Key Teaching Points.
-
•
The variant p.Tyr473Cys in the ACTN2 gene, coding for the Z-disk protein alpha-actinin-2, leads to a dominantly inherited phenotype suggestive of left-dominant arrhythmogenic cardiomyopathy (ACM). This implies that analysis of ACTN2 might be considered in patients with ACM in whom genetic screening was inconclusive.
-
•
The signal for plakoglobin was markedly reduced in the myocardial biopsy of our index case. This further supports the concept that plakoglobin deficiency may be a common feature of ACMs, independently of their molecular origin.
-
•
The decreased signal for plakoglobin in the myocardial biopsy of our index case suggests a link between Z-disc proteins and the desmosome. This remains to be investigated.
Introduction
Arrhythmogenic cardiomyopathy (ACM) is a heritable heart disease characterized by fibrofatty replacement of the cardiomyocytes. It can result in life-threatening arrhythmias and sudden cardiac death (SCD). Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is the most common form of ACM, while left-dominant or biventricular ACM is less frequent.1 Genes involved in ACM pathology mainly encode desmosomal protein,2 but anomalies in other genes have been described.3,4
Variants in the alpha-actinin-2 (ACTN2) gene have been associated with different cardiac manifestations, including hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM),5, 6, 7, 8, 9 atrial arrhythmia,6 left ventricular noncompaction (LVNC), idiopathic ventricular fibrillation, and SCD.7 Alpha-actinin-2, the protein encoded by ACTN2, is a major component of the sarcomere Z-disc expressed in the cardiac and skeletal muscle. Its supposed role is to bind actin filaments and to stabilize the muscle contractile apparatus.10,11 Changes in its scaffolding function have been proposed to be pathogenic and lead to DCM and HCM. Additionally, alpha-actinin-2 interacts with several signaling proteins and ion channels.11,12 Some speculate that mutant alpha-actinin-2 affects the localization and function of cardiac ion channels and thus might contribute to arrhythmic phenotypes. We describe here 4 members of a Swiss family in which a missense ACTN2 variant cosegregates with a cardiomyopathy suggestive of left-dominant ACM.
Case reports
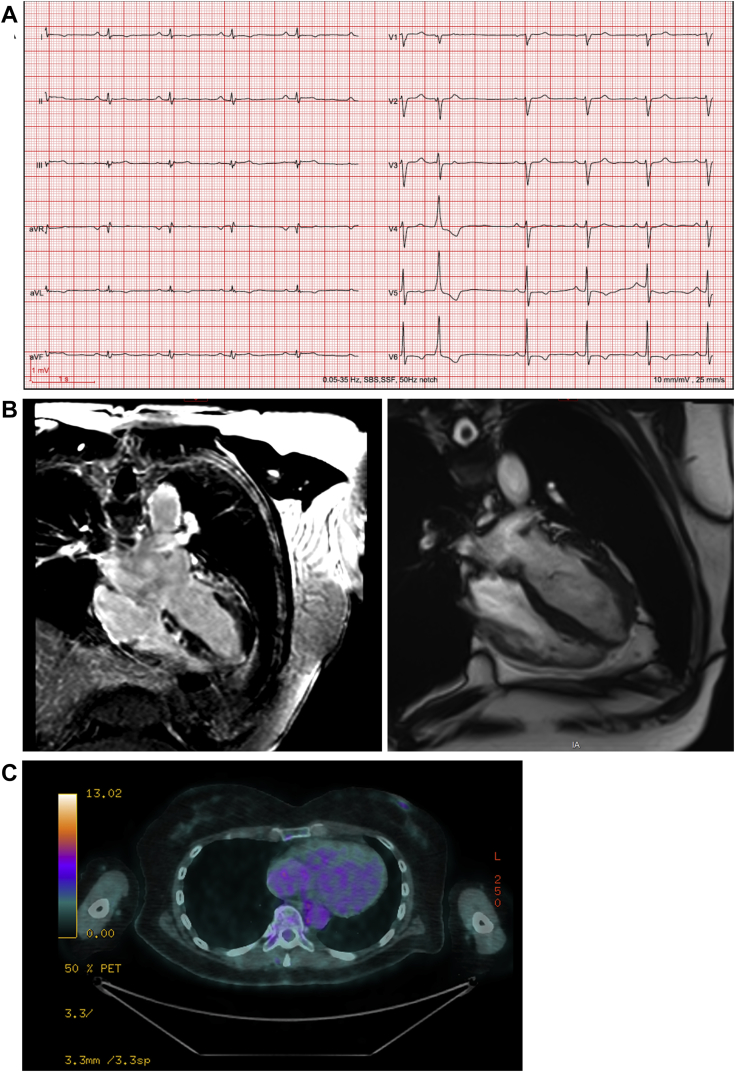
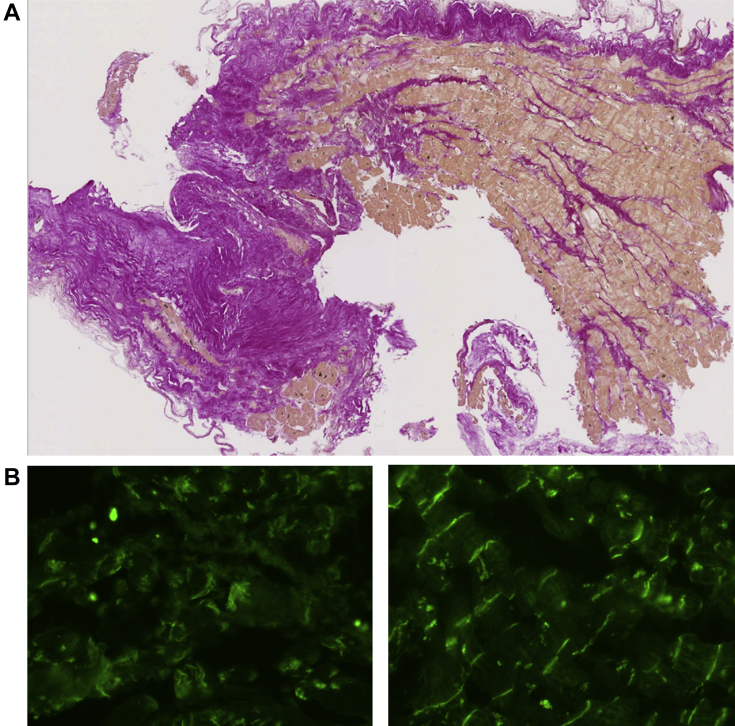
The female proband (II-2 in Figure 1B) presented with polymorphic ventricular premature beats at age 25 years. At age 62, she experienced tiredness, dyspnea, and thoracic discomfort. The electrocardiogram revealed low voltage in peripheral leads and T-wave inversion in lead V5 and V6 (Figure 2A). Coronary angiography showed no evidence of coronary artery disease. The cardiovascular magnetic resonance demonstrated a diffusely hypokinetic nondilated left ventricle with a reduced ejection fraction (41%) and widespread areas of late gadolinium enhancement predominantly in the inferior and anterolateral walls (Figure 2B). The right ventricle was considered normal. A metabolic positron emission tomography scan did not reveal any inflammatory component (Figure 2C). A left ventricle endomyocardial biopsy showed diffuse fibrosis without fatty replacement (Figure 3A). Immunofluorescence staining revealed a markedly diminished signal for plakoglobin (Figure 3B), while the signal level was preserved for other desmosomal proteins. The diagnosis of left-dominant ACM was strongly suspected based on the ventricular arrhythmias, T-wave inversions in the precordial leads, diffuse hypokinesia with late gadolinium enhancement, and the diffuse areas of fibrosis. Following some episodes of symptomatic nonsustained ventricular tachycardia, a cardioverter-defibrillator was implanted.
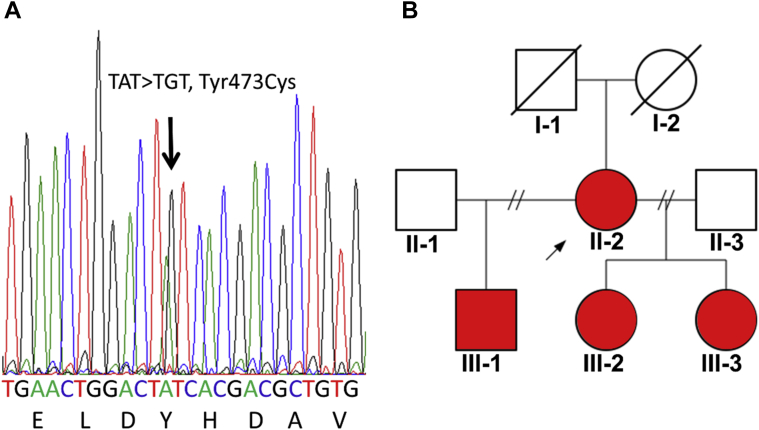
Figure 1.
Genetic investigations. A: Sanger sequence confirmation of the ACTN2 variant c.1418A>G (p.Tyr473Cys). B: Family pedigree. Lines through symbols indicate deceased subject; arrow indicates proband; red symbols indicate heterozygous carriers of p.Tyr473Cys in ACTN2 and affected by left-dominant arrhythmogenic cardiomyopathy.
Figure 2.
Cardiologic investigations in subject II-2. A: Electrocardiogram showing low voltage in peripheral leads and T-wave inversion in lead V5 and V6. B: Magnetic resonance imaging showing late gadolinium enhancement. C: Metabolic positron emission tomography scan showing no inflammatory component.
Figure 3.
Left ventricle endomyocardial biopsy from subject II-2. A: Microscopic appearance showing diffuse fibrosis. B: Immunofluorescence microscopy showing a diminished signal for plakoglobin in subject II-2 (left) and a normal signal in a control (right).
The offspring (III-1, III-2, III-3; Figure 1B) of the proband (II-2), aged 44, 36, and 35 years, respectively, all presented with right bundle branch block morphology ventricular premature beats. They reported fatigue, dyspnea, and/or palpitations. Their electrocardiograms were all characterized by low voltages in peripheral leads, T-wave inversion in lateral leads (III-1), incomplete right bundle branch block (III-3), and otherwise normal traces (III-2). Cardiovascular magnetic resonance was suggestive of a left-dominant ACM in all 3. The ejection fractions were reduced in III-1 (ventricle left: 47%, right: 49%) and preserved in III-2 and III-3. Areas of late gadolinium enhancement restricted to the left ventricle were observed with a predominance for the subepicardial layer of the heart basal regions in the anteroseptal, anterolateral, inferior, and inferoseptal walls (III-1); anteroseptal, inferior, and inferoseptal walls (III-2); and anterior, anteroseptal, and anterolateral walls (III-3).
Given the familial nature of the cardiomyopathy, high-throughput DNA sequencing (using TruSight One panel from Illumina, San Diego, CA) was performed to screen 10 genes (ACTN2, DSG2, DSC2, PKP2, DSP, LDB3, JUP, RYR2, TGFβ3, TMEM43) involved in arrhythmogenic and related cardiomyopathies. In the proband (II-2) and in the 3 children (III-1, III-2, III-3), the ACTN2 missense variant c.1418A>G, p.Tyr473Cys was identified in heterozygosity (Figure 1A). No variants were found in the other genes of the panel, notably in JUP, encoding plakoglobin. For the reasons explained in the discussion section (below), the variant p.Tyr473Cys in ACTN2 was considered likely pathogenic and causative of the familial left-dominant ACM.
Discussion
Several ACTN2 mutations located in different regions of the protein, including the actin binding, spectrin-like, and calmodulin-like domains, have been associated to HCM.5,6,8 Among them, the p.Thr495Met variant, located in the second spectrin-like domain similarly as p.Tyr473Cys, was observed in 3 different families.5,8 The spectrin-like domain of alpha-actinin-2 is known to interact with different structural and signaling proteins.11 Less frequently, ACTN2 mutations have been associated with the phenotype of DCM, as for example in carriers of the p.Gln9Arg variant.9 In addition, members of 2 families with variants in the actin binding domain of alpha-actinin-2 presented with a more complex and heterogenous cardiac phenotype: the p.Ala119Thr variant was linked to DCM, LVNC, idiopathic ventricular fibrillation, and SCD,7 and the p.Met228Thr variant cosegregated with HCM, early-onset supraventricular arrhythmias, and atrioventricular block, as well as with LVNC.6 Finally, an association between ACTN2 and ARVD/C has already been reported in the ClinVar database (unpublished; p.Glu829Ter, SCV000740535). In summary, heterozygous missense mutations of ACTN2 are associated with a range of cardiac phenotypes, including structural and rhythmic disorders with a highly variable expressivity, even for a given variant.
The variant p.Tyr473Cys has been recently reported in association with a phenotype of DCM/HCM but was considered of “uncertain significance” owing to insufficient evidence to determine its role in the disease (unpublished; reported in the ClinVar database, SCV000834859). However, several arguments suggest a pathogenic role. First, p.Tyr473Cys segregates with the phenotype of left-dominant ACM in 4 members of our family. Second, the Tyr473 residue is highly conserved across various species, and its replacement with cysteine is estimated as deleterious by the prediction programs PolyPhen-2 and SIFT. Finally, this amino acid substitution has not been observed in the public database gnomAD as of April 2019. For these reasons, we consider p.Tyr473Cys as likely pathogenic even in the absence of direct functional proof.
The phenotype in affected members of our family is homogenous and corresponds well with left-dominant ACM as described by Sen-Chowdhry and colleagues1: ventricular arrhythmia of right bundle block configuration (ie, arising from the left ventricle), T-wave inversions in inferior or lateral leads, mild left ventricular dilation and/or systolic impairment, myocyte loss with fibrofatty or fibrotic replacement confirmed by biopsy, or late gadolinium enhancement in the left ventricle on cardiac magnetic resonance. While left-dominant ACM has not been associated with ACTN2 mutations so far, DCM, which is part of the phenotypic spectrum of ACTN2-related disease, shares common features with left-dominant ACM. The distinction between these 2 entities is mainly based on the degree of ventricular arrhythmias compared to the extent of morphologic abnormalities.1 It is hence not surprising to find a common genetic cause for these 2 pathologies, and we cannot exclude that the subjects of this study will develop some signs of DCM in the future. The exact mechanism by which the substitution of a tyrosine by a cysteine residue in position 473 could lead to an ACM remains to be determined. The double function of alpha-actinin-2 as a scaffold as well as an interactor with signaling proteins and ion channels11,12 might explain why its dysfunction leads to a phenotype (ie, ACM) in which both structural remodeling of cardiac muscle and ventricular arrhythmias are present. While ACMs are mainly caused by variants in desmosomal genes, left-dominant forms have often been related to anomalies of other genes.3,4 Interestingly, at least 2 of these genes, DES and FLNC, encode Z-disc-related proteins (desmin and filamin C).3,4 Thus, it is tempting to speculate that a primary disruption of the sarcomeric Z-disc can be, at least in some cases, central to the pathogenesis of left-dominant ACM.
Interestingly, immunohistology of the endomyocardial biopsy in the proband revealed a markedly diminished signal for plakoglobin, one of the desmosomal proteins. Asimaki and colleagues13 have already observed a decreased plakoglobin signal in ARVD/C. In our family, in which ACM appears to be caused by the nondesmosomal gene ACTN2, the observation of reduced plakoglobin signal was unexpected (of note, the plakoglobin and other desmosomal protein genes were normal). In this regard, our report further supports the concept that a reduced plakoglobin signal is characteristic of all ACMs independently of their origin and is not only the consequence of a primary alteration of desmosomal complex. Furthermore, our observations suggest that the decrease in plakoglobin signal is not specific for ARVD/C but also characterizes left-dominant ACM. The concept of common final pathway expresses the idea that the disruption of certain genes or proteins sharing a common function is central to the pathogenesis of a given cardiogenetic disease with a specific phenotype (for example, the alteration of sarcomere proteins in HCM).14 Our finding that plakoglobin was reduced without causative desmosomal gene abnormality supports the concept of a common final pathway and the hypothesis that ACMs are desmosomopathies. Although no direct interaction is known so far, it is tempting to speculate that alpha-actinin-2 might somehow interact with desmosomal proteins. Such an interaction might be mediated by desmin, a component of intermediate filaments that connects the Z-disc complex to desmosomes.15
Conclusion
Clinical investigations of a family with a phenotype compatible with a left-dominant ACM led to the identification of a likely pathogenic ACTN2 variant. This finding further expands the spectrum of human diseases linked with ACTN2. The description of a similar association in other families would help to confirm this hypothesis. Our report also suggests that a primary dysfunction of Z-disc could be central to the pathogenesis of left-dominant ACM. On the other hand, the decreased signal for plakoglobin in the myocardial biopsy of our index case supports the existence of a close link between Z-disc and desmosome. For the clinical practice, this suggests that ACTN2 variant screening might be considered in patients with ACM in whom ACM gene panel testing was negative.
Acknowledgments
The authors thank Prof. Andrea Superti-Furga for his thorough review and comments, which significantly contributed to improve the quality of the manuscript. The authors are grateful to the patients for their participation in this study.
References
- 1.Sen-Chowdhry S., Syrris P., Prasad S.K. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol. 2008;52:2175–2187. doi: 10.1016/j.jacc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Bauce B., Nava A., Beffagna G. Multiple mutations in desmosomal proteins encoding genes in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2010;7:22–29. doi: 10.1016/j.hrthm.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 3.Ortiz-Genga M.F., Cuenca S., Dal Ferro M. Truncating FLNC mutations are associated with high-risk dilated and arrhythmogenic cardiomyopathies. J Am Coll Cardiol. 2016;68:2440–2451. doi: 10.1016/j.jacc.2016.09.927. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez-Jimenez F.J., Carriel V., Brodehl A. Novel desmin mutation p.Glu401Asp impairs filament formation, disrupts cell membrane integrity, and causes severe arrhythmogenic left ventricular cardiomyopathy/dysplasia. Circulation. 2018;137:1595–1610. doi: 10.1161/CIRCULATIONAHA.117.028719. [DOI] [PubMed] [Google Scholar]
- 5.Chiu C., Bagnall R.D., Ingles J. Mutations in alpha-actinin-2 cause hypertrophic cardiomyopathy: a genome-wide analysis. J Am Coll Cardiol. 2010;55:1127–1135. doi: 10.1016/j.jacc.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Girolami F., Iascone M., Tomberli B. Novel alpha-actinin 2 variant associated with familial hypertrophic cardiomyopathy and juvenile atrial arrhythmias: a massively parallel sequencing study. Circ Cardiovasc Genet. 2014;7:741–750. doi: 10.1161/CIRCGENETICS.113.000486. [DOI] [PubMed] [Google Scholar]
- 7.Bagnall R.D., Molloy L.K., Kalman J.M., Semsarian C. Exome sequencing identifies a mutation in the ACTN2 gene in a family with idiopathic ventricular fibrillation, left ventricular noncompaction, and sudden death. BMC Med Genet. 2014;15:99. doi: 10.1186/s12881-014-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theis J.L., Bos J.M., Bartleson V.B. Echocardiographic-determined septal morphology in Z-disc hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2006;351:896–902. doi: 10.1016/j.bbrc.2006.10.119. [DOI] [PubMed] [Google Scholar]
- 9.Mohapatra B., Jimenez S., Lin J.H. Mutations in the muscle LIM protein and alpha-actinin-2 genes in dilated cardiomyopathy and endocardial fibroelastosis. Mol Genet Metab. 2003;80:207–215. doi: 10.1016/s1096-7192(03)00142-2. [DOI] [PubMed] [Google Scholar]
- 10.Frank D., Kuhn C., Katus H.A., Frey N. The sarcomeric Z-disc: a nodal point in signalling and disease. J Mol Med (Berl) 2006;84:446–468. doi: 10.1007/s00109-005-0033-1. [DOI] [PubMed] [Google Scholar]
- 11.Sjoblom B., Salmazo A., Djinovic-Carugo K. Alpha-actinin structure and regulation. Cell Mol Life Sci. 2008;65:2688–2701. doi: 10.1007/s00018-008-8080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cukovic D., Lu G.W., Wible B., Steele D.F., Fedida D. A discrete amino terminal domain of Kv1.5 and Kv1.4 potassium channels interacts with the spectrin repeats of alpha-actinin-2. FEBS Lett. 2001;498:87–92. doi: 10.1016/s0014-5793(01)02505-4. [DOI] [PubMed] [Google Scholar]
- 13.Asimaki A., Tandri H., Huang H. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- 14.Towbin J.A., McKenna W.J., Abrams D.J. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. doi: 10.1016/j.hrthm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Paulin D., Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 2004;301:1–7. doi: 10.1016/j.yexcr.2004.08.004. [DOI] [PubMed] [Google Scholar]