore be more correctly described as having lower (PDP−) higher (PDP+) rates of PDP rather than PDP being present or absent. The risk factors for PDP in the PPMI cohort may also not be representative of those typical in PD as PPMI participants are relatively younger and cognitively intact and have higher educational achievement than other PD cohorts.
In conclusion, our findings suggest that alongside medication, dopamine‐related disease mechanisms may be involved with other neurotransmitter systems in the hallucinations and delusions of PDP. The same may not be true of illusions. It remains unclear how drug and disease effects interact to cause psychosis in early‐stage PD as we did not find support for an association between PDP and a specific DRT regime or for a temporal relationship between PDP onset and DRT onset or levodopa dose increase.
Author Roles
(1) Research and statistical analysis: A. Conception and design, B. Organization and execution, C. Review and critique. (2) Manuscript: A. Writing of the first draft, B. Review and critique.
S.D.: 1A, 1B, 2A
D.W.: 1A, 1C, 2B
D.A.: 1C, 2B
D.H.f.f.: 1A, 1B, IC, 2A, 2B
Disclosures
Ethical Compliance Statement
The authors confirm that each participating PPMI site received approval from an ethical standards committee on human experimentation before study initiation and written informed consent for research was obtained from all participants in the study. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
No relevant funding sources or conflicts of interest given the nature of the article.
Financial Disclosures for the Previous 12 Months
D.A. has served as a paid consultant and speaker for Pfizer, AstraZeneca, Eli Lilly, Eisai, Lundbeck, and Janssen and has received grant support from Novartis, Pfizer, and Janssen. D.W. has received research funding or support from Michael J. Fox Foundation for Parkinson's Research, National Institutes of Health, Novartis Pharmaceuticals, Department of Veterans Affairs, Avid Radiopharmaceuticals and Alzheimer's Disease Cooperative Study; honoraria from AbbVie, Biotie, Teva Pharmaceuticals, Otsuka, UCB, Clintrex LLC and the CHDI Foundation; license fee payments from the University of Pennsylvania for the Questionnaire for Impulsive‐Compulsive Disorders in Parkinson's Disease (QUIP) and QUIP–Rating Scale; royalties from Wolters Kluweland; and fees for legal consultation for lawsuit related to antipsychotic prescribing in a patient with Parkinson's disease. D.H.f.f. has received funding from National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London; National Institute for Health Research Programme Grants for Applied Research (RP‐PG‐0610‐10100 SHAPED) and the Macular Society. S.D. has no financial disclosures to report.
Acknowledgments
Data used in the preparation of this article were obtained from the Parkinson's Progression Markers Initiative database (http://www.ppmi-info.org/). D.A. is a Royal Society Wolfson Research Merit Award Holder and thanks the Wolfson Foundation and the Royal Society for their support. This paper represents independent research. D.A. and D.H.f.f. are partly funded by the National Institute for Health Research Biomedical Research Centre at South London and Maudsley National Health Service (NHS) Foundation Trust and King's College London and National Institute for Health Research Programme Grants for Applied Research (RP‐PG‐0610‐10100 SHAPED). The views expressed are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Forsaa EB, Larsen JP, Wentzel‐Larsen T. A 12‐year population‐based study of psychosis in Parkinson disease. Arch Neurol 2010;67(8):996–1001. [DOI] [PubMed] [Google Scholar]
- 2. ffytche DH, Creese B, Politis M. The psychosis spectrum in Parkinson disease. Nat Rev Neurol 2017;13(2):81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKinlay A, Grace RC, Dalrymple‐Alford JC, Anderson T, Fink J, Roger D. A profile of neuropsychiatric problems and their relationship to quality of life for Parkinson's disease patients without dementia. Parkinsonism Relat Disord 2008;14(1): 37–42. [DOI] [PubMed] [Google Scholar]
- 4. Aarsland D, Brønnick K, Ehrt U. Neuropsychiatric symptoms in patients with Parkinson's disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry 2007;78(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population‐based, prospective study. J Am Geriatr Soc 2000;48(8):938–942. [DOI] [PubMed] [Google Scholar]
- 6. Collerton D, Perry E, McKeith I. Why people see things that are not there: a novel perception and attention deficit model for recurrent complex visual hallucinations. Behav Brain Sci 2005;28(6):737–757; discussion 757–794. [DOI] [PubMed] [Google Scholar]
- 7. Ballanger B, Strafella AP, van Eimeren T. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol 2010;67(4):416–421. [DOI] [PubMed] [Google Scholar]
- 8. Kiferle L, Ceravolo R, Giuntini M. Caudate dopaminergic denervation and visual hallucinations: evidence from a (1)(2)(3)I‐FP‐CIT SPECT study. Parkinsonism Relat Disord 2014;20(7):761–765. [DOI] [PubMed] [Google Scholar]
- 9. Jaakkola E, Joutsa J, Makinen E, Johansson J, Kaasinen V. Ventral striatal dopaminergic defect is associated with hallucinations in Parkinson's disease. Eur J Neurol 2017;24(11):1341–1347. [DOI] [PubMed] [Google Scholar]
- 10. Ravina B, Marek K, Eberly S. Dopamine transporter imaging is associated with long‐term outcomes in Parkinson's disease. Mov Disord 2012;27(11):1392–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marek K, Jennings D, Lasch S. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011;95(4):629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith KM, Xie SX, Weintraub D. Incident impulse control disorder symptoms and dopamine transporter imaging in Parkinson disease. J Neurol Neurosurg Psychiatry 2016;87(8):864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barnes J, Boubert L. Executive functions are impaired in patients with Parkinson's disease with visual hallucinations. J Neurol Neurosurg Psychiatry 2008;79(2):190–192. [DOI] [PubMed] [Google Scholar]
- 14. Goetz, CG , Vogel C, Tanner CM, Stebbins GT. Early dopaminergic drug‐induced hallucinations in parkinsonian patients. Neurology 1998;51(3):811–814. [DOI] [PubMed] [Google Scholar]


