Abstract
Torsade de Pointes (TdP) is a rare cardiac arrhythmia that is associated with prolonged QTc interval. Hypocalcemia is a common cause of prolonged QTc. Although vitamin D deficiency (VDD) is a common disorder in elderly patients with an incidence rate of >40% and can cause hypocalcemia, it has never been linked to TdP.
We report a patient with severe VDD that resulted in TdP and cardiac arrest. Post-resuscitation work up illustrated prolonged QTc interval of 620 ms, significant hypocalcemia, and severe VDD of 4 (normal 30–80) ng/mL. After high dose vitamin D/calcium supplements, repeat electrocardiogram revealed normal QTc interval of 423 ms. During hospitalization, the patient suffered no additional arrhythmias and QTc continued to be normal.
<Learning objective: Severe vitamin D deficiency is common especially in elderly patients. The diagnosis and treatment of this disorder are simple, but the consequences of severe depletion of vitamin D storage can lead to deep electrolyte abnormalities and life-threatening arrhythmia such as Torsade de Pointes. A simple screening test is effective in preventing a dreadful outcome.>
Keywords: Torsade de pointes, Vitamin D deficiency, Prolonged QTc
Introduction
Torsade de Pointes (TdP) is a well-known ventricular arrhythmia associated with prolonged QTc. This condition can be congenital or iatrogenic. Acquired long QT syndrome (LQTS) can be triggered by drug therapy, electrolyte abnormalities, or bradycardia [1]. Hypocalcemia, a common finding with many systemic diseases, is a known cause of long QTc and it commonly results from vitamin D deficiency (VDD) [2]. VDD has never been reported as a cause of life-threatening arrhythmia. We present a unique case of severe VDD leading to significant electrolyte imbalance, TdP, and sudden cardiac death.
Case report
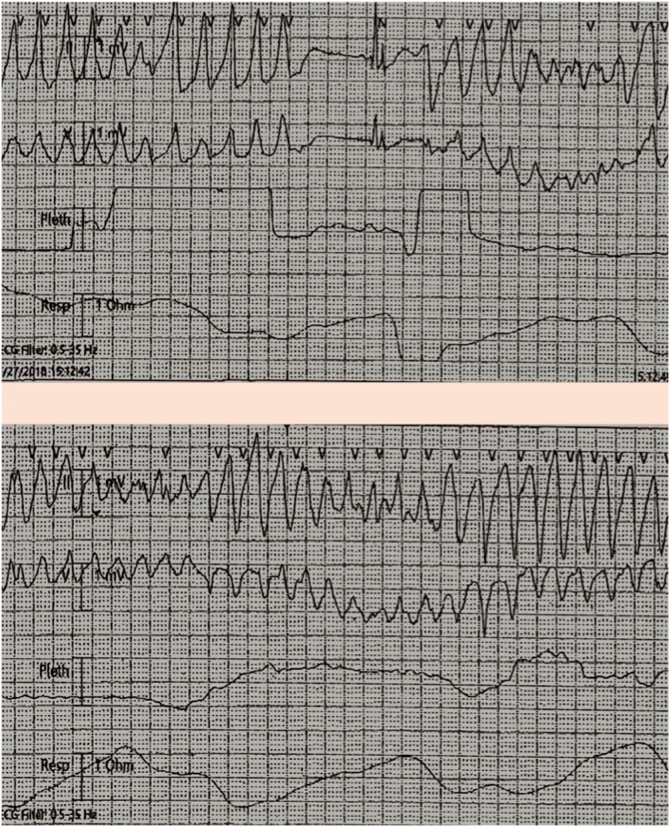
The patient was a 74-year-old Caucasian female who was transferred to our facility because of two episodes of syncope. She was initially hospitalized because of muscle cramps, numbness in the hands, and weakness. Cardiac monitoring during the first syncopal episode showed TdP which resolved spontaneously without any intervention. The second episode (Fig. 1) of polymorphic ventricular tachycardia required cardiac resuscitation and usage of an external defibrillator to regain sinus rhythm.
Fig. 1.
Telemetry strips showing recurrent polymorphic ventricular tachycardia during the first and the second episodes of syncope.
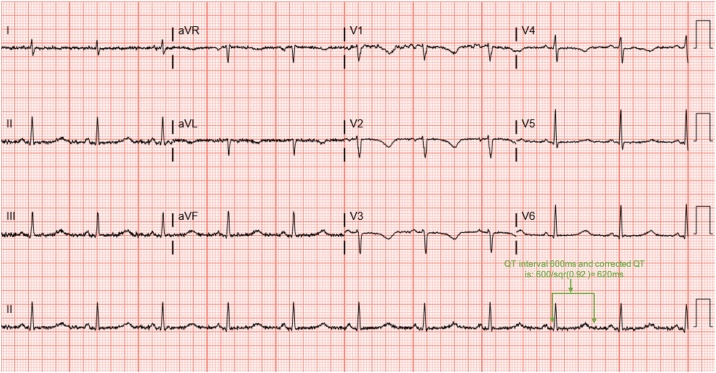
At our facility, the patient underwent emergent cardiac catheterization that showed no significant coronary artery disease. Transthoracic echocardiogram showed normal left ventricular functions. Upon presentation, her laboratory data were consistent with severe hypocalcemia 5.6 mg/dL (normal 8.6–10.2 mg/dL) with ionized calcium 0.83 mmol/L (normal 1.12–1.30 mmol/L) and mild hypokalemia 3.3 mmol/L (normal 3.5–5.1 mmol/L). The electrocardiogram (ECG) was significant for QTc prolongation of 620 ms (Fig. 2). She was not on any known QTc prolonging medications. She denied any recent illness nor a family history of sudden cardiac death. Other causes of long QTc such as family history, bradycardia, ischemia, and medications were excluded.
Fig. 2.
Electrocardiogram on admission to our facility showing T wave inversion in the antero-lateral leads and prolonged QTc 620 ms.
Additional work up showed high intact-parathyroid hormone (iPTH) 173.1 pg/mL (normal 15.0–65.0 pg/mL), low 25-OH-vitamin D level 4 ng/mL (normal 30–80 ng/mL) with significantly low 24-h urinary calcium 56 mg/24 h (normal 100–250 mg/24 h). The patient was diagnosed with severe VDD secondary to severe malnutrition. Work up for celiac disease and other malabsorption diseases was negative.
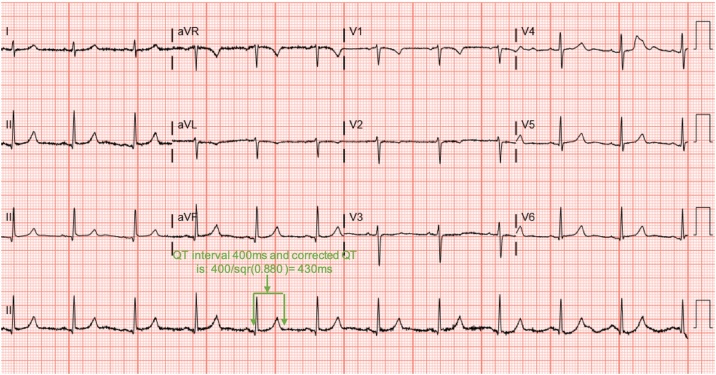
High-dose supplementation of vitamin D/calcium was started. Post supplementation laboratory findings indicated appropriate response to replacement with increased calcium level and decreased iPTH level. These findings were correlated with a significant shortening of QTc on multiple ECGs (Fig. 3).
Fig. 3.
Electrocardiogram seven days after admission to our facility showing normalization after aggressive supplementation of calcium and vitamin D.
No further ventricular arrhythmia was noted on continued cardiac monitoring after QTc normalization. No anti-arrhythmic medications were started. After arrhythmia-free observation in the hospital, she was discharged home and remained stable on follow up.
Discussion
To the best of our knowledge, this is the first case report of TdP caused by hypocalcemia due to severe VDD. TdP is a malignant rhythm that can degenerate to ventricular fibrillation and result in sudden cardiac death if left untreated [3]. This malignant rhythm has been linked to prolonged QTc interval which represents prolongation of ventricular repolarization, usually due to delayed activation of calcium, IKs, or IKr channels. It is known that hypocalcemia prolongs QTc and calcium replacement could correct this cardiac electrical pathology [4]. The mechanism of QTc prolongation is through extension of the cardiac action potential at the plateau phase. Calcium ion channels remain open longer and allow late inflow of calcium forming early after-depolarizations [5]. This myocardial electrical disturbance leaves the myocardium vulnerable to different ventricular arrhythmias including TdP [1]. In our patient, the QTc prolongation was caused by severe VDD with resultant severe hypocalcemia. Vitamin D and calcium supplementation resulted in complete elimination of ventricular arrhythmia without requiring any anti-arrhythmic medications.
In general, QTc prolongation is either congenital or secondary to using certain medications, bradycardia, or electrolyte abnormalities [3]. In previous reports, endocrine etiology of hypocalcemia such as hypothyroidism, has been shown to cause prolonged QTc [6]. However, it has never been reported to cause sustained ventricular arrhythmia.
Vitamin D and parathyroid hormone play an important role in the maintenance of calcium homeostasis. In the absence of sufficient vitamin D, only 10%–15% of the dietary calcium is absorbed, which can lead to hypocalcemia [7]. VDD is common above the age of 65 years and some think that all elderly patients are vitamin D deficient [7]. Despite the high prevalence of VDD, there are no published reports of acute life-threatening arrhythmia such as TdP in this population. VDD, with incremental effect, has been associated with significant increase in sudden cardiac death in coronary artery disease patients [8], but it is not clear if arrhythmia is the cause of this connection. We believe that the hypocalcemic effect of VDD was the cause of our patient’s symptoms. We noted that as we replaced vitamin D and calcium, there was a significant shortening of the QTc, and the patient became arrhythmia-free with no other interventions.
It is important to mention that our patient may benefit from genetic testing. Patients with acquired long QTc may still have a genetic defect responsible for this abnormality and deserve genetic evaluation.
Finally, screening for VDD plays a major role in protecting high-risk populations from multiple health consequences. Our case report emphasizes the importance of that and indicates that it could be lifesaving. Screening should be focused on the elderly, people with inadequate exposure to natural sunlight, bariatric patients, osteoporosis/osteomalacia, hepatic failure, chronic kidney disease, parathyroid disturbance, and malabsorption disorders [7].
Conclusion
We present a unique case report of VDD presenting with TdP. This case report highlights the importance of precise laboratory analysis in patients presenting with polymorphic ventricular tachycardia, and also the importance of screening elderly patients for VDD.
Conflict of interests
The authors declare that they have no competing interests.
Acknowledgment
None.
References
- 1.Itoh H., Crotti L., Aiba T., Spazzolini C., Denjoy I., Fressart V. The genetics underlying acquired long QT syndrome: impact for genetic screening. Eur Heart J. 2016;37:1456–1464. doi: 10.1093/eurheartj/ehv695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong J., Khan A. Hypocalcemia: updates in diagnosis and management for primary care. Can Fam Physician. 2012;58:158–162. [PMC free article] [PubMed] [Google Scholar]
- 3.Drew B.J., Ackerman M.J., Funk M., Gibler W.B., Kligfield P., Menon V. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121:1047–1060. doi: 10.1161/CIRCULATIONAHA.109.192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eryol N.K., Colak R., Ozdoğru I., Tanriverdi F., Unal S., Topsakal R. Effects of calcium treatment on QT interval and QT dispersion in hypocalcemia. Am J Cardiol. 2003;91:750–752. doi: 10.1016/s0002-9149(02)03423-9. [DOI] [PubMed] [Google Scholar]
- 5.Bradley T.J., Metzger D.L., Sanatani S. Long on QT and low on calcium. Cardiol Young. 2004;14:667–670. doi: 10.1017/S1047951104006134. [DOI] [PubMed] [Google Scholar]
- 6.Rometo A.B., Beerman L., Arora G. Electrolyte screening in the evaluation of prolonged QTc interval. Cardiol Young. 2015;25:398–399. doi: 10.1017/S1047951114000705. [DOI] [PubMed] [Google Scholar]
- 7.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 8.Pilz S., Marz W., Wellnitz B., Seelhorst U., Fahrleitner-Pammer A., Dimai H.P. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;25:3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]