https://onlinelibrary.wiley.com/page/journal/23301619/homepage/mdc312854-sup-v001.htm
Dystonia‐deafness syndrome (DDS) is a well‐known clinical entity, with sensorineural deafness typically manifesting earlier than dystonia, and associated with several genetic and acquired causes.1 We report on a case of DDS caused by ACTB gene mutation, emphasizing an expansion of phenotype compared to previous similar reported cases.
Case Report
A 52‐year‐old Brazilian woman presented with generalized dystonia with onset at age 25, characterized by left lower‐limb dystonia that gradually progressed to involve other regions of the body. She became wheelchair‐bound at age 47. Born from nonconsanguineous parents, she had a developmental delay characterized by poor language skills acquisition and progressive bilateral sensorineural hearing loss since early childhood. There was no other family member affected. At age 5, the patient developed a focal epilepsy (dystonic posture in arms, head version to the right, followed by generalized seizure) that occurred once or twice a month, controlled with phenytoin (100 mg three times per day). During the following years, she presented with one seizure every year in adulthood, and recently we changed the medication for carbamazepine 1,200 mg daily, with improvement. She is free from seizures for the last 6 months. Neurological examination showed severe generalized dystonia with dystonic tremor in her upper limbs, dystonic gait (Video S1), and a severe scoliosis (Fig. 1). The patient had a progressive abnormal posture of the spine since the beginning of the dystonia, and scoliosis had a late onset (scoliosis was observed around 4 years after the beginning of the dystonia). There was no facial dysmorphism. EEG showed right focal epileptiform paroxysmal activity. Brain and spinal MRI showed no abnormalities. DDS was suspected, and whole‐exome sequencing revealed a pathogenic variant mutation p.Arg183Trp (OMIM *102630) in the ACTB gene. Dystonia was treated with levodopa 600 mg with no response, and biperiden 8 mg daily, also with no improvement. Clonazepam 4 mg daily was tried, and there was mild and temporary improvement. Clonazepam was discontinued because of somnolence. Carbamazepine, that was used to control her epilepsy, resulted in a mild improvement in dystonia. The patient did not complain of pain. Botulinum toxin was tried for three times, but was ineffective.
Figure 1.
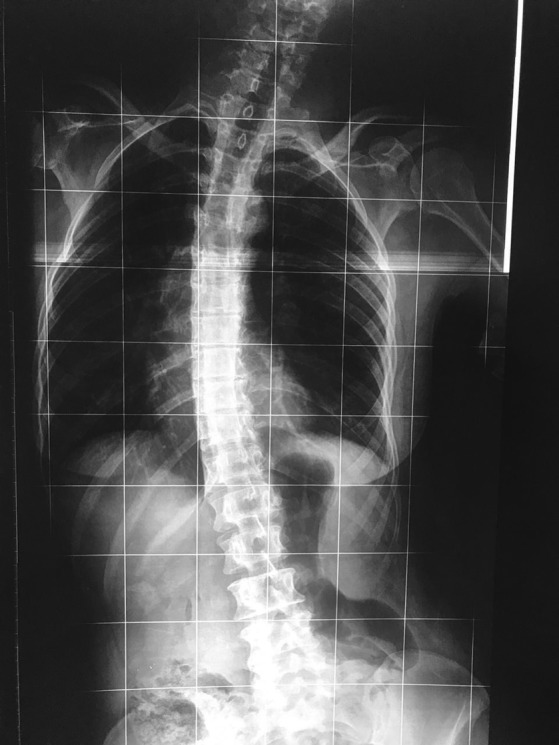
Spine radiography in supine position showing severe scoliosis.
Discussion
DDS is a well‐known clinical entity, with sensorineural deafness typically manifesting earlier than dystonia. Several genetic and acquired causes are associated with DDS, but etiology remains undetermined in many patients. Known genetic causes of DDS include Mohr‐Tranebjærg syndrome, organic acidurias, Woodhouse‐Sakati syndrome, and rare mitochondrial disorders.1
Mutations in ACTB, the gene encoding cytoplasmic b‐actin, have been described in Baraitser‐Winter syndrome, a developmental disorder characterized by congenital ptosis, hypertelorism, high‐arched eyebrows, and ocular colobomata, and frequently associated with sensorineural deafness.2 When combined with muscular, visceral, and craniofacial involvement, it is named Baraitser‐Winter cerebrofrontofacial syndrome (BWCFF). Cytoplasmic b‐actin supports fundamental cellular processes in healthy and diseased cells, including cell adhesion, migration, cytokinesis, and maintenance of cell polarity. Two dominant heterozygous gain‐of‐function b‐actin mutations, p.R183W and p.E364K, were already identified in patients with developmental malformations, deafness, and juvenile‐onset dystonia (p.R183W) and neutrophil dysfunction (p.E364K).3 The p.Arg183Trp mutation in the ACTB gene appears to be associated with an autosomal‐dominant syndrome of DDS, with minimal or no classic characteristics of BWCFF syndrome. This variant was originally first identified in a 2006 study seeking the etiology for generalized dystonia and deafness in a monozygotic twin‐pair.4 Also, p.Arg183Trp ACTB has demonstrated a gain‐of‐function effect, with slower actin filament growth, higher ATP hydrolysis, and faster depolymerization with impaired formation of long, stable filaments.4 Supporting Information Table S1 describes the additional clinical features of the 7 other cases of heterozygous p.Arg183Trp mutation in the ACTB gene reported to date.
ACTB p.Arg183Trp heterozygosity has been reported in 7 patients,5 none with epilepsy or scoliosis. All had combined infant‐onset deafness and dystonia manifesting in adolescence or young adulthood. Three of these cases have received beneficial pallidal stimulation. The most recent report showed a striatal neuronal/dopaminergic dysfunction as the possible underlying cause of the dystonia in these patients.5 This is the reason why the pallidal stimulation provided substantial improvement of the severe generalized dystonia in these patients.
In conclusion, our report expands the phenotype of DDS related to ACTB gene mutation, by including epilepsy with frontal paroxysmal activity and severe scoliosis. Patients with dystonia, deafness, epilepsy, and scoliosis should be investigated for ACTB gene mutations.
Author Roles
(1) Conception and design; (2) Organization and execution; (3) Manuscript preparation; (4) Manuscript review and critique.
J.L.F.: 1, 2, 3, 4
T.C.V.: 1, 2, 3, 4
J.L.P.: 1, 3, 4
O.G.P.B.: 1, 4
Disclosures
Ethical Compliance Statement: Full consent was obtained from the patient for the case report publication. The patient's father has signed the written informed consent. The authors confirm that the approval of an institutional review board was not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: The authors declare that there are no disclosures to report.
Supporting information
Video S1. Patient with dystonia‐deafness syndrome related to ACTB gene mutation presenting with severe generalized dystonia with dystonic tremor in her upper limbs and a dystonic gait. There was also orofacial dystonia (J.L.P., one of the authors, examines the patient).
Table S1. Clinical features of heterozygous variant p.Arg183Trp mutation in the ACTB gene.
Julian Letícia Freitas and Thiago Cardoso Vale contributed equally to this work.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Kojovic M, Pareés I, Lampreia T, et al. The syndrome of deafness‐dystonia: clinical and genetic heterogeneity. Mov Disord 2013;28:795–803. [DOI] [PubMed] [Google Scholar]
- 2. Baraitser M, Winter RM. Iris coloboma, ptosis, hypertelorism, and mental retardation: a new syndrome. J Med Genet 1988;25:41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hundt N, Preller M, Swolski O, et al. Molecular mechanisms of disease‐related human b‐actin mutations p.R183W and p.E364K. FEBS J 2014;281:5279–5291. [DOI] [PubMed] [Google Scholar]
- 4. Procaccio V, Salazar G, Ono S, et al. A mutation of beta‐actin that alters depolymerisation dynamics is associated with autosomal dominant developmental malformations, deafness, and dystonia. Am J Hum Genet 2006;78:947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skogseid IM, Rosby O, Konglund A, et al. Dystonia‐deafness syndrome caused by ACTB p.Arg183Trp heterozygosity shows striatal dopaminergic dysfunction and response to pallidal stimulation. J Neurodev Disord 2018;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Patient with dystonia‐deafness syndrome related to ACTB gene mutation presenting with severe generalized dystonia with dystonic tremor in her upper limbs and a dystonic gait. There was also orofacial dystonia (J.L.P., one of the authors, examines the patient).
Table S1. Clinical features of heterozygous variant p.Arg183Trp mutation in the ACTB gene.


