Cerebrotendinous xanthomatosis (CTX) is a treatable, autosomal‐recessive, multisystemic lipid storage disorder with various neurological and nonneurological manifestations. We report a case of CTX with progressive parkinsonism involving postsynaptic striatal iron deposition and intact dopaminergic presynaptic terminals.
A 40‐year‐old man with a 10‐year history of progressive gait difficulties, bradykinesia, and upper‐limb tremors developed cataracts at age 9, epileptic seizure at age 26, and mild chronic diarrhea at age 38. He was diagnosed with parkinsonian syndrome. Levodopa therapy was initiated (400 mg/d, gradually increased to 900 mg/d), but was ineffective.
Neurological examination revealed gait difficulties; symmetrical rigidity and bradykinesia, particularly of the legs; pronounced upper‐limb postural and action tremors and mild left‐arm resting tremor; left‐side incoordination; bilateral incoordination; balance‐related problems; lower‐limb weakness, particularly of the left leg; hyperreflexia of all limbs; and bilateral Babinski reflex. His Unified Parkinson's Disease Rating Scale part II and part III scores were 28 and 49, respectively. He had impaired cognitive function but no personality changes, slow voice without aphasia, symmetrical reduction of graphic perception, 2‐point discrimination perception in his lower limbs, Mini‐Mental State Examination score of 18, impaired orientation, difficulties with simple calculations, mild enlargement of right Achilles tendon, and mild pes cavus.
[18F]‐9‐fluoropropyl‐(+)‐dihydrotetrabenazine positron emission tomography revealed normal uptake in the bilateral putamen and caudate (Fig. 1A,B); brain susceptibility‐weighted imaging (Fig. 1C and E) and quantitative susceptibility mapping (Fig. 1D,F) showed elevated iron deposition in the substantia nigra, red nucleus, bilateral globus pallidus, and cerebellar dentate nucleus. These results indicated intact presynaptic dopaminergic input function and potentially lesioned postsynaptic outputs. Brain magnetic resonance imaging revealed mild atrophy and symmetric abnormal signals in the cerebellar dentate nucleus and mild white matter degeneration (Fig. 1G,H). Sagittal T1‐weighted right‐ankle magnetic resonance imaging revealed fusiform thickening (Fig. 1I,J); Achilles tendon biopsy showed accumulation of multinucleated giant cells, xanthoma cells, and dispersed lipid crystal clefts (Fig. 1L,M). The patient's cholesterol level was 45.79 μmol/L (normal range, <10μmol/L); Sanger sequencing revealed 2 heterozygous CYP27A1 mutations—chr2:219677818 c.1016C>T and chr2:219679182 c.1263+1G> A.
Figure 1.
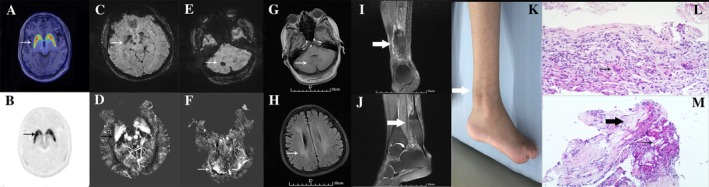
Imaging results. (A,B) [18F]‐9‐fluoropropyl‐(+)‐dihydrotetrabenazine positron emission tomography revealed normal striatal uptake of [18F]‐9‐fluoropropyl‐(+)‐dihydrotetrabenazine (arrows). Susceptibility‐weighted imaging (C,E) and quantitative susceptibility mapping (D,F) revealed iron deposition within several brain regions (arrows). T1‐weighted brain magnetic resonance imaging revealed abnormal, symmetric signals in the cerebellar dentate nucleus (G) as well as white matter degeneration (H). T1‐weighted right‐ankle magnetic resonance imaging revealed fusiform thickening (I,J; arrows). (K) Photograph of the enlarged Achilles tendon. Hematoxylin‐and‐eosin stained biopsy specimen of the Achilles tendon shows multinucleated giant cells (L; arrow), xanthoma cells (M; thick arrow), and dispersed lipid crystal clefts (M; thin arrow). Magnifications: G, 200×; H, 100×.
Initially, ursodeoxycholic acid was administered and subsequently chenodeoxycholic acid for 6 months.1 His gait difficulties and action tremors ameliorated initially; however, his condition steadily deteriorated. He refused follow‐up metabolic investigations.
Chronic diarrhea, bilateral cataracts, tendon xanthomas, and neurologic dysfunctions are pathognomonic of CTX. Our patient developed bilateral cataracts early. However, late onset of diarrhea and mild tendon xanthoma (Fig. 1K) potentially delayed diagnosis. Parkinsonism can occur in patients with CTX (prevalence, ~9%).1 The neurological signs are restricted to parkinsonism and misdiagnosed with Parkinson's disease in some patients. Chenodeoxycholic acid and antiparkinsonian drugs were ineffective despite reported dopaminergic presynaptic denervation in CTX.2 Our patient's intact dopaminergic presynaptic terminals corresponded with his nonresponsiveness to levodopa.
Furthermore, brain susceptibility‐weighted imaging and quantitative susceptibility mapping revealed bilateral iron deposition, which suggests postsynaptic disruption of the striatal dopaminergic loop as causal to his parkinsonism. Many neurodegenerative diseases, including Parkinson's disease, manifest iron dyshomeostasis.3 Iron deposition may be a potential predictor of parkinsonism.4 Excessive neuronal iron deposition potentially induce apoptosis, autophagy, necrosis, or ferroptosis and impair neuronal functions.5 Our patient's parkinsonian syndrome might have involved postsynaptic iron deposition, but not presynaptic denervation. However, identifying the role of iron deposition in CTX requires further investigation.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
J.L.: 1A, 1B, 1C, 2A, 2B, 3A
E.‐H.X.: 1A, 1B, 1C
W.M.: 1B, 1C, 2C
H.‐W.Q.: 2A, 2B, 2C
Y.‐T.Z.: 1A, 1B, 1C
Q.Y.: 2A, 2B, 2C
S.‐Y.L.: 1A, 1B, 1C, 2A, 2B, 3B
P.C.: 1A, 2C, 3B
Disclosures
Ethical compliance statement
This study was conducted in accordance with the principles of the Declaration of Helsinki. The patient gave written informed consent for all treatments and the publication of this case report. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest
This study was funded by the Beijing Municipal Administration of Hospitals’ Mission Plan (Grant SML20150803), the Beijing Municipal Science & Technology Commission (Grant Z171100000117013), and the National Key R&D Program of China (Grants 2017YFC0840105 and 2018YFC1312000). The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
The authors declare that there are no additional disclosures to report.
Acknowledgments
We thank the patient and his family for their longtime cooperation with his treatment and the preparation of this case report. We also thank Dr. Jun‐Wu Zhao for collecting valuable information about the patient.
Relevant disclosures and conflicts of interest are listed at the end of this article.
Contributor Information
Shu‐Ying Liu, Email: Liu_Shu-Ying@hotmail.com.
Piu Chan, Email: pbchan@hotmail.com.
References
- 1. Stelten BML, Warrenburg BPCVD, Wevers RA, Verrips A. Movement disorders in cerebrotendinous xanthomatosis. Parkinsonism Relat Disord 2019;58:12–16. [DOI] [PubMed] [Google Scholar]
- 2. Makary MS, Kisanuki YY, Amin NN, Slone HW. Teaching neuroimages: cerebrotendinous xanthomatosis: a rare treatable adult‐onset lipid storage disease. Neurology 2018;90(7):e637–e638. [DOI] [PubMed] [Google Scholar]
- 3. Ndayisaba A, Kaindlstorfer C, Wenning GK. Iron in neurodegeneration–cause or consequence? Front Neurosci 2019;13:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brar S, Henderson D, Schenck J, Zimmerman EA. Iron accumulation in the substantia nigra of patients with Alzheimer disease and parkinsonism. JAMA Neurology 2009;66(3):371–374. [DOI] [PubMed] [Google Scholar]
- 5. Hider RC, Longo DL, Hoffbrand AV. The role of deferiprone in iron chelation. N Engl J Med 2018;379(22):2140–2150. [DOI] [PubMed] [Google Scholar]


