Abstract
A 59-year-old female was brought to our emergency room with severe chest pain. Based on the electrocardiogram (ECG) and echocardiography, an acute coronary syndrome (ACS) was suspected. Her initial ECG showed ST elevation in the inferior leads (II, III, and aVF), which had progressed to involve the anterior leads (V2-V4) by the time she was shifted to the catheterization room. A coronary angiogram revealed total occlusion of the mid-left anterior descending (LAD) artery and a filling defect of the distal right coronary artery. Although we had emergently treated her using thrombus aspiration following stent implantation, lots of thrombi re-formed on the stent. We surmised her ACS was primarily caused by thrombus formation due to polycythemia vera (PV) based on the presence of increased blood consistency on admission. We performed repetitive long-inflation using a perfusion balloon and repeated thrombus aspiration. Finally, she was diagnosed as an untreated case of PV as a result of detailed blood investigations. Thereafter, we successfully treated her using the combination of dual antiplatelet therapy and direct oral anticoagulant therapy. Our experience highlights the importance of an urgent identification of PV. Effective management strategies should be successfully implemented in such patients as soon as possible.
<Learning objective: Polycythemia vera (PV) is an idiopathic, chronic myeloproliferative disease characterized by an increased red blood cell count and hematocrit, which in turn causes systematic thrombosis. A resultant acute myocardial infarction is therefore complicated and difficult to manage, due to the patient’s continuous hypercoagulable state. In the absence of a defined treatment approach, newer and successfully implemented strategies for the management of consequent thrombotic events in PV patients are indispensable to clinicians.>
Keywords: Thrombus, Polycythemia vera, Acute myocardial infarction
Introduction
Polycythemia vera (PV) is a chronic myeloproliferative disease, resultingfrom clonal abnormalities in pluripotent hematopoietic stem cells. The clinical symptoms of PV are insignificant. However, increased red blood cells over time may lead to complications such as thrombosis. An excessive number of red blood cells in the circulation may cause systemic thrombosis and patients may present with a stroke or an acute myocardial infarction (AMI). To date, there is no established treatment approach to this condition. Here, we report a case of PV complicated with AMI, following sequential multi-vessel occlusion.
Case report
A 59-year-old female presented to us at midnight with a complaint of sudden onset, severe chest pain for more than 30 min (at rest). She had been transported to our hospital by an ambulance within 1 h of the onset of symptoms. She had a history of complaints of burning sensation over her face and severe dizziness over the past 6 months. However, she was not aware of PV. The only positive coronary risk factor was a history of hypertension for 5 years, for which she was on treatment with calcium antagonists.
The blood pressure and the heart rate were 92/63 mmHg and 56 beats/min, respectively, with a regular pulse. Her oxygen saturation was maintained at 96% on supplementary oxygen (10 L/min). There were no special findings in physical examination.
Table 1 shows the patient’s laboratory data. The counts of leukocytes were elevated. The levels of enzymes, such as aspartate transaminase, creatinine kinase, and troponin I were not elevated at the time of admission. Her chest radiograph showed no acute abnormalities. An echocardiogram revealed akinesis of the mid-to-apex anteroseptal wall.
Table 1.
The patient’s clinical laboratory data.
| Blood test | Result |
|---|---|
| Leucocytes (/μl) | 15,200 (nv: 5200–12400) |
| Erythrocytes (/μl) | 7.39 × 105 (nv: 4.2–5.2) |
| Hemoglobin (g/dl) | 18.5 (nv: 10–14) |
| Hematocrit (%) | 55.4 (nv: 32–42) |
| Platelet (/μl) | 84.3 × 104 (nv: 13–40) |
| MCV (fl) | 75.0 (nv: 83–100) |
| Prothrombin time (sec) | 14.8 (nv: 11–13) |
| Activated partial thromboplastin time (sec) | 31.7 (nv: 26.1–35.6) |
| D-dimer (μg/mL) | 0.53 (nv: 0–0.4) |
| Albumin (g/dl) | 4.0 (nv: 4.0–5.2) |
| Aspartate transaminase (U/I) | 21 (nv: <50) |
| Alanine transaminase (U/I) | 28 (nv: <50) |
| Lactate dehydrogenase (IU/L) | 348 (nv: 135–460) |
| Triglycerides (mg/dl) | 161 (nv: <150) |
| Low density lipoproteins (mg/dl) | 91 (nv: <130) |
| High density lipoproteins (mg/dl) | 35 (nv: >45) |
| Glucose (mg/dl) | 90 (nv: 60–100) |
| Uric acid (mg/dl) | 6.9 (nv: 3.5–7.0) |
| Blood urea nitrogen (mg/dl) | 20.8 (nv: 10–50) |
| Creatinine (mg/dl) | 0.92 (nv: 0.8–1.4) |
| eGFR (ml/min/1.7 m2) | 49 (nv: >90) |
| Creatinine kinase (IU/L) | 49 (nv: 0–173) |
| CKMB (IU/L) | 13 (nv: 7–25) |
| Sodium (mmol/l) | 142 (nv: 136–146) |
| Potassium (mmol/l) | 4.6 (nv: 3.5–5.1) |
| Chloride (mmol/l) | 106 (nv: 98–108) |
| Iron (μg/dl) | 35 (nv: 50–170) |
| Ferritin (ng/ml) | 9.8 (nv: 5–152) |
| Total iron binding capacity (μg/dl) | 342 (nv: 246–410) |
| Troponin-I (pg/ml) | 13 (nv: <26.2) |
| Thyroxine (ng/ml) | 0.87 (nv: 0.7–1.5) |
| Thyroid stimulating hormone (μU/ml) | 5.6 (nv: 0.4–4.9) |
| Erythropoietin (mU/ml) | 1.0 (nv: 4.2–23.7) |
| Neutrophil alkaline phosphatase score | 360 |
| Glycosylated hemoglobin (%) | 5.9 (nv: 4.9–6.0) |
MCV, mean corpuscular volume; eGFR, estimated glomerular filtration rate; CKMB, creatine kinase myocardial band; nv, normal value.
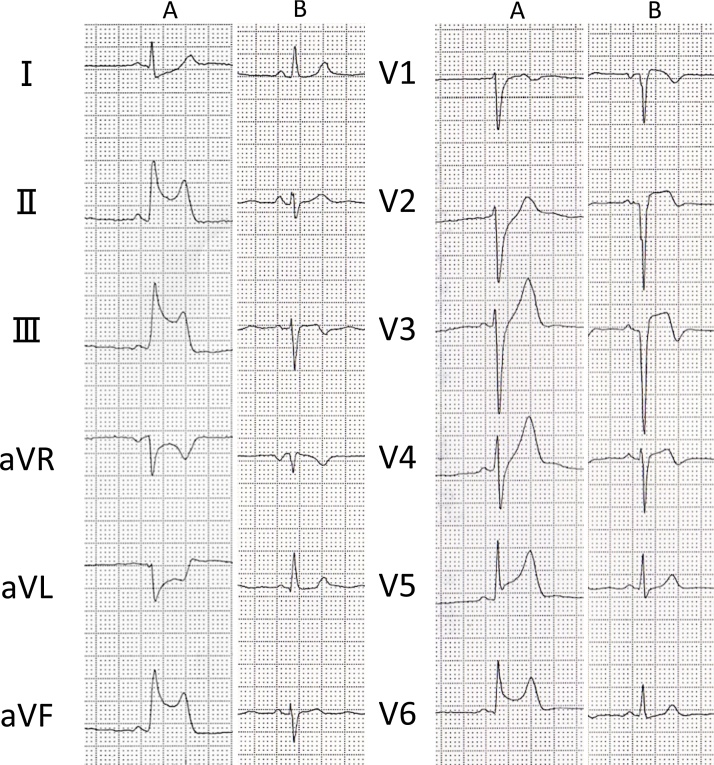
In the emergency room, an initial electrocardiogram (ECG) revealed ST-elevation in leads II, III, aVF, and ST-depression in leads I, aVR, and aVL. However, the ECG repeated after moving the patient to the catheterization room showed that the changes had progressed with ST-elevation now additionally seen in leads V2, V3, and V4. (Fig.1A, B).
Fig. 1.
Electrocardiogram (ECG) images. (A) Initial ECG performed in the emergency room. (B) Repeat ECG performed after shifting patient to catheterization room.
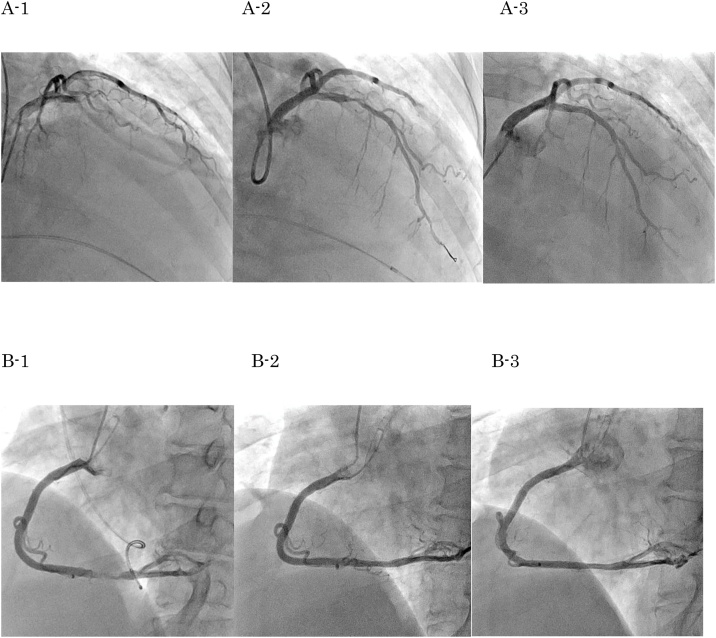
After checking renal function and presence of anemia, an urgent coronary angiogram (CAG) was performed. It showed total occlusion at segment 6 and 90% stenosis with a huge filling defect at segment 3 (Fig. 2A-1,B-1). There was no collateral artery. The thrombolysis in myocardial infarction (TIMI) score of the right coronary artery (RCA) was grade 3. Therefore, we performed an emergency primary percutaneous coronary intervention (PCI) to correct the blockage of the left anterior descending (LAD) artery. Since the patient had severe bradycardia and was in shock, we inserted a temporary pacemaker and placed an intra-aortic balloon pump. She had been administered oral aspirin (200 mg) and prasugrel (20 mg) before the procedure, and intravenous heparin (5000 units) was infused during the PCI. We passed the guidewire through the LAD lesion. The thrombus was aspirated, and we were able to achieve reperfusion (Fig. 2A-2). The door to balloon time was 85 min. As her vital signs were very unstable accompanied by persistent chest pain, we prioritized implanting a drug-eluting stent (Synergy 3.0 × 20 mm; Boston Scientific, Natick, MA, USA) for the LAD lesion without intravascular ultrasound guidance. However, even after proper stent placement, lots of thrombi re-formed on the stent. We re-checked the laboratory data at that time and noticed that she had polycythemia. We started continuous nicorandil and heparin administration. The activated clotting time (ACT) was >300 s. We repeated the thrombus aspiration and performed repetitive prolonged inflations (600 s) with a perfusion balloon (Ryusei 3.0 × 20 mm; Kaneka Corporation, Osaka, Japan) 3 times. Once the thrombus resolved, we completed the PCI to LAD artery. Following the LAD PCI, the thrombus at the distal RCA was observed to have already disappeared (Fig. 2B-2).
Fig. 2.
Coronary angiogram images. (A) Coronary angiogram images of the left anterior descending artery showing: A1- The total occlusion of left anterior descending artery. A2- Reperfusion achieved after implanting stent. As many thrombi appeared, a perfusion balloon was applied. A3- Two weeks later, the thrombus has disappeared. (B) Coronary angiogram images of the right coronary artery showing: B1- Right coronary artery with 90% stenosis due to thrombus. B2- The thrombus has spontaneously disappeared on completion of percutaneous coronary intervention. B3- Two weeks later.
After PCI, we checked the erythropoietin levels and abdominal computed tomography. The patient demonstrated splenomegaly. Bone marrow aspiration revealed three blood cell proliferations and bone marrow hyperplasia, and the erythroblasts were poorly dysplastic. Neutrophil alkaline phosphatase score was normal level. Based on the diagnostic criteria of the World Health Organization classification of tumors of the hematopoietic and lymphoid tissues 2016, we diagnosed the patient with PV because of Hb > 16 g/dL and bone marrow findings; however, erythropoietin level was normal. Unfortunately, we did not perform the JAK2 mutation test.
Following the PCI, we continued heparin administration for 16 days to maintain activated partial thromboplastin time at 60–80 s. Thereafter, we changed heparin to edoxaban (30 mg/day). We discontinued edoxaban after we confirmed the absence of thrombosis within the stent 6 months following the CAG and when her hematocrit (Hct) was under 45%. We administered oral aspirin (100 mg) and prasugrel (3.75 mg) for 6 months and then aspirin monotherapy. Simultaneously, for cytoreduction, we performed multiple phlebotomy procedures during hospitalization, to maintain the Hct at an optimum level (<45%), along with an administration of hydroxyurea (1500 mg/day) for 1 year.
The CAG showed no in-stent re-stenosis and no thrombus in coronary arteries during the 16-day and 6-month follow-up. At the 6-month follow-up, we also performed an ergonovine loading test, which was negative. (Fig. 2A-3, B-3).
A histopathological examination of the thrombus, which was aspirated during PCI showed that it was mainly composed of fibrin and blood components, e.g. a few neutrophils. It was therefore considered to be a red thrombus, without substrate formation.
Discussion
PV is a chronic myeloproliferative disease, resulting from clonal abnormalities of the pluripotent hematopoietic stem cells. There is an abnormal increase in the number of red blood cells and an absolute increase in the number of hematopoietic cells. Leukocytosis, thrombocytosis, splenomegaly, and bone marrow fibrosis is also observed at various stages.
Bleeding due to thrombotic and vascular complications is the major cause of morbidity and mortality in PV [1]. Thrombosis was found to have occurred in 19% of the total 1213 cases of PV observed over a period of 20 years. Of these, 21.7% cases were reported to have developed myocardial infarction [2].
The definitive pathophysiology of thromboembolic events in PV has not been elucidated, but many factors are recognized as contributory, such as an increase in the Hct and the resultant blood hyperviscosity, a stimulation of platelet aggregation and thrombogenesis, the presence of leukocytosis, rigidity of the vascular membrane, and intimal proliferation [3]. Pearson et al. examined the relationship between Hct, platelet count, and the frequency of vascular disorder in 69 patients with PV and found a significant correlation between increase in Hct and frequency of vascular disorders [4].
Advanced age and prior history of a thrombotic episode are the two most important risk factors to predict future vascular complications. Additionally, hypercholesterolemia, hypertension, smoking, and diabetes have been identified as predictors of thrombosis [5].
Based on her clinical symptoms and blood test results, this case matched the diagnostic criteria of PV. Therefore, this case was diagnosed as an AMI, complicated by PV. Other thrombosis-promoting risk factors (such as atrial fibrillation or a malignant tumor) were not detected. An increase in the blood viscosity, subsequent to an increase in Hct, is considered to be the major cause of thrombotic events. As the patient had a history of hypertension, it was conceivable that the presence of arteriosclerosis due to hypertension may also have contributed to the onset. In order to reduce the risk of thrombosis, it is necessary to maintain the Hct level at <45%, but as our patient was untreated, the Hct was remarkably high at 55%. This translates to the patient being at a very high risk of imminent thrombosis at the time.
As for the treatment, cytoreductive therapy using phlebotomy and hydroxyurea administration are a prerequisite. The European collaboration on low-dose aspirin in polycythemia vera (ECLAP) investigators have reported that aspirin is also effective for the prevention of thrombosis in PV and can lead to a 60% reduction in the risk of non-fatal myocardial infarction, nonfatal stroke, and death from cardiovascular complications [6], [7]. Barbui et al. also reported that long-term oral anticoagulation led to 63% reduction in the risk of recurrence after the first venous thrombosis compared with patients without antithrombotic treatment [7]. Ianotto et al. reported that the direct administration of an oral anticoagulant (DOAC) can reduce the number of thrombotic events to the same extent as antiplatelet therapy [8]. In addition, DOAC suppresses the occurrence of bleeding complications that are seen with warfarin administration. The DOAC is expected to become the norm for the prevention of thrombosis in the future.
In this case, if we were able to be aware of PV before PCI, we should avoid implanting stent. However, we used not only cytoreductive therapy but also anticoagulation therapy to manage PV and prevent thrombus formation. We utilized heparin in the acute phase and DOAC in the maintenance phase to prevent thrombosis. The patient was administered DOAC for 6 months, and no further recurrence of thrombosis or bleeding complications were observed.
There is no established treatment for PV as yet. The search for newer and effective strategies for the management of cardiovascular events in PV patients will undoubtedly be a priority for researchers in the future.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
None.
References
- 1.Murphy S., Iland H., Rosenthal D., Laszlo J. Essential thrombocythemia: an interim report from the polycythemia vera study group. Semin Hematol. 1986;23:177–182. [PubMed] [Google Scholar]
- 2.Barbui T., Finazzi G., De Gaetano G., Marchioli R., Tognoni G., Patrono C. Polycythemia vera: the natural history of 1213 patients followed for 20 years. Ann Intern Med. 1995;123:656–664. doi: 10.7326/0003-4819-123-9-199511010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert H.S. Current management in polycythemia vera. Semin Hematol. 2001;38:25–28. doi: 10.1016/s0037-1963(01)90137-4. [DOI] [PubMed] [Google Scholar]
- 4.Pearson T.C., Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet. 1978;2:1219–1222. doi: 10.1016/s0140-6736(78)92098-6. [DOI] [PubMed] [Google Scholar]
- 5.Landolfi R., Di Gennaro L., Barbui T., De Stefano V., Finazzi G., Marfisi R. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109:2446–2452. doi: 10.1182/blood-2006-08-042515. [DOI] [PubMed] [Google Scholar]
- 6.Landolfi R., Marchioli R., Kutti J., Gisslinger H., Tognoni G., Patrono C. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114–124. doi: 10.1056/NEJMoa035572. [DOI] [PubMed] [Google Scholar]
- 7.Barbui T., Finazzi G., Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122:2176–2184. doi: 10.1182/blood-2013-03-460154. [DOI] [PubMed] [Google Scholar]
- 8.Ianotto J.C., Couturier M.A., Galinat H., Mottier D., Berthou C., Guillerm G. Administration of direct oral anticoagulants in patients with myeloproliferative neoplasms. Int J Hematol. 2017;106:517–521. doi: 10.1007/s12185-017-2282-5. [DOI] [PubMed] [Google Scholar]