Summary
Selectively breaking the C-O bonds within biomass during catalytic fast pyrolysis (CFP) is desired, but extremely challenging. Herein, we develop a series of metal-oxide nanocomposites composed of W, Mo, Zr, Ti, or Al. It is demonstrated that the nanocomposites of WO3-TiO2-Al2O3 exhibit the highest deoxygenation ability during CFP of lignin, which can compete with the commercial HZSM-5 catalyst. The nanocomposites can selectively cleave the C-O bonds within lignin-derived phenols to form aromatics by direct demethoxylation and subsequent dehydration. Moreover, the nanocomposites can also achieve the selective breaking of the C-O bonds within xylan and cellulose to form furans by dehydration. The Brønsted and Lewis acid sites on the nanocomposites can be responsible for the deoxygenation of lignin and polysaccharides, respectively. This study provides new insights for the rational design of multifunctional catalysts that are capable of simultaneously breaking the C-O bonds within lignin and polysaccharides.
Subject Areas: Catalysis, Biomass, Chemical Engineering
Graphical Abstract
Highlights
-
•
WO3-TiO2-Al2O3 (WTA) can selectively break the C-O bonds within biomass
-
•
Lignin and polysaccharides are respectively converted into aromatics and furans
-
•
Brønsted and Lewis acid sites of WTA are responsible for the C-O bond cleavages
Catalysis; Biomass; Chemical Engineering
Introduction
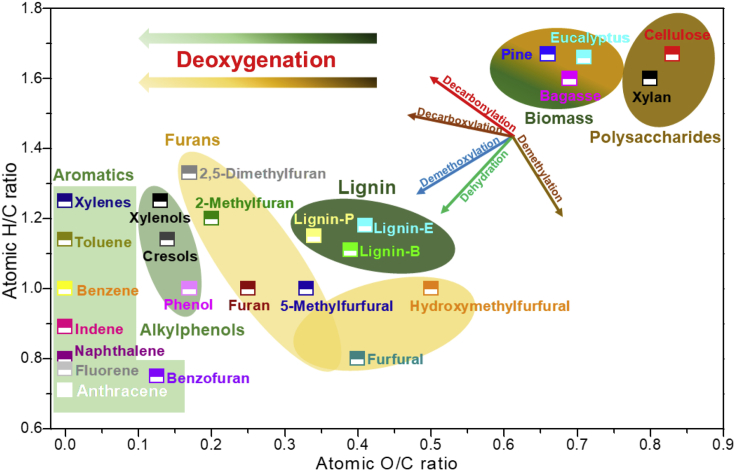
With the increasing societal concerns regarding fossil fuel shortages and climate change, lignocellulosic biomass has emerged as a potential alternative to fossil fuels for the production of chemicals and liquid fuels (Custodis et al., 2014, Huber et al., 2006, Liu et al., 2015, Meng et al., 2017, Rahimi et al., 2014, Roman-Leshkov et al., 2007). Lignocellulosic biomass generally consists of three major components: hemicellulose, cellulose, and lignin. Lignin is an amorphous, cross-linked heteropolymer composed of three phenylpropane units that vary in their degree of methoxylation: sinapyl, coniferyl, and p-coumaryl alcohol (Li et al., 2015a, Liu et al., 2016, Wang et al., 2015b). Cellulose and hemicellulose, the most typical structural polysaccharides, are polymers composed of six-carbon and five-carbon sugar units, typically glucose and xylose (Patwardhan et al., 2010, Patwardhan et al., 2011, Wang et al., 2017). The pre-existing aromatic structures of lignin and the pyranose/furanose ring structures of polysaccharides tend to render them very suitable for the production of aromatics and furans, respectively. However, one fundamental challenge is that both the lignin and polysaccharides are highly oxygen-rich when compared with their respective platform chemicals (aromatics and furans) (Kusumoto and Nozaki, 2015, Shiramizu and Toste, 2012) (as shown in Figure 1).
Figure 1.
The van Krevelen Diagram of Feedstocks and Their Respective Target Products
The organosolv lignin derived from pine, eucalyptus, and bagasse is denoted lignin-P, lignin-E, and lignin-B, respectively.
The C-O bonds within lignin, hemicellulose, and cellulose exist in very different chemical environments. The major oxygen-containing functional groups within lignin are aryl ethers, methoxyls, hydroxyls (phenolic and aliphatic), and carbonyls (Wang et al., 2009). The oxygen within cellulose and hemicellulose exists predominantly in the forms of aliphatic ethers (glycosidic bond) and aliphatic hydroxyls (Li et al., 2012). The deoxygenation of biomass proceeds by the cleavage of the C-O and C-C bonds. The former comprises dehydration (the elimination of H2O) and demethoxylation (the elimination of CH3OH), whereas the latter consists of decarbonylation (the elimination of CO) and decarboxylation (the elimination of CO2) (Dayton et al., 2015). The deoxygenation of biomass via decarbonylation and decarboxylation can cause unnecessary carbon losses, resulting in a reduction in both the liquid yield and energy recovery. Hence, selectively and simultaneously breaking the C-O bonds present in the different chemical environments is crucial to achieving the efficient deoxygenation of biomass. Recently, oxophilic metals have exhibited promising activities and selectivities for the cleavage of the C-O bonds within biomass through the strong interactions between the oxygen-containing functional groups and the oxophilic metal (Chen et al., 2018b, Robinson et al., 2016, Wan et al., 2018). Wang and coworkers reported that the simultaneous hydrodeoxygenation of cellulose, hemicellulose, and lignin into hexane, pentane, and alkylcyclohexanes could be achieved by a multifunctional Pt/NbOPO4 catalyst under solvothermal conditions (Xia et al., 2016). Lu demonstrated that Re2O7 can selectively cleave the C-O bonds within lignin (Qi et al., 2018). Sanna reported that the catalytic pyrolysis of biomass over activated olivine and activated serpentine was able to reduce the oxygen content of bio-oil by up to 40% (Sanna and Andrésen, 2012). Shanks, Nimlos, and Román-Leshkov showed that the pyrolysis vapors from biomass can be hydrodeoxygenated to form alkanes and aromatics using MoO3 or supported MoO3 under H2 atmosphere (Murugappan et al., 2016, Nolte et al., 2016).
Despite these advances, there remains a challenge in designing a multifunctional catalyst capable of simultaneously converting lignin, hemicellulose, and cellulose into their respective aromatics and furans by deoxygenation reactions. Catalytic fast pyrolysis (CFP) is one of the most promising methods to directly convert biomass into highly deoxygenated molecules over catalysts at middle temperature (450°C–650°C), high heating rate (>1,000°C/s), short reaction time (several seconds), and atmospheric pressure without the need for a H2 supply (Carlson et al., 2008, Li et al., 2015b, Wang et al., 2009, Wang et al., 2014, Zhang et al., 2009a). The deactivated solid catalysts can be recycled after burning off the coke in a regenerator. Currently, renewable aromatics can be produced by the CFP of biomass over various zeolite catalysts, such as HZSM-5, Hβ, HY, HUSY, MCM-41, FCC, and hierarchical zeolites (Du et al., 2013, Iliopoulou et al., 2012, Jae et al., 2011, Jia et al., 2017, Kelkar et al., 2015, Wang et al., 2014, Wang et al., 2018, Zhang et al., 2013). HZSM-5 has exhibited the highest aromatic yield due to its strong acidity and shape selectivity (Cheng et al., 2012). During the CFP of biomass over zeolites, the first step is the thermally induced cleavage of the C-C and C-O bonds within the entire biomass to generate various pyrolysis intermediates (e.g., anhydrosugars, furans, phenols, alcohol, aldehydes, and ketones). The pyrolysis intermediates subsequently diffuse into the pore channels of the zeolite and further undergo a variety of cracking, deoxygenation, oligomerization, cyclization, and aromatization reactions at the Brønsted acid sites to form olefins and aromatics via hydrocarbon pool mechanism (Carlson et al., 2011, Hoff et al., 2017, Yang et al., 2017, Zhang et al., 2009b). In addition, renewable furans (e.g., furfural) can be produced by CFP. Blasi and Lu have shown Lewis acids, such as Fe2(SO4)3 and ZnCl2, to be the most effective catalysts for the selective production of furfural from the fast pyrolysis of corncob (Branca et al., 2012, Lu et al., 2011). Hence, a catalyst comprising both Brønsted and Lewis acid sites could be a suitable candidate for the coproduction of aromatics and furans from the CFP of biomass. Lewis acidity appears in γ-Al2O3, γ-Ga2O3, and TiO2. Brønsted acidity exists in oxides of elements with formal valences higher than four (WO3, MoO3, V2O5, Nb2O5, and S-containing oxides) (Fernández-García and Rodriguez, 2011).
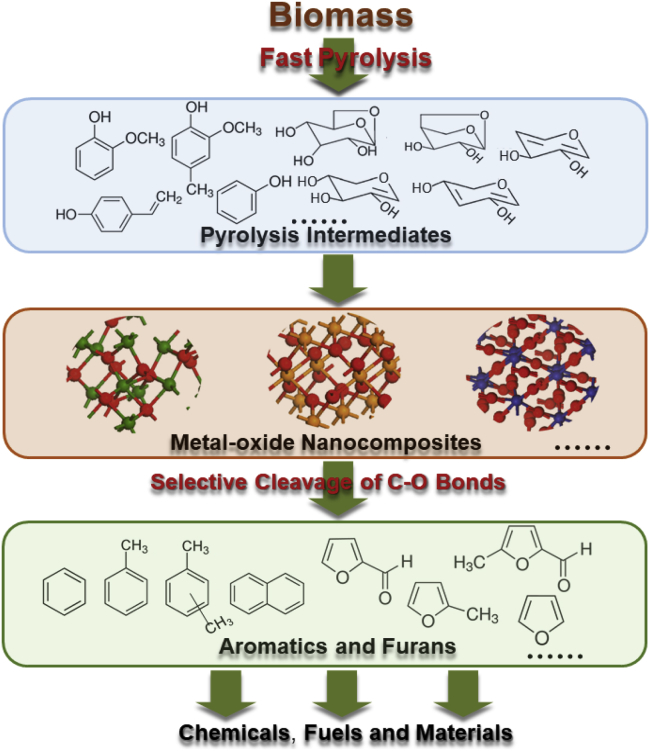
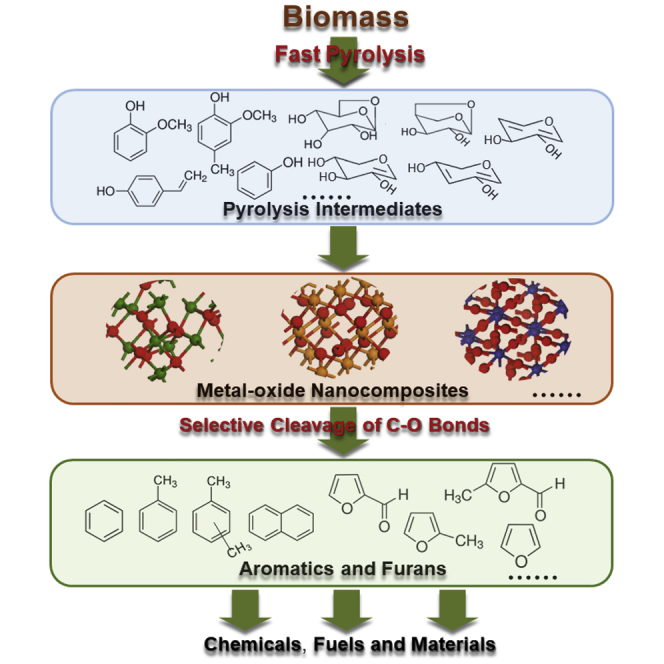
Here, we propose a new pyrolytic strategy for the production of value-added chemicals and platform chemicals from biomass (as shown in Figure 2). The CFP of three main components of biomass (organosolv lignin, xylan, and cellulose) over metal-oxide nanocomposites is first investigated for catalyst screening. Then the performance of the CFP of real biomass (bagasse, eucalyptus, and pine) over the metal-oxide nanocomposites was verified. It is found that lignin and polysaccharides can be, respectively, converted into aromatics and furans over the metal-oxide nanocomposites composed of W, Ti, and Al. The nanocomposites can selectively break the C-O bonds within lignin-derived phenols to generate aromatics by direct demethoxylation and dehydration reactions, and they can also selectively cleave the C-O bonds within xylan and cellulose to form furans by dehydration reactions. The Brønsted and Lewis acid sites of the nanocomposites may be responsible for the deoxygenation of lignin and polysaccharides, respectively.
Figure 2.
The Proposed Pyrolytic Strategy for Producing Aromatics and Furans from Biomass
Results and Discussion
Synthesis and Characterization of the Metal-Oxide Nanocomposites
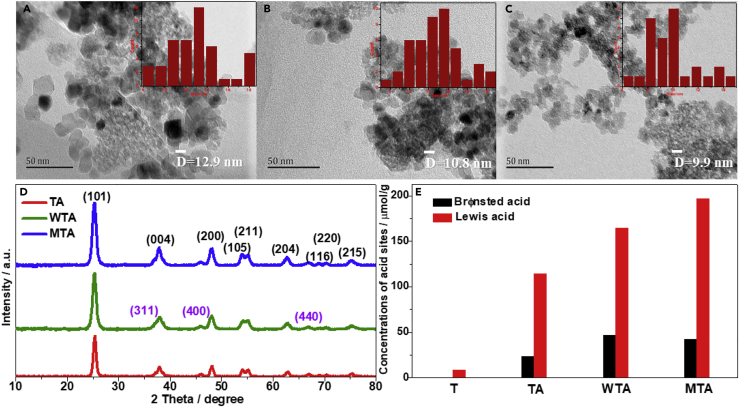
The metal-oxide nanocomposites, e.g., TiO2-Al2O3 (TA), ZrO2-Al2O3 (ZA), ZrO2-TiO2 (ZT), WO3-TiO2-Al2O3 (WTA), WO3-ZrO2-Al2O3, MO3-TiO2-Al2O3 (MTA), and WO3-ZrO2-TiO2 were prepared using a coprecipitation method (see “Transparent Methods” for preparation and characterization). The acronyms in parentheses are formed by combining the initial letters of these metal oxides. Transmission electron microscopic images (Figures 3A–3C) show that the average sizes of TA, WTA, and MTA samples are estimated to be 12.9, 10.8, and 9.9 nm, respectively. The X-ray diffraction (XRD) patterns of TA, WTA, and MTA are shown in Figure 3D. It is evident that the three samples have very similar XRD patterns. The diffraction peaks at 2θ = 25.2°, 37.8°, 48.1°, 54.0°, 55.1°, 62.8°, 68.9°, 70.4°, and 75.2° are assigned to the reflections from the (101), (004), (200), (105), (211), (204), (116), (220), and (215) crystal planes of anatase TiO2 (JCPDS No. 21-1272), respectively (Chen et al., 2018a). The small diffraction peaks at 37.1°, 45.8°, and 66.8° are attributed to the reflections from the (311), (400), and (440) crystal planes of γ-Al2O3 (JCPDS No.29-0063), respectively (Xiong et al., 2019). No independent peaks corresponding to the crystalline planes of WO3 or MO3 are observed in the XRD patterns of WTA and MTA, indicating that the incorporated WO3 and MO3 are dispersed homogeneously over the TiO2 and Al2O3 supports and exist in poorly crystalline and amorphous forms. The concentrations of Brønsted and Lewis acid sites on the metal-oxide nanocomposites are given in Figure 3E. Only small quantities of Lewis acid sites are found in nano-TiO2 (T), whereas both Brønsted and Lewis acid sites are observed in TA, WTA, and MTA. The rank order for the concentrations of Brønsted acid sites is WTA > MTA > TA > T, whereas the rank order for the concentrations of Lewis acid sites is MTA > WTA > TA > T. It is generally accepted that the oxygen vacancies exposed on the surface of metal-oxide nanocomposites supply coordinatively unsaturated metal cations that act as Lewis acid sites. The interaction with water converts the surface oxygens of the metal-oxide nanocomposites into hydroxyl groups, and the hydrogens in the hydroxyl groups behave as Brønsted acid sites (Guntida et al., 2019, Kim et al., 2019, Koodali, 2006). As WTA as an example, interaction of the tungsten species with the surface of TiO2 or Al2O3 (wetting phenomenon) can result in the formation of surface mono-oxo wolframyl groups with coordinatively unsaturated structure, which act as strong Lewis acid sites. As a result of adsorption of water, the coordination number of tungsten increases and the site behaves as a strong Brønsted acid site (Cristiani et al., 1993, Guntida et al., 2019, Gutiérrez-Alejandre et al., 1998). It is thus concluded that the mono-oxo wolframyl groups and hydrated wolframyl groups on the surface of WTA are, respectively, responsible for the formation of strong Lewis and Brønsted acid sites.
Figure 3.
Characterization of the Metal-Oxide Nanocomposites
(A–C) Transmission electron microscopic images of (A) TA, (B) WTA, and (C) MTA.
(D) Powder XRD patterns of TA, WTA, and MTA.
(E) The concentrations of acid sites in T, TA, WTA, and MTA.
Catalytic Fast Pyrolysis of Lignin over the Nanocomposites
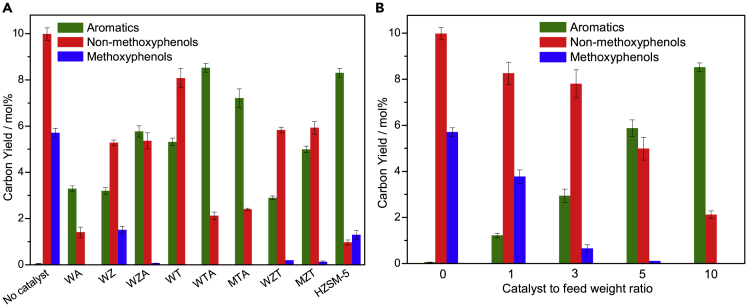
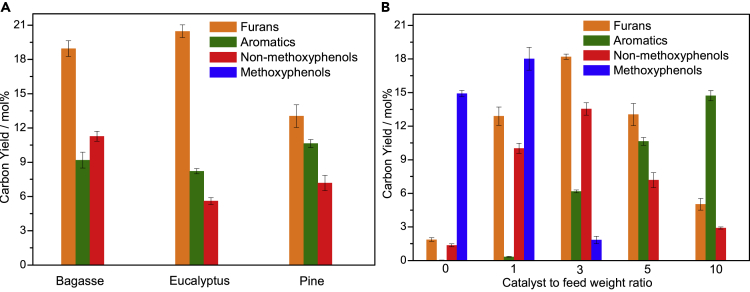
Organosolv lignin derived from bagasse was selected as the feedstock. The major products from the CFP of the organosolv lignin include various methoxyphenols, non-methoxyphenols, aromatics, char/coke, and permanent gas. In this study, we mainly focus on the formation of target products: monophenols (methoxyphenols and non-methoxyphenols) and aromatics. Methoxyphenols are defined as a class of compounds that comprise one or more methoxy groups (OCH3) directly bound to the aromatic ring in phenols. Non-methoxyphenols are defined as a class of monophenols without methoxy groups. The non-methoxyphenols from the fast pyrolysis of organosolv lignin without the use of nanocomposites are mainly composed of alkenylphenols (mainly vinyl, propenyl, and allyl phenols), phenol, and alkylphenols (mainly cresols, ethylphenols, and xylenols). Herein, nanoscale TiO2 (T), ZrO2 (Z), and Al2O3(A) are used as catalysts in the CFP of organosolv lignin. However, no aromatics are observed in the pyrolysis products. Surprisingly, it is found that the metal-oxide nanocomposites, TA, ZA, and ZT, are able to produce aromatics and non-methoxyphenols. The non-methoxyphenols from CFP consist of phenol and alkylphenols (mainly cresols, ethylphenols, xylenols, ethyl cresols, and trimethylphenols). The aromatics comprise benzene, toluene, xylenes, alkylbenzenes, naphthalenes, benzofurans, indenes, anthracenes, fluorenes, and phenanthrenes. As shown in Figure S1, the rank order for the aromatic yield is TA > ZT > ZA. The aromatic yield from the CFP of lignin can be further improved by the introduction of WO3 (W) and MoO3 (M) into the Zr-, Ti-, or Al-based nanocomposites. It is well known that the metal-oxide nanocomposites of WO3, MoO3, ZrO2, TiO2, or Al2O3 are robust solid acidic catalysts with high thermal stabilities (Barton et al., 1998). As shown in Figure 4A, WTA and MTA exhibit the best catalytic performance of the nanocomposites in terms of their deoxygenation abilities. The aromatic yields from the CFP of lignin over WTA are close to those over HZSM-5, indicating that these catalysts can compete with HZSM-5 in the CFP of lignin. It should be noted that no aromatics are found during the CFP of lignin over T. The catalysts produce aromatics when Al2O3 is incorporated into T. Further incorporation of WO3 or MO3 into TA enhances the aromatic yield. The results suggest that the rank order for the deoxygenation activities appears to be the same as the concentrations of Brønsted acid sites present in the nanocomposites. The aromatic selectivity from the CFP of lignin over the different nanocomposites is shown in Figure S2. WZ, MTA, and WTA show the highest selectivities toward benzene, toluene, and xylenes (BTX). A significant proportion of alkylaromatics, especially alkylbenzenes, are formed via alkylation reactions during the CFP of lignin. Among the nanocomposites, WT exhibits the best performance in terms of alkylation.
Figure 4.
The Carbon Yields of Aromatics and Phenols from the CFP of Organosolv Lignin Derived from Bagasse at 600°C
(A and B) (A) CFP over different nanocomposites with a catalyst-to-feedstock weight ratio of 10 and (B) CFP over WTA with different catalyst-to-feedstock weight ratios, where no catalyst is denoted as a catalyst-to-feedstock weight ratio of 0.
The effects of the catalyst-to-feedstock weight ratio on the carbon yields of the aromatics and phenols from the CFP of lignin are illustrated in Figure 4B. The yields of aromatics, methoxyphenols, and non-methoxyphenols are dependent upon the catalyst-to-feedstock weight ratio, indicating that the extent of deoxygenation during CFP of lignin, that is, the selectivity toward aromatics, methoxyphenols, or non-methoxyphenols, can be readily tuned by controlling the catalyst-to-feedstock weight ratio. As the catalyst-to-feedstock weight ratio increases from 0 to 10, the yields of the methoxyphenols and non-methoxyphenols decrease, whereas the yields of the aromatics significantly increase. The results are in accordance with the CFP of lignin over zeolites (Jan et al., 2015, Kim et al., 2015, Luo et al., 2019). It should be noted that the sum of the yields of the methoxyphenols, non-methoxyphenols, and aromatics decreases with increasing catalyst-to-feedstock weight ratio. These results may be due to the increased severities of deoxygenation in the hydrogen-deficient environments of CFP promoting hydrogen transfer reactions. As shown in Figure S3, the carbon yields of char/coke from the CFP of organosolv lignin are approximately 50%–71%. The results are in line with those in literatures, which reported that the carbon yields of char/coke and permanent gas from the CFP of biomass over zeolites or metal oxides were 30%–75% and 20%–50%, respectively (Carlson et al., 2011, Murugappan et al., 2016, Wang et al., 2015a).
Possible Reaction Pathways for the Catalytic Fast Pyrolysis of Lignin
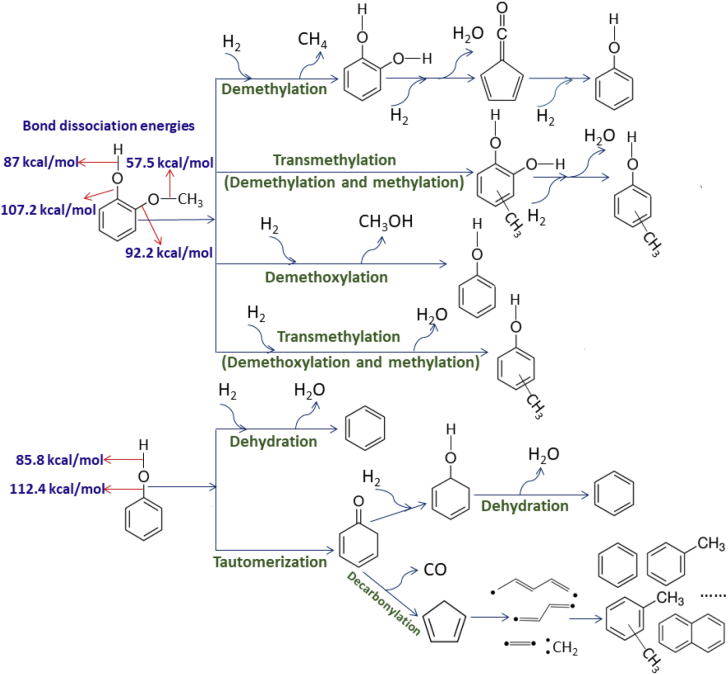
In the CFP of lignin, the first step is the thermal depolymerization of lignin to form various phenols. The main oxygen-containing groups in these phenols are phenolic hydroxyl and methoxy groups. These phenols subsequently diffuse onto the catalyst surface to undergo deoxygenation reactions. As shown in Figure 5, the deoxygenation of phenol to form benzene can proceed by three distinct pathways: the tautomerization-dehydration and tautomerization-decarbonylation routes starting from the cleavage of the O-H bond and a direct dehydration route via the breaking of the Caryl–OH bond (Lup et al., 2017, Rogers and Zheng, 2016, Teles et al., 2018). There are four generally accepted pathways for removing the methoxy group from guaiacol: (1) cleavage of the O–CH3 bond to produce methane (demethylation) and catechol, (2) transmethylation reactions that can be considered to be the results of demethylation of guaiacol and subsequent methylation of catechol, (3) splitting of the Caryl–OCH3 bond to produce methanol (demethoxylation) and phenol, and (4) transmethylation reactions that can be considered to be the results of demethoxylation of guaiacol and subsequent methylation of phenol (Rogers and Zheng, 2016). The bond dissociation energies for O–H, Caryl–OH, O–CH3, and Caryl–OCH3 in guaiacol are 87.0, 107.2, 57.5, and 97.2 kcal/mol, respectively (Kubiak, 1995, Schlaf and Zhang, 2015, Vuori and Bredenberg, 1987). Although the bond dissociation energies can change by 1–10 kcal/mol between double substituted benzenes (e.g., guaiacol) and mono-substituted benzenes (e.g., phenol), the rank order of the bond dissociation energies is approximately the same: O–CH3 < O–H < Caryl–OCH3 < Caryl–OH. The higher bond dissociation energies of the Caryl–OCH3 and Caryl–OH bonds make their preferential cleavage very difficult to achieve.
Figure 5.
The Bond Dissociation Energies for Typical Bonds within Phenol and Guaiacol, and the Possible Pathways for the Catalytic Conversion of Phenol and Guaiacol into Aromatics
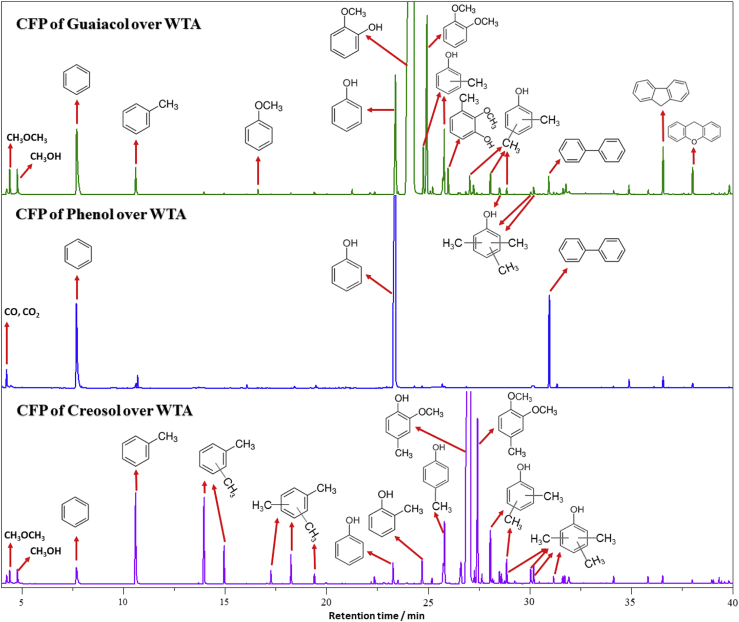
To further understand the reaction mechanism of the CFP of lignin over the nanocomposites, phenol, guaiacol, and creosol are selected as probe molecules to identify the possible pathways. The total ion chromatograms resulting from the CFP of guaiacol, phenol, and creosol over WTA are shown in Figure 6. The major products from the CFP of guaiacol are 1,2-dimethoxy benzene, cresols, creosol, phenol, benzene, toluene, xylenols, methanol, dimethyl ether (DME), biphenyl, fluorene, xanthene, and trimethylphenols. Very small amounts of CO and CO2 are also observed in the product stream, indicating that decarbonylation and decarboxylation are not the primary reactions that occur during the CFP of guaiacol. Large amounts of 1,2-dimethoxy benzene, cresols, creosol, xylenols, and trimethylphenols present in the product stream indicate that the transmethylation reaction is one of the major reactions during CFP. There are only two possible pathways for the formation of transmethylation products: (1) demethylation and subsequent methylation and (2) demethoxylation and subsequent methylation (as shown in Figure 5). Considerable quantity of phenol and the very small quantity of anisole present in the product stream imply that demethoxylation is the initiating step for the CFP of guaiacol. Catechol is not found from the CFP of guaiacol, suggesting that demethylation is not a predominant reaction pathway during the CFP of guaiacol. The 1,2-dimethoxy benzene, cresols, creosol, xylenols, and trimethylphenol products are presumed to be obtained via the C-methylation and O-methylation of various reactants and intermediates with methanol and DME over the nanocomposites. The 1,2-dimethoxy benzene is generated by the O-methylation of guaiacol with methanol and DME, whereas the cresols, xylenols, and trimethylphenols are produced by consecutive C-methylation reactions of phenol with methanol and DME. Cooperation of the acid and base sites is typically required for C-methylation and O-methylation reactions (Vishwanathan et al., 2008). As shown in Figure S4, CO2 desorption confirms the basicity of TA, WTA, and MTA. Toluene is obtained by either the dehydration of cresols or the methylation of benzene with methanol. These C-methylation and O-methylation reactions, taken together with the phenol, methanol, and DME present in the product stream, strongly suggest that direct demethoxylation of guaiacol is the major pathway for the deoxygenation of guaiacol over WTA. The main products from the CFP of creosol include 3,4-dimethoxytoluene, toluene, xylenes, trimethylbenzene, cresols, xylenols, and trimethylphenols. The similar product distribution from the CFP of creosol as that from guaiacol further verifies the proposed mechanism.
Figure 6.
Total Ion Chromatograms Resulting from the CFP of Guaiacol, Phenol, and Creosol over WTA at 600°C with a Catalyst-to-Feedstock Weight Ratio of 5
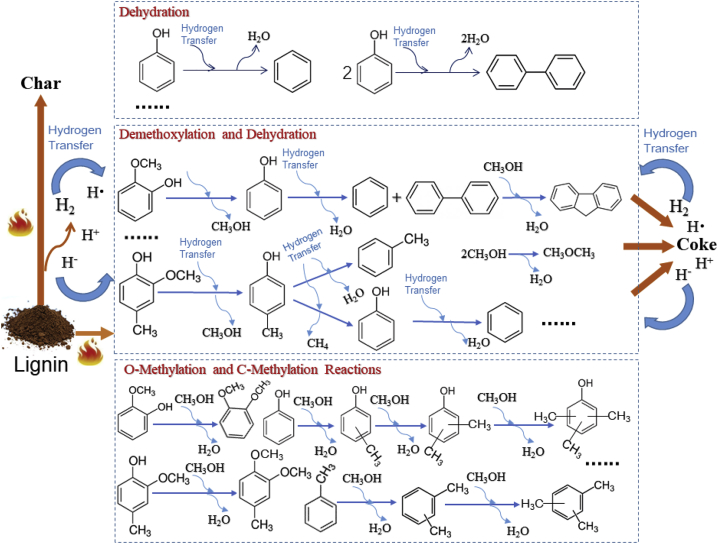
The possible pathways for the CFP of lignin are illustrated in Figure 7. It is postulated that the hydrogen transfer reactions that occur during the CFP of lignin eliminate the need for an external hydrogen source. H2 and active hydrogen species (hydrogen radicals, hydrides, and protons) derived from the dehydrogenation, polycondensation, and carbonization reactions during the CFP of lignin can serve as hydrogen donors to cleave the C-O bonds within the various phenols. The proposed pathways for CFP of guaiacol over WTA are significantly different from those over zeolites. Hemberger and coworkers have reported that fulvenone, generated by the catalytic demethylation and subsequent dehydration of guaiacol, is the central reactive intermediate during the CFP of guaiacol over HUSY (Hemberger et al., 2017). The primary products from the CFP of phenol are benzene and biphenyl, strongly indicating that benzene is mainly formed by the direct dehydration of phenol via a free radical mechanism. Phenol first undergoes homolytic fission of the Caryl–OH bond to form aryl and hydroxyl radicals. The hydrogen radicals are subsequently added to the aryl and hydroxyl radicals to form benzene and water. Biphenyl is generated by the recombination of two aryl radicals. Thilakaratne and coworkers demonstrated that the major products from the CFP of phenol over HZSM-5 are benzene, naphthalene, biphenyl, alkylated naphthalenes, higher polyaromatic hydrocarbons, and olefins. They proposed that the aromatics produced from the CFP of phenol over HZSM-5 are generated via both a direct dehydration route and a tautomerization-decarbonylation route, with the latter being the predominant route (Thilakaratne et al., 2016). Olefins are not observed from CFP of phenol, guaiacol, and creosol over WTA. These results strongly suggest that the aromatics are predominantly produced by the direct demethoxylation and dehydration of lignin-derived phenols. However, olefins are detected during CFP of lignin. The olefins may be formed by the removal of alkenyl side chains from alkenylphenols. It is supposed that the nanocomposites are capable of selectively reducing the dissociation energies of the Caryl–OCH3 and Caryl–OH bonds of the adsorbed phenols. The high temperature present during CFP facilitates the selective cleavage of these bonds (Shao et al., 2017). However, the actual mechanisms for the CFP of lignin are much more complex than those for the CFP of model compounds. Large quantities of polycyclic aromatics are detected during the CFP of lignin over the nanocomposites. These results may be due to the rearrangement, recombination, and polycondensation of various lignin-derived reactive intermediates (e.g., radicals and carbenium ions) during the CFP of lignin (Custodis et al., 2014).
Figure 7.
The Possible Pathways for the CFP of Lignin over the Metal-Oxide Nanocomposites
Catalytic Fast Pyrolysis of Hemicellulose and Cellulose over the Nanocomposites
Xylan is selected as a representative of hemicellulose. The CFP of xylan and cellulose are also performed over these nanocomposites. The ion chromatograms resulting from the CFP of xylan and cellulose are graphed in Figure S5. The product distributions from the fast pyrolysis of xylan and cellulose are significantly altered by these nanocomposites. The CFP of xylan and cellulose over these nanocomposites favors the formation of furans. The furans from the CFP of xylan are mainly composed of furfural along with small quantities of furan and 2-methylfuran. In addition to the furans mentioned above, large quantities of 5-methylfurfural are also observed from the CFP of cellulose. In addition, aldehydes, ketones, and carboxylic acids, such as formaldehyde, acetaldehyde, acetone, butanone, 2-methyl-2-cyclopenten-1-one, and acetic acid, are found during the CFP of xylan and cellulose. Furans, aldehydes, and ketones are important precursors for hydrocarbon fuels. No aromatics and olefins are observed from the CFP of xylan and cellulose, suggesting that aromatics cannot be generated over the nanocomposites via hydrocarbon pool mechanism.
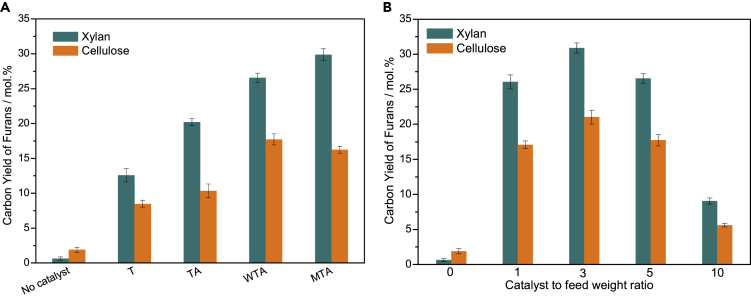
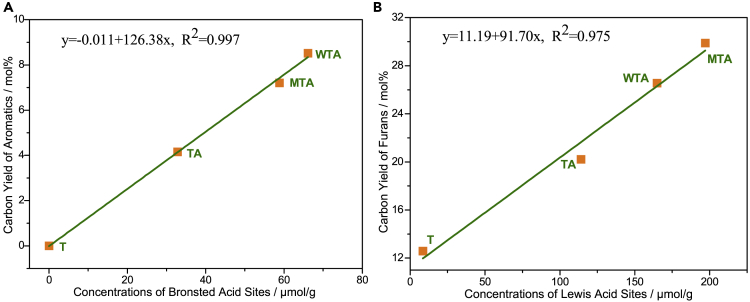
The yields of furans from CFP of xylan and cellulose catalyzed by the different nanocomposites are depicted in Figure 8A. These nanocomposites can drastically improve the yields of furans. The maximum carbon yields of furans from xylan (29.9%) and cellulose (17.7%) are achieved by CFP over MTA and WTA, respectively. The rank order of the carbon yields of furans from the CFP of xylan is MTA > WTA > TA > T. The rank order of the deoxygenation activities for xylan appears to be the same as that for the concentration of Lewis acid sites present in the nanocomposites. The carbon yields of furans from the CFP of cellulose are lower than those from the CFP of xylan under the same reaction conditions. The rank order of the carbon yields of furans from the CFP of cellulose is WTA > MTA > TA > T. It is worth noting that T can effectively catalyze the formation of furans from xylan and cellulose, whereas no aromatics are detected during the T-catalyzed CFP of lignin. Considering that T possesses only Lewis acid sites, it can be inferred that the formation of furans and aromatics is catalyzed by Lewis and Brønsted acid sites, respectively. As shown in Figure 9, the carbon yields of furans from the CFP of xylan is a linear function of the concentration of Lewis acid sites, whereas the carbon yields of aromatics from the CFP of lignin is a linear function of the concentration of Brønsted acid sites. These results suggest that the formation of furans and aromatics is catalyzed by Lewis and Brønsted acid sites, respectively. The carbon yields of furans as a function of the catalyst-to-feedstock weight ratio are graphed in Figure 8B. The carbon yields of furans reach a maximum value at a catalyst-to-feedstock weight ratio of 3, and then the yields decrease with increasing catalyst-to-feedstock weight ratios. The possible reaction mechanism for the formation of furans from the CFP of xylan and cellulose is shown in Figure S6. It is widely accepted that the first step is the thermal depolymerization of cellulose and xylan to form anhydrosugars (e.g., levoglucosan [LG] and anhydroxylopyranoses [AXP]) through the cleavage of the C-O bonds (glycosidic bonds) (Lin et al., 2009, Liu et al., 2014, Patwardhan et al., 2010, Patwardhan et al., 2011, Shen and Gu, 2009, Wang et al., 2012). LG can undergo dehydration reactions to generate 5-hydroxymethylfurfural, which can undergo subsequent dehydration and decarboxylation reactions to form 5-methylfurfural and 2-methylfuran. 5-Hydroxymethylfurfural can be converted into furfural and formaldehyde by removing the hydroxymethyl group. This result is strongly supported by the fact that formaldehyde is only found in the product stream of the CFP of cellulose. AXP can undergo dehydration reactions to form dianhydroxylopyranoses (DAXP). AXP and DAXP can be further dehydrated to produce furfural. In addition, a small quantity of furans may be derived from glucose and xylose, which can be formed through the acid-catalyzed hydrolysis of cellulose and xylan during CFP.
Figure 8.
The Carbon Yields of Furans from the CFP of Xylan and Cellulose at 600°C
(A and B) (A) CFP over different nanocomposites with a catalyst-to-feedstock weight ratio of 5 and (B) CFP over WTA with different catalyst-to-feedstock weight ratios, where no catalyst is denoted as a catalyst-to-feedstock weight ratio of 0.
Figure 9.
The Carbon Yield of Aromatics or Furans as a Function of the Concentration of Acid Sites
(A) The carbon yield of aromatics from CFP of organosolv lignin as a function of the concentration of Brønsted acid sites.
(B) The carbon yield of furans from CFP of xylan as a function of the concentration of Lewis acid sites.
Catalytic Fast Pyrolysis of Biomass over WTA
Bagasse, eucalyptus, and pine, as representatives of herbaceous, hardwood, and softwood plants, were selected as the feedstocks for CFP. The yields of aromatics and phenols from the CFP of these feedstocks over WTA are given in Figure 10A. WTA is capable of simultaneously converting the polysaccharides and lignin fractions in the different biomass into furans and aromatics, indicating that CFP over WTA is a versatile and feedstock-flexible method for the coproduction of aromatics and furans from biomass. At a catalyst-to-feedstock weight ratio of 5, the maximum carbon yield of furans (20.4%) is achieved by the CFP of the eucalyptus feedstock. Under the same conditions, the CFP of the pine feedstock exhibits the lowest carbon yield of furans (13.0%) and the highest carbon yield of aromatics (10.7%). The results can be ascribed to the different chemical structures of bagasse, eucalyptus, and pine, especially the chemical structures of their hemicellulose and lignin fractions. In softwood plants, galactoglucomannan and guaiacyl units are the major components of the hemicellulose and lignin fractions, respectively. The effects of the catalyst-to-feedstock weight ratios on the product distribution from the CFP of pine feedstock over WTA are illustrated in Figure 10B. The phenols from the fast pyrolysis of pine without the use of the nanocomposites are mainly methoxyphenols. As the catalyst-to-feedstock weight ratio increases, the yields of furans and non-methoxyphenols first increase and then begin to decrease as the catalyst-to-feedstock ratio passes 3. The non-methoxyphenols from the CFP of the pine feedstock are mainly phenol and alkylphenols (mainly cresols, ethylphenols, xylenols, ethyl cresols, and trimethylphenols). In addition, the yields of the aromatics gradually increase and reach a maximum value at a catalyst-to-feedstock weight ratio of 10. These results further suggest that the methoxyphenols first undergo demethoxylation reactions to form phenol and alkylphenols, which then undergo dehydration reactions to generate aromatics. Also, the yield and selectivity toward aromatics, methoxyphenols or non-methoxyphenols can be tuned by controlling the catalyst-to-feedstock weight ratio. Notably, the sum of the carbon yields of the aromatics, non-methoxyphenols, and methoxyphenols increases significantly from 16.3% to 28.4% when the catalyst-to-feedstock weight ratio increases from 0 to 1. These results are the opposite of those from the CFP of individual lignin over WTA. As shown in Figures 7 and S6, the deoxygenation of phenols requires the hydrogen transfer reactions, in which hydrogen is supplied by the in situ dehydrogenation, polycondensation, and carbonization reactions of lignin and phenols. In contrast, the furans are formed by intramolecular dehydration, which does not require an external hydrogen source. It can be hypothesized that the hydrogen transfer reactions between the polysaccharides and lignin are significantly promoted by WTA, resulting in the formation of a greater amount of phenols and aromatics from the CFP of raw biomass via the hydrogenolysis reaction. Lercher reported that the presence of Lewis acid sites in zeolites can enhance both the strength of Brønsted acid sites and the rate of hydrogen transfer reactions (Müller et al., 2016, Wichterlová et al., 1999). Xiao found that the hydrogen transfer reactions between cellulose and lignin occurred during co-fast pyrolysis of cellulose and lignin without any catalysts (Wu et al., 2016). Lignin and polysaccharides typically require different reaction conditions to be completely converted into their respective platform chemicals. The optimal catalyst-to-feedstock weight ratios for maximizing the yields of furans, non-methoxyphenols, and aromatics are 3, 3, and 10, respectively. By comprehensively considering the yields of the furans and aromatics, the optimal catalyst-to-feedstock weight ratio for the CFP of biomass is 3–5. The yield of aromatics and furans from the CFP of biomass and its components over the catalysts reported in the literature are given in Tables S1 and S2 for comparison. As shown in Table S1, the catalysts used in the CFP of biomass for aromatic production include zeolites and mixed metal oxides, such as HZSM-5, modified HZSM-5, and Mo-containing mixed metal oxides, which are well-known strong Brønsted acids (Edmunds et al., 2019, Lu et al., 2019, Murugappan et al., 2016, Wang et al., 2015a). CFP of biomass over different catalysts for furanic production are provided in Table S2. It is found that both Brønsted and Lewis acid catalysts, such as zeolites, H2SO4, and ZnCl2, can effectively catalyze the dehydration of polysaccharides to form furans (Branca et al., 2012, Chen et al., 2018c, Lu et al., 2011). However, the simultaneous conversion of lignin and polysaccharides into respective aromatics and furans has not yet been achieved in the literature. Our results demonstrate that WTA with suitable Brønsted and Lewis acid sites could be the key for achieving efficient and simultaneous deoxygenation of lignin and holocellulose.
Figure 10.
The Carbon Yields of the Furans, Aromatics, and Phenols from the CFP of Raw Biomass over WTA at 600°C
(A and B) (A) CFP of the different feedstocks over WTA with a catalyst-to-feedstock weight ratio of 5 and (B) CFP of the pine feedstock over WTA with varying catalyst-to-feedstock weight ratios, where no catalyst is denoted as a catalyst-to-feedstock weight ratio of 0.
Conclusions
Lignocellulosic biomass is a highly heterogeneous polymer, and controlling the complex pyrolysis pathways of this biomass is challenging. Metal-oxide nanocomposites comprising W, Mo, Ti, Zr, or Al are successfully synthesized and applied to simultaneously control the deoxygenation pathways of lignin, cellulose, hemicellulose. The mono-oxo wolframyl groups and hydrated wolframyl groups on surface of WTA act as strong Lewis and Brønsted acid sites, respectively. It is demonstrated that the nanocomposites of WTA can achieve the selective breaking of the C-O bonds within the lignin-derived phenols to generate aromatics by direct demethoxylation and subsequent dehydration reactions, while also enabling the selective cleavage of the C-O bonds within xylan and cellulose to produce furans by dehydration reactions. Methanol, produced from the direct demethoxylation of lignin-derived pyrolysis intermediates, can further undergo methylation reactions to form alkylated phenols and aromatics. It is inferred that the Brønsted acid sites and Lewis acid sites of the nanocomposites can be responsible for the formation of the aromatics and furans, respectively. Moreover, during the CFP of raw biomass feedstocks, the nanocomposites can promote hydrogen transfer reactions between the polysaccharides and lignin, resulting in the formation of a greater amount of phenols and aromatics. However, the high-temperature and H-deficient environment of CFP can lead to low yields of the target products. To overcome these disadvantages, an additional H2 supply or low-temperature hydrothermal/solvothermal conditions should be explored in further studies.
Limitations of the Study
The mass balances for CFP of lignin, xylan, cellulose, and biomass are not provided in this study. In addition, the stability and recyclability of the metal-oxide nanocomposites during CFP are also not considered. We will conduct CFP of biomass and its components over metal-oxide nanocomposites in a fixed bed reactor for providing relevant information.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors gratefully acknowledge the National Key R&D Program of China (Grant 2017YFE0124200), the National Natural Science Foundation of China (Grants 51776209, 51661145011 and 21406227), the Natural Science Foundation of Shenzhen (JCYJ20170818164006890), the Science and Technology Planning Project of Guangdong Province (2015A020215024), Youth Innovation Promotion Association, CAS (2018383), and Pearl River S&T Nova Program of Guangzhou (Grant 201806010061) for their financial support of this work. The authors also express their sincere thanks to the Analytical and Testing Center of Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences for the assistance of pyrolysis experiments.
Author Contributions
A.Z., Z.Z., Y.T., and H.L. conceived of the research and designed the experiments, A.Z. carried out the preparation, characterization, and catalytic fast pyrolysis. A.Z., Z.H., G.W., and K.Z. analyzed and discussed the data; A.Z. wrote the manuscript, and Z.H., G.W., K.Z., L.J., Z.Z., Y.T.; and H.L. discussed and revised the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: January 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100814.
Contributor Information
Zengli Zhao, Email: zhaozl@ms.giec.ac.cn.
Yuanyu Tian, Email: tianyy1008@126.com.
Supplemental Information
References
- Barton D.G., Soled S.L., Iglesia E. Solid acid catalysts based on supported tungsten oxides. Top. Catal. 1998;6:87–99. [Google Scholar]
- Branca C., Di Blasi C., Galgano A. Catalyst screening for the production of furfural from corncob pyrolysis. Energy Fuels. 2012;26:1520–1530. [Google Scholar]
- Carlson T.R., Cheng Y.-T., Jae J., Huber G.W. Production of green aromatics and olefins by catalytic fast pyrolysis of wood sawdust. Energy Environ. Sci. 2011;4:145–161. [Google Scholar]
- Carlson T.R., Vispute T.P., Huber G.W. Green gasoline by catalytic fast pyrolysis of solid biomass derived compounds. ChemSusChem. 2008;1:397–400. doi: 10.1002/cssc.200800018. [DOI] [PubMed] [Google Scholar]
- Chen M., Ma J., Zhang B., Wang F., Li Y., Zhang C., He H. Facet-dependent performance of anatase TiO2 for photocatalytic oxidation of gaseous ammonia. Appl. Catal. B Environ. 2018;223:209–215. [Google Scholar]
- Chen W.-T., Zhang Y., Lee T.H., Wu Z., Si B., Lee C.-F.F., Lin A., Sharma B.K. Renewable diesel blendstocks produced by hydrothermal liquefaction of wet biowaste. Nat. Sustain. 2018;1:702–710. [Google Scholar]
- Chen X., Chen Y., Chen Z., Zhu D., Yang H., Liu P., Li T., Chen H. Catalytic fast pyrolysis of cellulose to produce furan compounds with SAPO type catalysts. J. Anal. Appl. Pyrol. 2018;129:53–60. [Google Scholar]
- Cheng Y.-T., Jae J., Shi J., Fan W., Huber G.W. Production of renewable aromatic compounds by catalytic fast pyrolysis of lignocellulosic biomass with bifunctional Ga/ZSM-5 catalysts. Angew. Chem. Int. Ed. 2012;51:1387–1390. doi: 10.1002/anie.201107390. [DOI] [PubMed] [Google Scholar]
- Cristiani C., Bellotto M., Forzatti P., Bregani F. On the morphological properties of tungsta-titania de-NO x ing catalysts. J. Mater. Res. 1993;8:2019–2025. [Google Scholar]
- Custodis V.B., Hemberger P., Ma Z., van Bokhoven J.A. Mechanism of fast pyrolysis of lignin: studying model compounds. J. Phys. Chem. B. 2014;118:8524–8531. doi: 10.1021/jp5036579. [DOI] [PubMed] [Google Scholar]
- Dayton D.C., Carpenter J.R., Kataria A., Peters J.E., Barbee D., Mante O.D., Gupta R. Design and operation of a pilot-scale catalytic biomass pyrolysis unit. Green. Chem. 2015;17:4680–4689. [Google Scholar]
- Du Z., Ma X., Li Y., Chen P., Liu Y., Lin X., Lei H., Ruan R. Production of aromatic hydrocarbons by catalytic pyrolysis of microalgae with zeolites: catalyst screening in a pyroprobe. Bioresour. Technol. 2013;139:397–401. doi: 10.1016/j.biortech.2013.04.053. [DOI] [PubMed] [Google Scholar]
- Edmunds C.W., Mukarakate C., Xu M., Regmi Y.N., Hamilton C., Schaidle J.A., Labbé N., Chmely S.C. Vapor-phase stabilization of biomass pyrolysis vapors using mixed-metal oxide catalysts. ACS Sustain. Chem. Eng. 2019;7:7386–7394. [Google Scholar]
- Fernández-García M., Rodriguez J.A. Metal oxide nanoparticles. In: Scott R., editor. Encyclopedia of Inorganic Bioinorganic Chemistry. John Wiley & Sons, Ltd.; 2011. pp. 6–7. [Google Scholar]
- Guntida A., Suriye K., Panpranot J., Praserthdam P. Lewis acid transformation to Bronsted acid sites over supported tungsten oxide catalysts containing different surface WOx structures. Catal. Today. 2019 [Google Scholar]
- Gutiérrez-Alejandre A., Ramírez J., Busca G. A vibrational and spectroscopic study of WO3/TiO2− Al2O3 catalyst precursors. Langmuir. 1998;14:630–639. [Google Scholar]
- Hemberger P., Custodis V.B.F., Bodi A., Gerber T., van Bokhoven J.A. Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysis. Nat. Commun. 2017;8:15946. doi: 10.1038/ncomms15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff T.C., Gardner D.W., Thilakaratne R., Proano-Aviles J., Brown R.C., Tessonnier J.-P. Elucidating the effect of desilication on aluminum-rich ZSM-5 zeolite and its consequences on biomass catalytic fast pyrolysis. Appl. Catal. A Gen. 2017;529:68–78. [Google Scholar]
- Huber G.W., Iborra S., Corma A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 2006;106:4044–4098. doi: 10.1021/cr068360d. [DOI] [PubMed] [Google Scholar]
- Iliopoulou E.F., Stefanidis S.D., Kalogiannis K.G., Delimitis A., Lappas A.A., Triantafyllidis K.S. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B Environ. 2012;127:281–290. [Google Scholar]
- Jae J., Tompsett G.A., Foster A.J., Hammond K.D., Auerbach S.M., Lobo R.F., Huber G.W. Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal. 2011;279:257–268. [Google Scholar]
- Jan O., Marchand R., Anjos L.C.A., Seufitelli G.V.S., Nikolla E., Resende F.L.P. Hydropyrolysis of lignin using Pd/HZSM-5. Energy Fuels. 2015;29:1793–1800. [Google Scholar]
- Jia L.Y., Raad M., Hamieh S., Toufaily J., Hamieh T., Bettahar M.M., Mauviel G., Tarrighi M., Pinard L., Dufour A. Catalytic fast pyrolysis of biomass: superior selectivity of hierarchical zeolites to aromatics. Green Chem. 2017;19:5442–5459. [Google Scholar]
- Kelkar S., Saffron C.M., Andreassi K., Li Z., Murkute A., Miller D.J., Pinnavaia T.J., Kriegel R.M. A survey of catalysts for aromatics from fast pyrolysis of biomass. Appl. Catal. B Environ. 2015;174-175:85–95. [Google Scholar]
- Kim J.-Y., Lee J.H., Park J., Kim J.K., An D., Song I.K., Choi J.W. Catalytic pyrolysis of lignin over HZSM-5 catalysts: effect of various parameters on the production of aromatic hydrocarbon. J. Anal. Appl. Pyrol. 2015;114:273–280. [Google Scholar]
- Kim J., Sarma B.B., Andrés E., Pfänder N., Concepción P., Prieto G. Surface lewis acidity of periphery oxide species as a general kinetic descriptor for CO2 hydrogenation to methanol on supported copper nanoparticles. ACS Catal. 2019;9:10409–10417. [Google Scholar]
- Kubiak, C.P. (1995). Preconversion catalytic deoxygenation of phenolic functional groups. Quarterly technical progress report, July 1, 1995–September 30, 1995 (Purdue Univ., Lafayette, IN (United States). Dept. of Chemistry).
- Koodali R. Catalysis by metal oxides. In: Richards R., editor. Surface and Nanomolecular Catalysis. CRC press; 2006. pp. 48–50. [Google Scholar]
- Kusumoto S., Nozaki K. Direct and selective hydrogenolysis of arenols and aryl methyl ethers. Nat. Commun. 2015;6:6296. doi: 10.1038/ncomms7296. [DOI] [PubMed] [Google Scholar]
- Li C., Zhao X., Wang A., Huber G.W., Zhang T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015;115:11559–11624. doi: 10.1021/acs.chemrev.5b00155. [DOI] [PubMed] [Google Scholar]
- Li C.Z., Zheng M.Y., Wang A.Q., Zhang T. One-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: simultaneous conversion of cellulose, hemicellulose and lignin. Energy Environ. Sci. 2012;5:6383–6390. [Google Scholar]
- Li J., Yu Y., Li X., Wang W., Yu G., Deng S., Huang J., Wang B., Wang Y. Maximizing carbon efficiency of petrochemical production from catalytic co-pyrolysis of biomass and plastics using gallium-containing MFI zeolites. Appl. Catal. B Environ. 2015;172-173:154–164. [Google Scholar]
- Lin Y.C., Cho J., Tompsett G.A., Westmoreland P.R., Huber G.W. Kinetics and mechanism of cellulose pyrolysis. J. Phys. Chem. C. 2009;113:20097–20107. [Google Scholar]
- Liu C., Hu J., Zhang H., Xiao R. Thermal conversion of lignin to phenols: relevance between chemical structure and pyrolysis behaviors. Fuel. 2016;182:864–870. [Google Scholar]
- Liu C., Wang H., Karim A.M., Sun J., Wang Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014;43:7594–7623. doi: 10.1039/c3cs60414d. [DOI] [PubMed] [Google Scholar]
- Liu G., Wright M.M., Zhao Q., Brown R.C. Catalytic fast pyrolysis of duckweed: effects of pyrolysis parameters and optimization of aromatic production. J. Anal. Appl. Pyrol. 2015;112:29–36. [Google Scholar]
- Lu Q., Dong C.-q., Zhang X.-m., Tian H.-y., Yang Y.-p., Zhu X.-f. Selective fast pyrolysis of biomass impregnated with ZnCl2 to produce furfural: analytical Py-GC/MS study. J. Anal. Appl. Pyrol. 2011;90:204–212. [Google Scholar]
- Lu Q., Wang Z.-x., Guo H.-q., Li K., Zhang Z.-x., Cui M.-s., Yang Y.-p. Selective preparation of monocyclic aromatic hydrocarbons from ex-situ catalytic fast pyrolysis of pine over Ti(SO4)2-Mo2N/HZSM-5 catalyst. Fuel. 2019;243:88–96. [Google Scholar]
- Luo Z., Lu K., Yang Y., Li S., Li G. Catalytic fast pyrolysis of lignin to produce aromatic hydrocarbons: optimal conditions and reaction mechanism. RSC Adv. 2019;9:31960–31968. doi: 10.1039/c9ra02538c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lup A.N.K., Abnisa F., Daud W.M.A.W., Aroua M.K. A review on reaction mechanisms of metal-catalyzed deoxygenation process in bio-oil model compounds. Appl. Catal. A Gen. 2017;541:87–106. [Google Scholar]
- Meng Q., Hou M., Liu H., Song J., Han B. Synthesis of ketones from biomass-derived feedstock. Nat. Commun. 2017;8:14190. doi: 10.1038/ncomms14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S., Liu Y., Kirchberger F.M., Tonigold M., Sanchez-Sanchez M., Lercher J.A. Hydrogen transfer pathways during zeolite catalyzed methanol conversion to hydrocarbons. J. Am. Chem. Soc. 2016;138:15994–16003. doi: 10.1021/jacs.6b09605. [DOI] [PubMed] [Google Scholar]
- Murugappan K., Mukarakate C., Budhi S., Shetty M., Nimlos M.R., Román-Leshkov Y. Supported molybdenum oxides as effective catalysts for the catalytic fast pyrolysis of lignocellulosic biomass. Green Chem. 2016;18:5548–5557. [Google Scholar]
- Nolte M.W., Zhang J., Shanks B.H. Ex situ hydrodeoxygenation in biomass pyrolysis using molybdenum oxide and low pressure hydrogen. Green Chem. 2016;18:134–138. [Google Scholar]
- Patwardhan P.R., Brown R.C., Shanks B.H. Product distribution from the fast pyrolysis of hemicellulose. Chemsuschem. 2011;4:636–643. doi: 10.1002/cssc.201000425. [DOI] [PubMed] [Google Scholar]
- Patwardhan P.R., Satrio J.A., Brown R.C., Shanks B.H. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 2010;101:4646–4655. doi: 10.1016/j.biortech.2010.01.112. [DOI] [PubMed] [Google Scholar]
- Qi Z., Zhang B., Ji J., Li X., Dai T., Guo H., Wang A., Lu L., Li C. Selective cleavage of C− O bonds in lignin catalyzed by rhenium (VII) oxide (Re2O7) ChemPlusChem. 2018 doi: 10.1002/cplu.201700547. [DOI] [PubMed] [Google Scholar]
- Rahimi A., Ulbrich A., Coon J.J., Stahl S.S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature. 2014;515:249. doi: 10.1038/nature13867. [DOI] [PubMed] [Google Scholar]
- Robinson A., Ferguson G.A., Gallagher J.R., Cheah S., Beckham G.T., Schaidle J.A., Hensley J.E., Medlin J.W. Enhanced hydrodeoxygenation of m-cresol over bimetallic Pt–Mo catalysts through an oxophilic metal-induced tautomerization pathway. ACS Catal. 2016;6:4356–4368. [Google Scholar]
- Rogers K.A., Zheng Y. Selective deoxygenation of biomass-derived bio-oils within hydrogen-modest environments: a review and new insights. Chemsuschem. 2016;9:1750–1772. doi: 10.1002/cssc.201600144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Leshkov Y., Barrett C.J., Liu Z.Y., Dumesic J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature. 2007;447:982–U985. doi: 10.1038/nature05923. [DOI] [PubMed] [Google Scholar]
- Sanna A., Andrésen J.M. Bio-oil deoxygenation by catalytic pyrolysis: new catalysts for the conversion of biomass into densified and deoxygenated bio-oil. ChemSusChem. 2012;5:1944–1957. doi: 10.1002/cssc.201200245. [DOI] [PubMed] [Google Scholar]
- Schlaf M., Zhang Z.C. Springer; 2015. Reaction Pathways and Mechanisms in Thermocatalytic Biomass Conversion II: Homogeneously Catalyzed Transformations, Acrylics from Biomass, Theoretical Aspects, Lignin Valorization and Pyrolysis Pathways. [Google Scholar]
- Shao Y., Xia Q.N., Dong L., Liu X.H., Han X., Parker S.F., Cheng Y.Q., Daemen L.L., Ramirez-Cuesta A.J., Yang S.H. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 2017;8:16104. doi: 10.1038/ncomms16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D., Gu S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009;100:6496–6504. doi: 10.1016/j.biortech.2009.06.095. [DOI] [PubMed] [Google Scholar]
- Shiramizu M., Toste F.D. Deoxygenation of biomass-derived feedstocks: oxorhenium-catalyzed deoxydehydration of sugars and sugar alcohols. Angew. Chem. Int. Ed. 2012;51:8082–8086. doi: 10.1002/anie.201203877. [DOI] [PubMed] [Google Scholar]
- Teles C.A., de Souza P.M., Rabelo-Neto R.C., Griffin M.B., Mukarakate C., Orton K.A., Resasco D.E., Noronha F.B. Catalytic upgrading of biomass pyrolysis vapors and model compounds using niobia supported Pd catalyst. Appl. Catal. B Environ. 2018;238:38–50. [Google Scholar]
- Thilakaratne R., Tessonnier J.-P., Brown R.C. Conversion of methoxy and hydroxyl functionalities of phenolic monomers over zeolites. Green Chem. 2016;18:2231–2239. [Google Scholar]
- Vishwanathan V., Balakrishna G., Rajesh B., Jayasri V., Sikhwivhilu L.M., Coville N.J. Alkylation of catechol with methanol to give guaiacol over sulphate-modified zirconia solid acid catalysts: the influence of structural modification of zirconia on catalytic performance. Catal. Commun. 2008;9:2422–2427. [Google Scholar]
- Vuori A.I., Bredenberg J.B.-s. Thermal chemistry pathways of substituted anisoles. Ind. Eng. Chem. Res. 1987;26:359–365. [Google Scholar]
- Wan W., Ammal S.C., Lin Z., You K.-E., Heyden A., Chen J.G. Controlling reaction pathways of selective C–O bond cleavage of glycerol. Nat. Commun. 2018;9:4612. doi: 10.1038/s41467-018-07047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Kim K.H., Brown R.C. Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 2014;16:727–735. [Google Scholar]
- Wang K., Zhang J., Shanks B.H., Brown R.C. The deleterious effect of inorganic salts on hydrocarbon yields from catalytic pyrolysis of lignocellulosic biomass and its mitigation. Appl. Energy. 2015;148:115–120. [Google Scholar]
- Wang S., Cao B., Liu X., Xu L., Hu Y., Afonaa-Mensah S., Abomohra A.E.-F., He Z., Wang Q., Xu S. A comparative study on the quality of bio-oil derived from green macroalga Enteromorpha clathrata over metal modified ZSM-5 catalysts. Bioresour. Technol. 2018;256:446–455. doi: 10.1016/j.biortech.2018.01.134. [DOI] [PubMed] [Google Scholar]
- Wang S., Dai G., Yang H., Luo Z. Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog. Energ. Combust. 2017;62:33–86. [Google Scholar]
- Wang S., Guo X., Liang T., Zhou Y., Luo Z. Mechanism research on cellulose pyrolysis by Py-GC/MS and subsequent density functional theory studies. Bioresour. Technol. 2012;104:722–728. doi: 10.1016/j.biortech.2011.10.078. [DOI] [PubMed] [Google Scholar]
- Wang S., Ru B., Lin H., Sun W., Luo Z. Pyrolysis behaviors of four lignin polymers isolated from the same pine wood. Bioresour. Technol. 2015;182:120–127. doi: 10.1016/j.biortech.2015.01.127. [DOI] [PubMed] [Google Scholar]
- Wang S., Wang K., Liu Q., Gu Y., Luo Z., Cen K., Fransson T. Comparison of the pyrolysis behavior of lignins from different tree species. Biotechnol. Adv. 2009;27:562–567. doi: 10.1016/j.biotechadv.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Wichterlová B., Žilkova N., Uvarova E., Čejka J., Sarv P., Paganini C., Lercher J.A. Effect of Broensted and Lewis sites in ferrierites on skeletal isomerization of n-butenes. Appl. Catal. A Gen. 1999;182:297–308. [Google Scholar]
- Wu S., Shen D., Hu J., Zhang H., Xiao R. Cellulose-lignin interactions during fast pyrolysis with different temperatures and mixing methods. Biomass Bioenerg. 2016;90:209–217. [Google Scholar]
- Xia Q., Chen Z., Shao Y., Gong X., Wang H., Liu X., Parker S.F., Han X., Yang S., Wang Y. Direct hydrodeoxygenation of raw woody biomass into liquid alkanes. Nat. Commun. 2016;7:11162. doi: 10.1038/ncomms11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J., Luo Z., Yang J., Guo Y., Piyadasa A., Wang S., Hoang S., Fang Y., Hu S., Yang W. Robust and well-controlled TiO2–Al2O3 binary nanoarray-integrated ceramic honeycomb for efficient propane combustion. CrystEngComm. 2019;21:2727–2735. [Google Scholar]
- Yang H., Coolman R., Karanjkar P., Wang H., Dornath P., Chen H., Fan W., Conner W.C., Mountziaris T.J., Huber G. The effects of contact time and coking on the catalytic fast pyrolysis of cellulose. Green. Chem. 2017;19:286–297. [Google Scholar]
- Zhang H., Xiao R., Huang H., Xiao G. Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour. Technol. 2009;100:1428–1434. doi: 10.1016/j.biortech.2008.08.031. [DOI] [PubMed] [Google Scholar]
- Zhang H., Xiao R., Jin B., Shen D., Chen R., Xiao G. Catalytic fast pyrolysis of straw biomass in an internally interconnected fluidized bed to produce aromatics and olefins: effect of different catalysts. Bioresour. Technol. 2013;137:82–87. doi: 10.1016/j.biortech.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Zhang H., Xiao R., Wang D., Zhong Z., Song M., Pan Q., He G. Catalytic fast pyrolysis of biomass in a fluidized bed with fresh and spent fluidized catalytic cracking (FCC) catalysts. Energy Fuels. 2009;23:6199–6206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.