ABSTRACT
Introduction
Cognitive dysfunction is common in Parkinson's disease (PD) and associated with reduced functional abilities and increased dependence. To date, however, little is known about the relationship between performance of instrumental activities of daily living (IADLs) and cognitive stages in PD, and there are conflicting reports as to whether declines in specific cognitive domains predict IADL impairment.
Methods
Participants with PD were drawn from the Pacific Udall Center and included in the study if both participant and study partner IADL ratings and cognitive tests were completed (n = 192). Logistic regression analyses were performed to determine whether participant and/or study partner rating predicted mild cognitive impairment or dementia. Correlations are reported for the relationship between participant/study partner IADL reports as well as for specific cognitive tests.
Results
Although both participant and study partner ratings of IADL performance were associated with a diagnosis of PD with dementia, only participant self‐rating of functional ability was significantly associated with a diagnosis of PD with mild cognitive impairment. Functional ability correlated most strongly with measures of processing speed, auditory working memory, and immediate verbal recall for both the participant and study partner ratings.
Conclusion
For participants with PD in the early stages of cognitive decline, self‐rating may be more sensitive to the impact of cognitive changes on IADL function than ratings made by a knowledgeable study partner. Changes in executive function, processing speed, and learning may indicate a higher likelihood of IADL impairment. Careful assessment of cognition and IADL performance is recommended to permit individualized interventions prior to significant disability.
Keywords: Activities of daily living, Parkinson's disease, cognition, dementia, mild cognitive impairment, study partner
Cognitive impairment is pervasive in Parkinson's disease (PD), often resulting in devastating impacts on quality of life, functional performance, and independence.1 A decline in the ability to perform instrumental activities of daily living (IADL) related to cognitive impairment, although often initially subtle, can negatively impact disease outcome if not identified and adequately compensated.2 Although significant functional impairment may be more apparent in PD‐related dementia (PDD), subtle deficits in complex IADLs occur frequently among those with PD‐related mild cognitive impairment (PD‐MCI), and the presence of such deficits may help to reveal the presence of early cognitive decline.3
The assessment of functional change in PD presents challenges, however. Performance‐based measures are not often feasible in a clinic setting, and thus there is heavy reliance on self and care partner reporting. Anosognosia, a lack of awareness of the effects of a disease, is common in all dementias including PDD, and people diagnosed with PD may be more likely to overestimate their functional performance as compared to clinician rating.4 Less is known about the accuracy of self‐appraisal in PD‐MCI, although recent reports suggest that anosognosia may be less common in this group.5, 6 To augment the evaluation of functional status in those with cognitive impairment, a knowledgeable care partner is often consulted. There is support for the widespread use of a care partner report to accurately reflect cognitive and functional changes in Alzheimer's disease (AD),7, 8 although the quality of study partner reports vary, and care partners may overestimate functional performance specifically among those with higher cognitive scores.9 Despite these reports in AD, to date little is known about the relationship between self and study partner reports concerning the performance of IADLs during the course of PD.
Although it is recognized that impaired cognitive status (PDD and PD‐MCI) is associated with reduced performance on IADLs, there are conflicting reports as to whether declines in specific cognitive domains predict functional impairment. Memory, executive function, processing speed, language, and visuospatial skills have been variably associated with more complex IADLs such as driving, management of finances, and medication management in PD10, 11; however, many of these studies have been limited by small sample sizes. Still others have found no connection between specific cognitive tasks and complex functional change.3 It is further unknown as to whether participant and study partner ratings differ with respect to their relationships to individual cognitive domains.
The primary aim of the current study was to determine whether self‐reported and study partner reported changes in IADLs as measured by the Penn Parkinson's Daily Activities Questionnaire–15 (PDAQ‐15)12 predict cognitive diagnosis (PDD and/or PD‐MCI) in the Pacific Udall Center cohort, with the goal of providing clinicians and researchers additional tools to identify potential cognitive impairment and its impact in PD. Second, we sought to describe the relationship between self‐report and/or study partner report of IADL change, specifically to determine if either source yielded a stronger association with cognitive diagnosis. Finally, we investigated whether participant and study partner reports of IADL change were associated with specific cognitive test performance in PD to provide clinicians with insight into the potential impact of impairment in specific cognitive domains on independent living and guide subsequent research into patient‐centered interventions.
Methods
Participants
Participants were drawn from the Pacific Udall Center of Excellence in Parkinson's Disease Research, which consists of the following 3 sites: University of Washington/Veterans Affairs Puget Sound Health Care System, Oregon Health and Sciences University/Veterans Affairs Portland Health Care System, and Stanford University. The cohort began enrolling participants in 2010; the PDAQ‐15 was added to the clinical scales collected in 2016. Participants in the current study were included if they met the U.K. Parkinson's Disease Society Brain Bank clinical diagnostic criteria for PD,13 had a cognitive diagnosis assigned (no cognitive impairment, PD‐MCI, PDD), and if PDAQ‐15 scores were available for both the participant and study partner (n = 214). Study partners were co‐enrolled with the participant and were a spouse or other first‐degree relative when available; otherwise a close friend or other relative with knowledge of the participant's daily functioning was recruited. A total of 22 participants were excluded as a result of missing data for 1 or more of the primary covariates (part III of the Unified Parkinson's Disease Rating Scale–Movement Disorders Society Revision,14 the 15 item Geriatric Depression Scale,15 levodopa equivalent daily dose [LEDD],16 or Montreal Cognitive Assessment17), for a total of 192 participants included in the analyses. The institutional review boards at all sites provided formal approval for the study. All participants and study partners provided written informed consent prior to study participation.
IADLs
The PDAQ‐15 is a questionnaire previously validated by Brennan and colleagues12 that measures the performance of IADLs in people diagnosed with PD both by self‐report and by the report of a knowledgeable care partner. The PDAQ‐15 assesses activities most likely to be impacted by cognitive decline in PD and includes items that measure how much difficulty a participant currently experiences as a result of PD on IADLs such as reading comprehension, medication management, navigation, learning to use new gadgets, financial management/understanding, and orientation. Each item is rated on a scale from 0 to 4 for a total score range of 0 to 60 (higher score = better performance) as follows: none = 4, a little = 3, somewhat = 2, a lot = 1, or cannot do = 0. The questionnaires were completed by the participant (PDAQ‐P) and study partner (PDAQ‐SP) separately.
Cognitive Variables
Cognitive and motor diagnoses were assigned at a clinical diagnostic consensus conference attended by at least 2 movement disorders specialists, a neuropsychologist, and study personnel, as previously described.18 Cognitive diagnoses were made according to published diagnostic criteria for PDD19 and PD‐MCI.20 At least 2 neuropsychological measures were available for each cognitive domain as required for PD‐MCI level II criteria20 at all sites using the core neuropsychological measures described later in combination with site‐specific instruments (Supplemental Table 1).
Core cognitive variables for the current analyses were those administered since the establishment of the cohort and given across all sites. These measures include the following: (1) global cognition (Montreal Cognitive Assessment), (2) verbal learning and memory (Hopkins Verbal Learning Test–Revised immediate and delayed recall,21 (3) visuomotor attention and working memory/divided attention (Trailmaking Test, parts A and B),22 (4) auditory working memory (Letter–Number Sequencing subtest from the Wechsler Adult Intelligence Scale–III),23 (5) processing speed/working memory (Digit Symbol subtest from the Wechsler Adult Intelligence Scale–Revised),24 (6) semantic verbal fluency (animal naming),22 (7) phonemic verbal fluency (F,A,S or C,F,L),22 and (8) visuospatial (Benton Judgment of Line Orientation).25 For analyses, Trailmaking B minus A was used to control for potential effects of motor slowing on visuomotor working memory. All participants taking PD medications were rated in the on state.
Statistical Analyses
Group differences in clinical, demographic, and cognitive variables were assessed using 1‐way analysis of variance and Scheffe's test for post hoc pairwise comparisons or chi‐square tests for categorical variables. To address the primary aim of the study, logistic regression models were conducted to test whether the PDAQ‐15 predicted cognitive diagnosis assignment (PD‐MCI vs. no cognitive impairment, and PDD vs. PD‐MCI), separately for the PDAQ‐P and the PDAQ‐SP, controlling for age, education, sex, PD disease duration since onset of motor symptoms, LEDD, motor severity (Unified Parkinson's Disease Rating Scale–Movement Disorders Society revision part III), depression (Geriatric Depression Scale), cognitive severity (Montreal Cognitive Assessment), and study site. Predicted probabilities as a function of PDAQ‐15 score (in increments of 5 units) if all covariates were held at the population mean and their 95% confidence intervals were estimated based on the fitted logistic regression model. Next, the determination of whether the resulting PDAQ‐P and PDAQ‐SP coefficients were significantly different was accomplished by estimating both equations, calculating the difference, and bootstrapping the data (1000 samples). Spearman's rank‐order correlations were performed to test the association between participant and study partner PDAQ‐15 ratings for each cognitive group. Finally, the associations between individual cognitive tests and PDAQ‐15 scores were assessed by regressing the PDAQ‐15 and cognitive test scores with age, sex, education, disease duration, Unified Parkinson's Disease Rating Scale–Movement Disorders Society part III, Geriatric Depression Scale, LEDD, and site and calculating the Spearman's rank‐order correlations between ranks of the residuals. The results are provided before and after correcting for multiple comparisons: the Bonferroni adjustment was used to control the family wise type I error set a priori at 0.05; because there were 9 cognitive variables, a significance level of 0.05/9 = 0.006 was used. All analyses were performed in Stata 15.1 (StataCorp, College Station, TX).
Results
Participant characteristics are provided in Table 1. In logistic regression analyses (Table 2), the PDAQ‐P was significantly associated with both PD‐MCI and PDD diagnoses after controlling for all confounders. In contrast, the PDAQ‐SP was significantly associated only with PDD, not PD‐MCI, over and above the included confounders (Fig. 1). This difference between the 2 logistic regression coefficients for the PDAQ‐P and PDAQ‐SP was statistically significant (P < 0.001). Correlations between the PDAQ‐P and PDAQ‐SP were positive and moderate in the overall sample (Spearman's ρ = 0.51, P < 0.0001), and for the not cognitively impaired (Spearman's ρ = 0.48, P < 0.0001) or PDD groups (Spearman's ρ = 0.51, P < 0.01). For the PD‐MCI group, the correlation was positive, but the relationship was less strong (Spearman's ρ = 0.32, P < 0.001).
Table 1.
Participant characteristics
| Variable | No Cognitive Impairment, n = 68 | PD‐MCI, n = 100 | PDD, n = 24 | P * |
|---|---|---|---|---|
| Age, y | ||||
| Mean (SD) | 66.1 (6.3) | 70.8 (7.4) | 72.4 (8.4) | <0.0001 |
| Range | 52.0–80.2 | 41.9–92.7 | 55.8–96.4 | NCI < PD‐MCI, NCI < PDD |
| Education years | ||||
| Mean (SD) | 16.5 (2.1) | 16.3 (2.5) | 16.3 (2.5) | 0.935 |
| Range | 12–20 | 12–20 | 12–20 | |
| Gender, male; n (%) | 29 (42.7) | 76 (76.0) | 19 (79.2) | <0.001 |
| NCI > PD‐MCI > PDD | ||||
| Disease duration, y | ||||
| Mean (SD) | 9.9 (6.2) | 11.3 (7.2) | 11.3 (5.8) | 0.385 |
| Range | 2–27 | 1–40 | 2–25 | |
| LEDD, mg/d | ||||
| Mean (SD) | 533.0 (407.6) | 686.6 (554.2) | 520.1 (515.1) | 0.099 |
| Range | 0–2300 | 0–2376 | 0–2205 | |
| MDS‐UPDRS, part III | ||||
| Mean (SD) | 20.7 (12.9) | 27.1 (11.6) | 30.9 (12.7) | <0.001 |
| Range | 1–64 | 7–67 | 6–60 | NCI < PD‐MCI, NCI < PDD |
| GDS | ||||
| Mean (SD) | 5.3 (1.3) | 5.7 (1.3) | 6.0 (1.7) | 0.029 |
| Range | 2–8 | 1–9 | 3–10 | NCI < PDD |
| PDAQ‐P | ||||
| Mean (SD) | 53.0 (7.0) | 48.8 (8.8) | 37.3 (11.3) | <0.0001 |
| Range | 30–60 | 26–60 | 19–54 | NCI > PD‐MCI > PDD |
| PDAQ‐SP | ||||
| Mean (SD) | 52.8 (8.7) | 49.9 (7.7) | 36.1 (13.7) | <0.0001 |
| Range | 28–60 | 28–60 | 11–56 | NCI > PDD PD‐MCI > PDD |
| MoCA | ||||
| Mean (SD) | 27.9 (1.7) | 24.5 (2.9) | 19.8 (3.2) | <0.0001 |
| Range | 24–30 | 17–30 | 11–25 | NCI > PD‐MCI > PDD |
| HVLT‐R total immediate recall | ||||
| Mean (SD) | 27.9 (3.4) | 21.4 (4.2) | 15 (4.4) | <0.0001 |
| Range | 21–35 | 11–32 | 6–23 | NCI > PD‐MCI > PDD |
| HVLT‐R delayed recall | ||||
| Mean (SD) | 10.1 (2.0) | 6.9 (2.6) | 4.2 (2.9) | <0.0001 |
| Range | 0–12 | 0–11 | 0–9 | NCI > PD‐MCI > PDD |
| Digit symbol | ||||
| Mean (SD) | 50.9 (9.0) | 38.8 (9.6) | 26.3 (10.3) | <0.0001 |
| Range | 35–73 | 19–70 | 0–45 | NCI > PD‐MCI > PDD |
| Trailmaking part Aa | ||||
| Mean (SD) | 28.9 (9.6) | 39.4 (13.0) | 64.9 (28.4) | <0.0001 |
| Range | 15–58 | 15–78 | 33–150 | NCI > PD‐MCI > PDD |
| Trailmaking part Ba | ||||
| Mean (SD) | 66.0 (21.1) | 108.4 (47.8) | 203.7 (75.3) | <0.0001 |
| Range | 26–131 | 40–300 | 77–300 | NCI > PD‐MCI > PDD |
| Letter number sequencing | ||||
| Mean (SD) | 10.8 (2.0) | 9.2 (1.8) | 6.7 (2.1) | <0.0001 |
| Range | 8–16 | 3–13 | 4–11 | NCI > PD‐MCI > PDD |
| Semantic verbal fluency | ||||
| Mean (SD) | 23.9 (4.3) | 17.5 (5.5) | 11.1 (4.2) | <0.0001 |
| Range | 14–33 | 5–34 | 3–18 | NCI > PD‐MCI > PDD |
| Phonemic verbal fluency | ||||
| Mean (SD) | 48.3 (11.6) | 40.2 (12.0) | 29.5 (9.5) | <0.0001 |
| Range | 24–74 | 15–71 | 17–45 | NCI > PD‐MCI > PDD |
| Judgment of line orientation | ||||
| Mean (SD) | 13.3 (1.7) | 11.6 (2.4) | 10.6 (2.3) | <0.0001 |
| Range | 7–15 | 5–15 | 7–15 | NCI > PD‐MCI > PDD |
P values based on 1‐way analysis of variance overall F for continuous variables or chi‐square for categorical variables. Pairwise post hoc tests were performed using Scheffe's or chi‐square tests.
Higher scores represent worse performance. For all other cognitive variables, higher scores represent better performance.
PDD, Parkinson's disease dementia; PD‐MCI, Parkinson's disease mild cognitive impairment; SD, standard deviation; LEDD, levodopa equivalent daily dose; MDS‐UPDRS, Uniform Parkinson Disease Rating Scale–Movement Disorders Society revision; NCI, no cognitive impairment; PDAQ‐P, Parkinson's Daily Activities Questionnaire–15, participant; PDAQ‐SP, Parkinson's Daily Activities Questionnaire–15, study partner; GDS, Geriatric Depression Scale; MoCA, Montreal Cognitive Assessment; HVLT‐R, Hopkins Verbal Learning Test‐Revised.
Table 2.
Association between PDAQ‐15 scores and cognitive diagnosis in the Pacific Udall Center of Excellence in Parkinson's Disease Research
| PD‐MCI vs. No Cognitive Impairment | PDD vs. PD‐MCI | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| PDAQ‐P | ||||
| Age | 0.96 (0.90–1.03) | 0.247 | 1.11 (1.00–1.24) | 0.059 |
| Education | 0.97 (0.79–1.20) | 0.803 | 0.84 (0.58–1.21) | 0.346 |
| Gender | 3.78 (1.41–10.10) | 0.008 | 2.64 (0.34–20.77) | 0.356 |
| Site | 1.31 (0.72–2.38) | 0.373 | 11.34 (1.98–64.80) | 0.006 |
| Disease duration | 0.99 (0.92–1.07) | 0.874 | 1.09 (0.97–1.23) | 0.159 |
| LEDD | 0.99 (0.99–1.00) | 0.545 | 1.00 (0.99–1.00) | 0.783 |
| MDS‐UPDRS, part III | 1.00 (0.96–1.04) | 0.925 | 1.05 (0.98–1.12) | 0.183 |
| GDS | 0.87 (0.61–1.24) | 0.435 | 0.87 (0.51–1.91) | 0.970 |
| MoCA | 1.78 (1.41–2.24) | <0.001 | 2.83 (1.67–4.79) | <0.001 |
| PDAQ‐P | 1.10 (1.04–1.17) | 0.001 | 1.23 (1.10–1.37) | <0.001 |
| Total AUCa | 0.90 | 0.96 | ||
| PDAQ‐SP | ||||
| Age | 0.96 (0.90–1.03) | 0.277 | 1.10 (0.99–1.22) | 0.078 |
| Gender | 2.91 (1.15–7.36) | 0.024 | 1.80 (0.30–10.85) | 0.521 |
| Education | 0.97 (0.79–1.19) | 0.742 | 0.90 (0.65–1.25) | 0.533 |
| Site | 1.14 (0.65–2.01) | 0.642 | 3.53 (0.97–12.80) | 0.055 |
| Disease duration | 0.97 (0.90–1.04) | 0.365 | 1.09 (0.98–1.21) | 0.122 |
| LEDD | 1.00 (1.00–1.00) | 0.299 | 1.00 (0.99–1.00) | 0.334 |
| MDS‐UPDRS, part III | 0.99 (0.95–1.03) | 0.683 | 1.03 (0.97–1.09) | 0.385 |
| GDS | 0.92 (0.66–1.27) | 0.605 | 0.88 (0.47–1.65) | 0.687 |
| MoCA | 1.71 (1.38–2.13) | <0.001 | 2.47 (1.53–3.97) | <0.001 |
| PDAQ‐SP | 1.04 (0.99–1.10) | 0.120 | 1.17 (1.07–1.27) | 0.001 |
| Total AUCa | 0.88 | 0.95 | ||
AUC for the fully adjusted model.
Bold text indicates statistical significance (P < 0.05).
PDAQ‐15, Penn Parkinson's Daily Activities Questionnaire–15; PUC, Pacific Udall Center of Excellence in Parkinson's Disease Research; PD‐MCI, Parkinson's disease mild cognitive impairment; PDD, Parkinson's disease dementia; PDAQ‐P, Parkinson's Daily Activities Questionnaire–15, participant; OR, odds ratio; CI, confidence interval; LEDD, levodopa equivalent daily dose; MDS‐UPDRS, Uniform Parkinson Disease Rating Scale–Movement Disorders Society Revision; GDS, Geriatric Depression Scale; MoCA, Montreal Cognitive Assessment; AUC, area under the receiver operating characteristic curve; PDAQ‐SP, Parkinson's Daily Activities Questionnaire–15, study partner.
Figure 1.
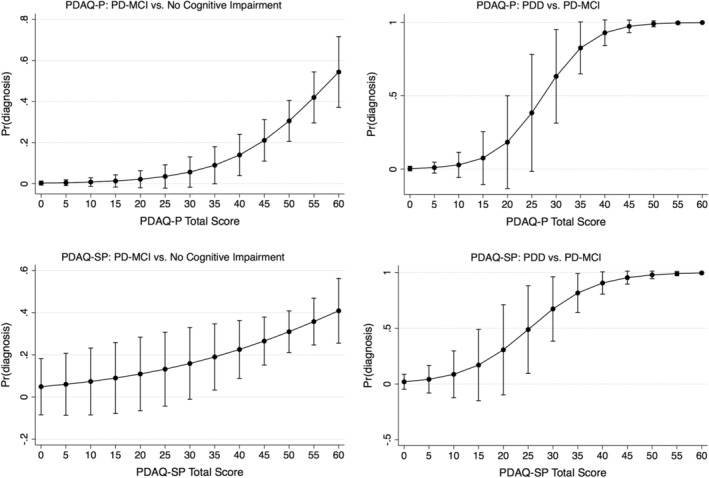
Predicted probabilities as a function of PDAQ‐15 score (in the increments of 5 units) if all covariates were held at the population mean; 95% confidence intervals were estimated based on the fitted logistic regression model. PDAQ‐15, Penn Parkinson's Daily Activities Questionnaire–15; PDAQ‐P, Penn Parkinson's Daily Activities Questionnaire–15, participant; PDAQ‐SP, Penn Parkinson's Daily Activities Questionnaire–15, study partner; PDD, Parkinson's disease dementia; PD‐MCI, Parkinson's disease mild cognitive impairment; Pr, predicted.
Spearman correlations between PDAQ‐P and PDAQ‐SP and the ranked residuals of individual cognitive tests are provided in Figure 2. Most cognitive tests had low to moderate positive correlations with the PDAQ‐P and the PDAQ‐SP. The strongest correlations (either at or approaching moderate correlation strength) for both the PDAQ‐P and PDAQ‐SP were with tests of processing speed, auditory working memory, and immediate verbal recall.
Figure 2.
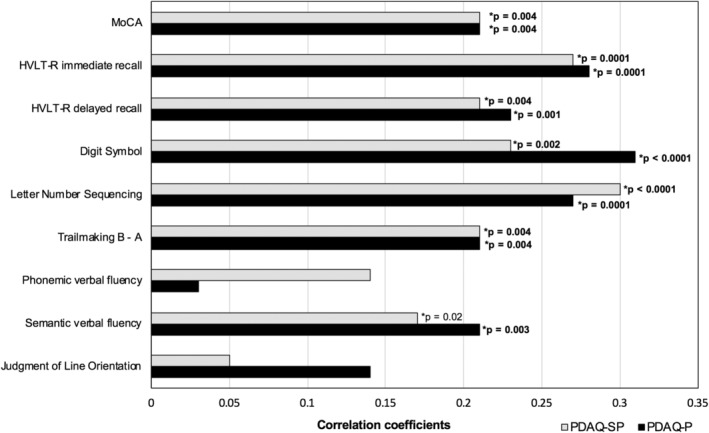
Spearman rank‐order correlations between ranked residuals of PDAQ‐15 scores and cognitive tests. P values are provided for statistically significant correlations between the cognitive tests and PDAQ‐SP or PDAQ‐P. Bold font indicates significance after correcting for multiple comparisons. Trailmaking B‐A scores were reversed in this figure for ease of presentation. HVLT‐R, Hopkins Verbal Learning Test‐Revised; MOCA, Montreal Cognitive Assessment; PDAQ‐15, Penn Parkinson's Daily Activities Questionnaire–15; PDAQ‐P, Penn Parkinson's Daily Activities Questionnaire‐15, participant; PDAQ‐SP, Penn Parkinson's Daily Activities Questionnaire–15, study partner.
Discussion
The current study examines the relationship between cognitive impairment and independent participant and study partner ratings of functional ability in a prevalent sample of participants with PD. We found that, although both participant and study partner ratings of IADL performance were associated with a diagnosis of PDD, only participant self‐ratings of functional ability were significantly associated with a diagnosis of PD‐MCI. Functional ability correlated most strongly with measures of processing speed, auditory working memory, and immediate verbal recall for both the PDAQ‐P and the PDAQ‐SP.
Our results support the use of the PDAQ‐15 as an additional tool to assess the possible presence and/or impact of PDD. These results are consistent with previous reports that the PDAQ‐15 discriminates between participants with and without PDD when administered to a study partner.12 Interestingly, our results do not support the presence of anosognosia reported by others among participants with more advanced cognitive impairment.26 However, our sample size in the PDD group was small (n = 24), and global cognitive scores overlapped with the PD‐MCI group (and was thus more likely to include participants with relatively preserved insight); as a result, this group may not adequately represent patients with more severe cognitive decline.
For participants with PD in earlier stages of cognitive decline (PD‐MCI), our results suggest that self‐ratings may be more sensitive to the impact of cognitive changes on IADL function than ratings made by a knowledgeable study partner. Thus, clinicians who rely on care partner report alone may not gain sufficient insight into the impact of early cognitive decline on IADLs in PD‐MCI. Early studies of dementia, including PDD, indicated that reduced awareness of dementia‐related symptoms is common,27, 28 leading perhaps to increased reliance on care partner report. Anosognosia of motor and cognitive deficits in PD has been reported,29, 30 although larger studies that focus on specific cognitive domains found generally accurate appraisals of memory and executive functions among those with preserved cognition.5, 31 With specific regard to accurate appraisals of functional ability, an earlier study that compared participant self‐ratings of activities of daily living/IADL functions to performance‐based measures found that participants with PD underrate their performance particularly in the areas of eating, medication management, and finances. However, the sample size was small (n = 76), and given the reported range of global cognitive screening scores, the study likely included more severely cognitively impaired participants.4 A larger, more recent study of 385 participants with PD found that anosognosia for nonmotor symptoms was frequent in both mild PDD and more advanced PD‐MCI, but uncommon in early PD‐MCI or in PD participants without cognitive impairment.6 Similarly, a recent study that specifically examined different MCI types (including PD) found that anosognosia was rare.32 Our results support these latter findings and extend these results to address specific differences between participant and study partner reports.
Although we found overall moderate agreement between participant and study partner reports in general, it was notably lower for participants diagnosed with PD‐MCI. A recent study aimed at assessing the degree of difference between participants and study partners on the PDAQ‐15 found discrepancies among the 2 groups that grew with increasing cognitive impairment.26 Conversely, we found the weakest correlation between the 2 sources to be within the PD‐MCI group, again potentially because of the overall higher global cognitive abilities in our cognitively impaired groups. Our results suggest that participants with PD‐MCI may be more acutely aware of cognitive changes and how they may subtly impact IADLs. This could be related to varying levels of interaction that study partners have with participants or that early and subtle functional changes may not provoke concern in family members. Indeed, in a previous study of participants with AD, care partners tended to overestimate IADL performance among those with higher global cognitive functioning.9 Prior studies in PD and MCI suggest generally good agreement between participants and study partners in relation to assessing cognitive abilities,29, 33 although participant self‐reports may be better than study partner reports in the assessment of more frankly concerning symptoms, such as visual hallucinations.34 Here, we provide unique data concerning differences between participants and study partners in the early stages of cognitive decline. Once a diagnosis of PDD is apparent, however, both participant and study partner reports are associated with cognitive diagnosis, suggesting increasing awareness among study partners as the cognitive dysfunction progresses.
We further found that many cognitive tests generally had low but consistent correlations with both the PDAQ‐P and PDAQ‐SP, and the strongest associations were with processing speed, auditory working memory, and verbal learning. Prior research on the relationship between individual cognitive tests and performance of IADLs is mixed. Among the participants with AD, MCI, and healthy older adults, reduced executive performance has been particularly associated with poorer IADLs,35, 36 and interventions to improve executive functions have been suggested as a means to increase independence.37 In PD, memory, executive function, processing speed, language, and visuospatial skills have been variably associated with complex IADLs,10, 11, 38 although other studies that evaluate performance‐based methods of IADL assessment have found no relationship between cognition and functional performance in participants with PD.3, 39 Here, we provide support for associations with both cognitive diagnosis and individual cognitive tests, particularly processing speed and working memory. Clinicians and care partners may thus want to keep a close watch for subtle changes in functional performance that may negatively impact health, safety, and social/intellectual engagement particularly among participants with slowed processing speed and other executive dysfunction. Furthermore, future research into efforts to enhance executive function in PD may eventually lead to more targeted and person‐centered interventions.
There are limitations in the current study. PDAQ ratings are based entirely on self and study partner report, and previous studies have found that performance‐based measures may be more sensitive to real‐world changes in functional abilities.40, 41 However, such measures are often time consuming, and such barriers to feasibility mean that these measures are unlikely to be implemented in clinic and research settings. To date, the PDAQ‐15 has been validated primarily for use with study partners12; in a recently published study, a smaller sample of participant–study partner pairs (n = 61) who completed both the PDAQ‐15 and a performance‐based functional measure found that study partner responses correlated more strongly with functional ability than did participant responses. However, the authors state that the majority of participants included in this sample had significant cognitive impairment.26 Additional validation of the PDAQ‐15 with performance‐based functional measures across all levels of cognitive function in PD will be an important future step. Additional limitations include that a larger sample size is needed to more adequately assess specific relationships between cognitive tests and IADL changes within cognitive groups. Overall correlations between individual tests and IADL function were generally low, suggesting that other factors may also be involved. Although we controlled for important factors such as motor symptom severity, disease duration, LEDD, and depression, other unknown factors have yet to be explored.
The current study provides unique data concerning the relationship between self and study partner report of IADL performance and cognitive impairment in PD and suggests that participants with MCI may be able to more accurately appraise the impact of their cognitive impairments on IADL performance than their study partners. Furthermore, specific cognitive changes may relate more strongly to decline in functional ability. These results support queries by medical professionals into the complex functional activities and specific cognitive functions even prior to the onset of PDD, when practical interventions may be most effective. Future investigations into cognitive interventions aimed at improving performance of complex daily activities is of vital importance and may help to prolong independence in people diagnosed with PD.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
B.C.: 1A, 1B, 1C, 2A, 2B, 3A
K.L.P.: 1C, 2C, 3B
L.T.: 1C, 2A, 3B
J.F.Q.: 1C, 2C, 3B
K.A.C.: 1C, 2C, 3B
A.L.H.: 1C, 2C, 3B
K.S.: 1C, 2C, 3B
S.‐C.H.: 1C, 2C, 3B
T.J.M.: 1A, 2C, 3B
K.L.E.: 1A, 2C, 3B
C.P.Z.: 1A, 1C, 2C, 3B
Disclosures
Ethical Compliance Statement
The institutional review boards at the University of Washington/Veterans Affairs Puget Sound, Oregon Health and Sciences University/Veterans Affairs Portland, and Stanford University provided formal approval for the study. Informed consent procedures were conducted by study personnel with all participants and study partners, who provided written informed consent prior to study participation. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm this work is consistent with those guidelines.
Funding Sources and Conflict of Interest
This work was supported by the Department of Veterans Affairs and National Institutes of Neurological Disorders and Stroke grant P50 NS062684. The funding sources did not provide scientific input for the study. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
Dr. Cholerton is supported by grants from the National Institutes of Health (NIH). Dr. Poston reports honoraria from invited scientific presentations to universities and professional societies not exceeding $5,000 per year, is reimbursed by AstraZeneca and Sangamo BioSciences, Inc. for the conduct of clinical trials, receives consulting fees from Allergan and Curasen, and is funded by grants from the NIH and Michael J. Fox Foundation. Dr. Tian is supported by grants from the NIH. Dr. Quinn is reimbursed by Prothena and Roche for the conduct of clinical trials and by vTv Pharmaceuticals for Data Safety Monitoring Board service. Dr. Quinn is also supported by grants from the NIH and Department of Veterans Affairs. Dr. Chung is funded by a Veterans Affairs Merit Grant. Dr. Hiller is reimbursed by Theravance Inc. for conducting clinical trials and supported by grants from NIH. Ms. Specketer is supported by grants from the NIH. Dr. Hu is funded by grants from the NIH and Michael J. Fox Foundation. Dr. Montine reports honoraria from invited scientific presentations to universities and professional societies not exceeding $5,000 per year and is funded by grants from the NIH and the Farmer Family Foundation, and a gift from Regina and John Scully. Dr. Edwards is funded by grants from the NIH. Dr. Zabetian was supported by grants from the American Parkinson Disease Association, Department of Veteran Affairs, and NIH, and a gift from the Dolsen Foundation.
Supporting information
Supplemental Table S1 Neuropsychological measures used for cognitive diagnosis across Pacific Udall Center of Excellence in Parkinson's Disease Research sites.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Puget Sound Health Care System. We sincerely thank our research subjects and family members for their participation in this study.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Leroi I, McDonald K, Pantula H, Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol 2012;25(4):208–214. [DOI] [PubMed] [Google Scholar]
- 2. Foster ER. Instrumental activities of daily living performance among people with Parkinson's disease without dementia. Am J Occup Ther 2014;68(3):353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pirogovsky E, Schiehser DM, Obtera KM, et al. Instrumental activities of daily living are impaired in Parkinson's disease patients with mild cognitive impairment. Neuropsychology 2014;28(2):229–237. [DOI] [PubMed] [Google Scholar]
- 4. Shulman LM, Pretzer‐Aboff I, Anderson KE, et al. Subjective report versus objective measurement of activities of daily living in Parkinson's disease. Mov Disord 2006;21(6):794–799. [DOI] [PubMed] [Google Scholar]
- 5. Lehrner J, Kogler S, Lamm C, et al. Awareness of memory deficits in subjective cognitive decline, mild cognitive impairment, Alzheimer's disease and Parkinson's disease. Int Psychogeriatr 2015;27(3):357–366. [DOI] [PubMed] [Google Scholar]
- 6. Orfei MD, Assogna F, Pellicano C, et al. Anosognosia for cognitive and behavioral symptoms in Parkinson's disease with mild dementia and mild cognitive impairment: Frequency and neuropsychological/neuropsychiatric correlates. Parkinsonism Relat Disord 2018;54:62–67. [DOI] [PubMed] [Google Scholar]
- 7. Farias ST, Lau K, Harvey D, Denny KG, Barba C, Mefford AN. Early functional limitations in cognitively normal older adults predict diagnostic conversion to mild cognitive impairment. J Am Geriatr Soc 2017;65(6):1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahman‐Filipiak AM, Giordani B, Heidebrink J, Bhaumik A, Hampstead BM. Self‐ and informant‐reported memory complaints: frequency and severity in cognitively intact individuals and those with mild cognitive impairment and neurodegenerative dementias. J Alzheimers Dis 2018;65(3):1011–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loewenstein DA, Arguelles S, Bravo M, et al. Caregivers' judgments of the functional abilities of the Alzheimer's disease patient: a comparison of proxy reports and objective measures. J Gerontol B Psychol Sci Soc Sci 2001;56(2):P78–P84. [DOI] [PubMed] [Google Scholar]
- 10. Anderson SW, Aksan N, Dawson JD, Uc EY, Johnson AM, Rizzo M. Neuropsychological assessment of driving safety risk in older adults with and without neurologic disease. J Clin Exp Neuropsychol 2012;34(9):895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manning KJ, Clarke C, Lorry A, et al. Medication management and neuropsychological performance in Parkinson's disease. Clin Neuropsychol 2012;26(1):45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brennan L, Siderowf A, Rubright JD, et al. The Penn Parkinson's Daily Activities Questionnaire‐15: psychometric properties of a brief assessment of cognitive instrumental activities of daily living in Parkinson's disease. Parkinsonism Relat Disord 2016;25:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988;51(6):745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 15. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 16. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 17. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 18. Cholerton BA, Zabetian CP, Quinn JF, et al. Pacific Northwest Udall Center of Excellence Clinical Consortium: study design and baseline cohort characteristics. J Parkinsons Dis 2013;3(2):205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22(12):1689–1707; quiz 1837. [DOI] [PubMed] [Google Scholar]
- 20. Litvan I, Aarsland D, Adler CH, et al. MDS Task Force on mild cognitive impairment in Parkinson's disease: critical review of PD‐MCI. Mov Disord 2011;26(10):1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benedict RHB, Schretlen D, Groninger L, Brandt J. The Hopkins Verbal Learning Test‐Revised: normative data and analysis of inter‐form and inter‐rater reliability. Clin Neuropsychol 1998;12:43–55. [Google Scholar]
- 22. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 23. Wechsler D. WAiS‐III® Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 24. Wechsler D. Wechsler Adult Intelligence Scale–Revised Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 25. Benton AL, Sivan AB, Hamsher K, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. New York, NY: Oxford University Press; 1994. [Google Scholar]
- 26. Deck BL, Xie SX, Choi G, et al. Cognitive functional abilities in Parkinson's disease: agreement between patients and informants. Mov Disord Clin Pract 2019;6(6):440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Starkstein SE, Sabe L, Chemerinski E, Jason L, Leiguarda R. Two domains of anosognosia in Alzheimer's disease. J Neurol Neurosurg Psychiatry 1996;61(5):485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wagner MT, Spangenberg KB, Bachman DL, O'Connell P. Unawareness of cognitive deficit in Alzheimer disease and related dementias. Alzheimer Dis Assoc Disord 1997;11(3):125–131. [DOI] [PubMed] [Google Scholar]
- 29. Copeland JN, Lieberman A, Oravivattanakul S, Troster AI. Accuracy of patient and care partner identification of cognitive impairments in Parkinson's disease‐mild cognitive impairment. Mov Disord 2016;31(5):693–698. [DOI] [PubMed] [Google Scholar]
- 30. Pietracupa S, Latorre A, Berardelli A, Fabbrini G. Parkinsonian patients and poor awareness of dyskinesias. Front Neurol 2014;5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kudlicka A, Clare L, Hindle JV. Awareness of executive deficits in people with Parkinson's disease. J Int Neuropsychol Soc 2013;19(5):559–570. [DOI] [PubMed] [Google Scholar]
- 32. Pillai JA, Bonner‐Jackson A, Floden D, Fernandez H, Leverenz JB. Lack of accurate self‐appraisal is equally likely in MCI from Parkinson's disease and Alzheimer's disease. Mov Disord Clin Pract 2018;5(3):283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other‐reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry 2005;20(9):827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muller AJ, Mills JMZ, O'Callaghan C, et al. Informant‐ and self‐appraisals on the Psychosis and Hallucinations Questionnaire (PsycH‐Q) enhances detection of visual hallucinations in Parkinson's disease. Mov Disord Clin Pract 2018;5(6):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McAlister C, Schmitter‐Edgecombe M. Everyday functioning and cognitive correlates in healthy older adults with subjective cognitive concerns. Clin Neuropsychol 2016;30(7):1087–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roy S, Ficarro S, Duberstein P, et al. Executive function and personality predict instrumental activities of daily living in Alzheimer disease. Am J Geriatr Psychiatry 2016;24(11):1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hunter EG, Kearney PJ. Occupational therapy interventions to improve performance of instrumental activities of daily living for community‐dwelling older adults: a systematic review. Am J Occup Ther 2018;72(4):7204190050p7204190051–7204190050p7204190059. [DOI] [PubMed] [Google Scholar]
- 38. Becker S, Baumer A, Maetzler W, et al. Assessment of cognitive‐driven activity of daily living impairment in non‐demented Parkinson's patients [published online ahead of print October 15, 2018]. J Neuropsychol. 10.1111/jnp.12173 [DOI] [PubMed] [Google Scholar]
- 39. Pirogovsky E, Martinez‐Hannon M, Schiehser DM, et al. Predictors of performance‐based measures of instrumental activities of daily living in nondemented patients with Parkinson's disease. J Clin Exp Neuropsychol 2013;35(9):926–933. [DOI] [PubMed] [Google Scholar]
- 40. Puente AN, Terry DP, Faraco CC, Brown CL, Miller LS. Functional impairment in mild cognitive impairment evidenced using performance‐based measurement. J Geriatr Psychiatry Neurol 2014;27(4):253–258. [DOI] [PubMed] [Google Scholar]
- 41. Rycroft SS, Giovannetti T, Divers R, Hulswit J. Sensitive performance‐based assessment of everyday action in older and younger adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2018;25(2):259–276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1 Neuropsychological measures used for cognitive diagnosis across Pacific Udall Center of Excellence in Parkinson's Disease Research sites.


