Abstract
Objective
The outcome of the androgen-deprivation therapy (ADT) may be affected by metabolic diseases such as diabetes mellitus (DM) and dyslipidemia and/or by their treatments. We aimed to evaluate the prognostic impact of these disorders and corresponding medications in Japanese men treated with ADT for prostate cancer.
Methods
This study retrospectively included 121 patients with metastatic prostate cancer who were treated with primary ADT at our hospital between 2001 and 2013. All patients received primary ADT with castration and/or an antiandrogen agent (bicalutamide or flutamide). Associations between clinicopathological factors, metabolic disease profiles, medication use, and prognosis (progression-free survival [PFS] and overall survival [OS]) were evaluated by univariate and multivariate analysis.
Results
The median follow-up time was 54.9 months, and the median PFS and OS were 23.9 months and 73.0 months, respectively. High serum glucose levels at baseline (hazard ratio [HR], 95% confidence interval [CI]: 2.12, 1.16–3.76; P = 0.015), and concurrent DM (HR, 95% CI: 2.07, 1.06–3.94; P = 0.034) were significantly associated with poorer OS after adjustment for age, prostate-specific antigen levels at diagnosis, Gleason score, and clinical stage. Treatment with sulfonylurea drugs was significantly associated with a reduced risk of disease progression in men with DM (HR, 95% CI: 0.36, 0.12–0.90; P = 0.028).
Conclusions
Impaired glucose tolerance and treatment with sulfonylureas have prognostic significance in prostate cancer. These findings demonstrate the importance of managing DM during ADT and point to a possible favorable effect of sulfonylureas on prostate cancer.
Keywords: Androgen-deprivation therapy, Diabetes mellitus, Dyslipidemia, Metastatic prostate cancer, Prognosis
1. Introduction
Androgen-deprivation therapy (ADT) is the standard treatment for recurrent or advanced prostate cancer.1 In 2015, docetaxel chemotherapy with ADT was reported to prolong both progression-free survival (PFS) and overall survival (OS) in the CHAARTED and STAMPEDE trials of men with metastatic hormone-sensitive prostate cancer.2,3 In 2017, combination treatment with the CYP17 inhibitor abiraterone and ADT was also reported to benefit survival in the LATITUDE and STAMPEDE trials of men with metastatic hormone-sensitive prostate cancer.4,5 Accordingly, upfront docetaxel chemotherapy or abiraterone combined with ADT have become standard therapies for metastatic hormone-sensitive prostate cancer.6
The metabolic diseases, diabetes mellitus (DM) and dyslipidemia, are established risk factors for atherosclerosis and cardiovascular disease.7 We previously reported an association between metabolic disorders and outcome after radical prostatectomy.8,9 Several studies have reported associations between an increased risk for the development of metabolic diseases and some prostate cancer treatments, including ADT and corticosteroids.10,11 Conversely, there is evidence that DM and dyslipidemia themselves affect the efficacy of ADT. For example, Shevach et al. reported worse outcomes of ADT in men with DM,12 and dyslipidemia has been associated with increased risk of recurrence after curative treatment.13 Similarly, antidiabetes and antidyslipidemia agents such as metformin and statins, respectively, have been suggested to have antitumor activity in prostate cancer.14,15 Thus, a number of associations have been reported between prostate cancer prognosis and metabolic diseases and their treatments. Accordingly, in this study, we investigated the prognostic significance of DM, dyslipidemia, and medication use in Japanese men receiving ADT for de novo metastatic prostate cancer.
2. Patients and methods
2.1. Patients
This study retrospectively enrolled 121 Japanese men with de novo metastatic prostate cancer who were treated with primary ADT at Kyushu University Hospital (Fukuoka, Japan) between 2001 and 2013, and who had full datasets available of pretreatment serum glucose, total cholesterol, and triglyceride levels; DM and dyslipidemia status; and medication status. We excluded (i) patients with ethnicities other than Japanese, (ii) patients who had received local treatment before primary ADT, and (iii) patients who received other treatments (such as chemotherapy) before disease progression. This study was approved by the Institutional Review Board of Kyushu University. A waiver of informed consent was granted by the Institutional Review Board, on the condition that the right of opt-out was provided to all patients.
All patients were histopathologically diagnosed with adenocarcinoma of the prostate. Of the 121 men, 109 (90.1%) were biopsied at Kyushu University Hospital, and 12 (9.9%) were biopsied at another institution (4 of the biopsy specimens were subsequently reviewed at our institution). Clinical staging was determined by the unified tumor, node, metastases (TNM) criteria, based on the results of digital rectal examinations, transrectal ultrasound, magnetic resonance imaging, computed tomography, and bone scans.16
2.2. Treatments and outcomes
All patients were treated by primary ADT with either surgical castration or medical castration using a luteinizing hormone-releasing hormone agonist/antagonist (goserelin acetate, leuprorelin acetate, or degarelix acetate) and/or an antiandrogen agent (bicalutamide or flutamide). Of the 121 men, 114 (94.2%) were treated with combined androgen blockade, and 7 (5.8%) were treated with castration alone. Progression was defined as an increase in prostate-specific antigen (PSA) levels of >2 ng/mL with a 25% increase over the nadir, or radiographic progression, which was defined as the appearance of two new lesions or the progression of one or more known lesions (as classified by the Response Evaluation Criteria in Solid Tumors).17 In case that PSA does not decline after ADT initiation, the PSA level before treatment was defined as nadir PSA. Serum testosterone level was measured in most cases when progression was observed, among whom the castrated level of serum testosterone (<50 ng/dL) was confirmed.
2.3. Statistical analysis
All statistical analyses were performed using JMP13 software (SAS Institute, Cary, NC, USA). Continuous and categorical data were analyzed by Wilcoxon's rank sum and Pearson's chi-square tests, respectively. Survival analyses were conducted by the Kaplan–Meier method with the log-rank test. The Cox proportional-hazards model was used to estimate hazard ratios (HRs). All P values were two-sided, and P < 0.05 was considered significant.
3. Results
The median follow-up time was 54.9 months (interquartile range [IQR], 24.9–80.9 months); during this time, 81 men (66.9%) experienced progression to castration-resistant prostate cancer (CRPC) and 59 men (48.8%) died from any cause. The median PFS and OS times were 23.9 months (IQR, 10.3–130.9 months) and 73.0 months (IQR, 35.9 months–not reached), respectively. Most patients presented with high PSA levels, high Gleason scores, and advanced TNM stage at initial diagnosis (Table 1). Serum glucose, total cholesterol, and triglyceride levels, as well as body mass index, were within normal range for most patients (Table 1). DM and dyslipidemia comorbidities were present in 27 (22.3%) and 22 (18.2%) men, respectively.
Table 1.
Patients' characteristics
| Characteristics | n = 121 |
|---|---|
| Age, years (IQR) | 72 (68-77) |
| PSA at diagnosis, ng/mL (IQR) | 202.9 (75.2-637.0) |
| Gleason score, n (%) | |
| ≤7 | 16 (14.0%) |
| 8-10 | 98 (86.0%) |
| NA | 7 |
| cT stage, n (%) | |
| T2/3a | 52 (44.1%) |
| T3b | 26 (22.0%) |
| T4 | 40 (33.9%) |
| NA | 3 |
| cN stage, n (%) | |
| N0 | 44 (36.7%) |
| N1 | 76 (63.3%) |
| cM stage, n (%) | |
| M1a | 8 (6.6%) |
| M1b | 108 (89.3%) |
| M1c | 5 (4.1%) |
| Glucose at diagnosis, mg/dL (IQR) | 106 (94-125) |
| NA | 1 |
| Total cholesterol at diagnosis, mg/dL (IQR) | 193 (163-218) |
| NA | 3 |
| Triglyceride, mg/dL (IQR) | 110 (76-158) |
| NA | 4 |
| Body mass index, kg/m2 (IQR) | 22.6 (20.5-24.4) |
| DM, n (%) | |
| Absence | 94 (77.7%) |
| Presence | 27 (22.3%) |
| Dyslipidemia, n (%) | |
| Absence | 99 (81.8%) |
| Presence | 22 (18.2%) |
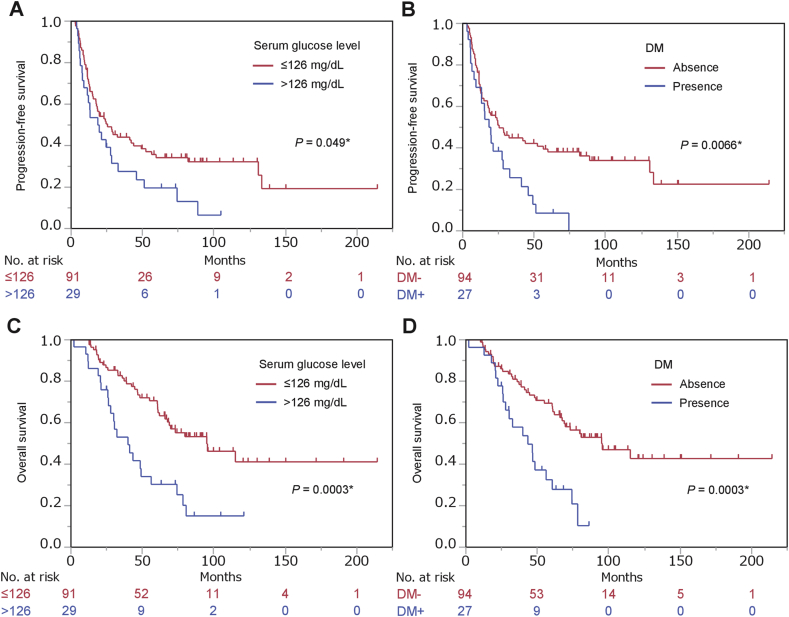
The prognostic significance of clinicopathological parameters for survival were evaluated by univariate and multivariate analysis. Gleason score and clinical T-stage were significantly associated with PFS, whereas age, PSA levels at diagnosis, clinical N-stage, and clinical M-stage were not associated in this cohort (Table 2). Among the parameters related to metabolic disorders, only the presence of DM was significantly associated with increased risk of progression in univariate analysis (HR, 95% confidence interval [CI]: 1.94, 1.17–3.12; P = 0.0002), although high serum glucose levels approached significance (HR, 95% CI: 1.61, 0.98–2.58; P = 0.059) (Fig. 1A and B, Table 2). However, in multivariate analysis, neither DM (HR, 95% CI: 1.25, 0.69–2.20; P = 0.45) nor high serum glucose (HR, 95% CI: 1.06, 0.61–1.79; P = 0.83) were associated with PFS after adjustment for age, PSA at diagnosis, Gleason score, and clinical stage (Table 2).
Table 2.
Univariate and multivariate analyses for progression-free survival
| Variable | Univariate |
P | Multivariatea |
P |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age (range) | 3.10 (0.98–10.03) | 0.055 | ||
| PSA at diagnosis (range) | 2.60 (0.71–7.39) | 0.14 | ||
| Gleason score | ||||
| ≤7 | Ref | |||
| 8–10 | 2.68 (1.26–6.94) | 0.0086* | ||
| cT stage | ||||
| T2/3a | Ref | |||
| T3b | 1.19 (0.64–2.13) | 0.57 | ||
| T4 | 2.25 (1.36–3.72) | 0.0016* | ||
| cN stage | ||||
| N0 | Ref | |||
| N1 | 1.56 (0.98–2.56) | 0.061 | ||
| cM stage | ||||
| M1a | Ref | |||
| M1b | 1.40 (0.62–3.98) | 0.45 | ||
| M1c | 0.32 (0.017–1.98) | 0.24 | ||
| Glucose | ||||
| <126 mg/dL | Ref | ref | ||
| ≥126 mg/dL | 1.61 (0.98–2.58) | 0.059 | 1.06 (0.61-1.79) | 0.83 |
| Total cholesterol | ||||
| <220 mg/dL | Ref | Ref | ||
| ≥220 mg/dL | 0.91 (0.51–1.53) | 0.73 | 1.21 (0.65–2.14) | 0.53 |
| Triglyceride | ||||
| <150 mg/dL | Ref | Ref | ||
| ≥150 mg/dL | 0.97 (0.57–1.58) | 0.97 | 1.28 (0.74–2.14) | 0.37 |
| Body mass index | ||||
| <25 kg/m2 | Ref | Ref | ||
| ≥25 kg/m2 | 0.81 (0.44–1.38) | 0.45 | 0.89 (0.47–1.56) | 0.69 |
| DM | ||||
| Absence | Ref | Ref | ||
| Presence | 1.94 (1.17–3.12) | 0.011* | 1.25 (0.69–2.20) | 0.45 |
| Dyslipidemia | ||||
| Absence | Ref | Ref | ||
| Presence | 1.10 (0.59–1.92) | 0.75 | 1.31 (0.67–2.41) | 0.42 |
*Statistically significant.
Adjusted by age, PSA at diagnosis, Gleason score, and clinical stage.
Fig. 1.
Survival curves of men with prostate cancer stratified by serum glucose concentration and comorbid diabetes mellitus (DM). Progression-free survival (A, B) and overall survival (C, D) of 121 men stratified by serum glucose levels (A, C) and comorbid DM (B, D).
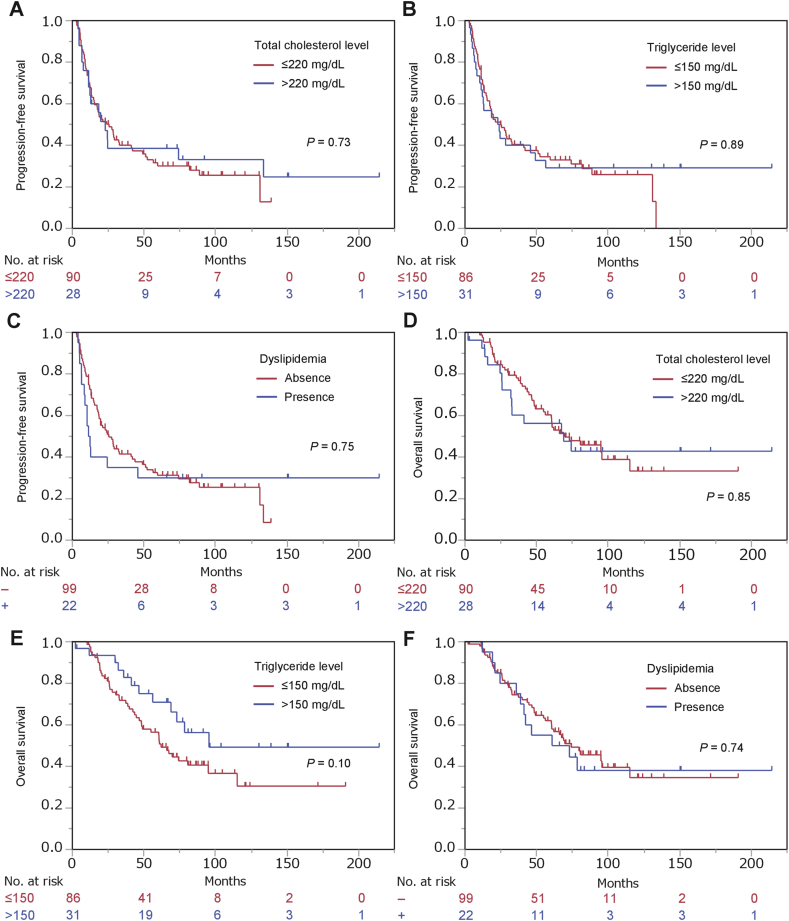
A significant association was detected between OS and Gleason score and clinical T-stage but not age, PSA levels at diagnosis, clinical N-stage, or clinical M-stage (Table 3). Among the parameters related to metabolic disorders, high serum glucose levels (HR, 95% CI: 2.60, 1.50–4.38; P = 0.0009) and DM (HR, 95% CI: 2.70, 1.52–4.68; P = 0.0010) were significantly associated with increased risk of progression in univariate analysis (Fig. 1C and D, Table 3). In multivariate analysis, both parameters (high serum glucose and DM) remained significantly associated with OS after adjustment for age, PSA at diagnosis, Gleason score, and clinical stage (HR, 95% CI: 2.12, 1.16–3.76; P = 0.015 and HR, 95% CI: 2.07, 1.06–3.94; P = 0.034, respectively) (Table 3). No parameters related to dyslipidemia were significantly associated with either PFS or OS (Supplementary Fig. 1, Table 2, Table 3) (see Table 4).
Table 3.
Univariate and multivariate analyses for overall survival
| Variable | Univariate |
P | Multivariatea |
P |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age (range) | 3.80 (0.93–15.92) | 0.063 | ||
| PSA at diagnosis (range) | 3.57 (0.87–10.83) | 0.073 | ||
| Gleason score | ||||
| ≤7 | Ref | |||
| 8-10 | 2.76 (1.12–9.16) | 0.025* | ||
| cT stage | ||||
| T2/3a | Ref | |||
| T3b | 1.45 (0.72–2.86) | 0.29 | ||
| T4 | 2.01 (1.11–3.67) | 0.022* | ||
| cN stage | ||||
| N0 | Ref | |||
| N1 | 1.20 (0.71–2.12) | 0.51 | ||
| cM stage | ||||
| M1a | Ref | |||
| M1b | 1.06 (0.43–3.52) | 0.91 | ||
| M1c | 0.41 (0.021–2.81) | 0.39 | ||
| Glucose | ||||
| <126 mg/dL | Ref | Ref | ||
| ≥126 mg/dL | 2.60 (1.50–4.38) | 0.0009* | 2.12 (1.16–3.76) | 0.015* |
| Total cholesterol | ||||
| <220 mg/dL | Ref | Ref | ||
| ≥220 mg/dL | 1.06 (0.56–1.89) | 0.85 | 1.26 (0.64–2.36) | 0.49 |
| Triglyceride | ||||
| <150 mg/dL | Ref | Ref | ||
| ≥150 mg/dL | 0.59 (0.30–1.08) | 0.090 | 0.76 (0.37–1.44) | 0.41 |
| Body mass index | ||||
| <25 kg/m2 | Ref | Ref | ||
| ≥25 kg/m2 | 0.60 (0.28–1.17) | 0.14 | 0.64 (0.28–1.31) | 0.24 |
| DM | ||||
| Absence | Ref | Ref | ||
| Presence | 2.70 (1.52–4.68) | 0.0010* | 2.07 (1.06–3.94) | 0.034* |
| Dyslipidemia | ||||
| Absence | Ref | Ref | ||
| Presence | 1.11 (0.56–2.03) | 0.75 | 1.46 (0.72–2.78) | 0.28 |
*Statistically significant.
Adjusted by age, PSA at diagnosis, Gleason score, and clinical stage.
Table 4.
Univariate analysis for progression-free survival and overall survival among men according to medication for diabetes mellitus
| Variable | n | Progression-free survival |
P | Overall survival |
P |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||||
| Sulfonylurea | |||||
| Absence | 16 | Ref | Ref | ||
| Presence | 11 | 0.36 (0.12–0.90) | 0.028* | 0.52 (0.18–1.30) | 0.17 |
| Biguanide | |||||
| Absence | 22 | Ref | Ref | ||
| Presence | 5 | 1.09 (0.36–2.76) | 0.87 | 1.06 (0.30–2.95) | 0.92 |
| DPP-4 inhibitor | |||||
| Absence | 20 | Ref | Ref | ||
| Presence | 7 | 0.94 (0.34–2.29) | 0.90 | 1.12 (0.39–2.90) | 0.82 |
| α-glucosidase inhibitor | |||||
| Absence | 22 | Ref | Ref | ||
| Presence | 5 | 0.40 (0.093–1.17) | 0.10 | 0.66 (0.15–2.03) | 0.50 |
| Insulin | |||||
| Absence | 24 | Ref | Ref | ||
| Presence | 3 | 0.54 (0.086–1.88) | 0.38 | 0.80 (0.13–2.82) | 0.76 |
*Statistically significant.
Finally, we analyzed the impact of medications for DM on PFS and OS. Among the six types of medications taken by the men with DM (sulfonylureas, biguanides, dipeptidyl peptidase-4 inhibitors, α-glucosidase inhibitors, and insulin), sulfonylurea treatment was significantly associated with longer PFS (HR, 95% CI: 0.36, 0.12–0.90; P = 0.028) and showed a trend, albeit not significant, toward association with longer OS (HR, 95% CI: 0.52, 0.18–1.30; P = 0.17). None of the other medications was significantly associated with PFS or OS.
4. Discussion
This study examined the prognostic significance of metabolic disease-related factors in men receiving ADT for metastatic prostate cancer. Our results indicate that high serum glucose and DM are possible adverse prognostic factors for OS, which is consistent with several retrospective studies identifying impaired glucose tolerance as an adverse prognostic factor in patients with prostate cancer when treated with ADT.12,18 In a study in the US, Shevach et al. reported that DM was associated with a shorter time to CRPC in men receiving ADT for non-metastatic advanced prostate cancer.12 Similarly, DM predicted worse OS for elderly Chinese men (>75 years of age) receiving ADT for advanced prostate cancer.18 Interestingly, androgen receptor expression has been reported to be higher in prostate cancer tissues from men with DM than those without DM.19 In a mouse xenograft study, a high carbohydrate diet resulted in impaired glucose tolerance and promoted progression to CRPC by increasing intra-tumoral androgen synthesis.20 These findings may at least partially explain our observation that the outcome of ADT was worse for men with DM. Notably, our study showed an association between impaired glucose tolerance and OS, but not PFS, suggesting that impaired glucose tolerance is strongly associated with survival after progression to CRPC. A meta-analysis of three phase 3 trials found that DM was not associated with prognosis for men with chemotherapy-naïve CRPC treated with docetaxel.21 It will be interesting to determine whether impaired glucose tolerance influences the outcome of agents that target the androgen receptor axis, such as abiraterone and enzalutamide. Indeed, a retrospective study of prostate cancer in the US showed that men with hyperglycemia, but not with hyperlipidemia, had a poor response to abiraterone and enzalutamide.22
In our study, dyslipidemia-associated factors had no significant impact on the outcome of ADT. To date, an association between dyslipidemia and prostate cancer outcome has only been reported for patients receiving curative therapy.13,23 To our knowledge, this is the first report of the prognostic impact of dyslipidemia in patients receiving ADT.
The adverse prognostic significance of impaired glucose tolerance identified in this study suggests the importance of close management of DM during ADT. Intriguingly, several antidiabetic agents have been suggested to affect the proliferation and survival of prostate cancer cells and the outcome of prostate cancer.14,24, 25, 26, 27, 28, 29 In particular, biguanide metformin was shown to suppress the incidence and progression of prostate cancer in preclinical and clinical studies.14,24 Metformin was found to be associated with reduced mortality risk in a US study of prostate cancer treated with ADT.25 Downregulation of dipeptidyl peptidase-4 (DPP-4) was recently reported to accelerate the progression to CRPC, suggesting that inhibitors of this enzyme may be detrimental for prostate cancer patients.26 However, we did not detect an association between survival and either biguanides or DPP-4 inhibitors in our cohort. Rather, we found that sulfonylurea treatment was associated with a reduced risk of progression to CRPC. The sulfonylurea glipizide was previously reported to suppress prostate cancer progression in the TRAMP transgenic mouse model by inhibiting tumor-associated angiogenesis.27 Sulfonylureas may also affect the androgen milieu by inhibiting aldo-keto reductase 1C,28 and an association between their use and a reduced incidence of prostate cancer was detected in a prospective study in Sweden.29 The present study is the first to show a possible anticancer effect of sulfonylureas for patients treated with ADT, which suggests that this class of compounds may be a favorable option for patients with impaired glucose tolerance.
This study has several limitations. The sample size was relatively small, and the study design was retrospective. The presence of metabolic disorders and medication use may have been overlooked in some cases. The changes on the status of DM and dyslipidemia after ADT initiation were not examined in this study. In addition, a small fraction of the cohort lacked information on either serum glucose or lipid level. The accrual period was long and included the period before several novel agents for CRPC were introduced (e.g., abiraterone acetate, enzalutamide, radium-233, docetaxel, and cabazitaxel). However, a strength of our study is that it included only Japanese men with de novo metastatic prostate cancer, which ensured a homogenous study population.
In conclusion, this study showed that impaired glucose tolerance, but not dyslipidemia, has prognostic significance for ADT-treated patients, suggesting that DM management is important. Especially, sulfonylureas may have a favorable effect on prognosis by antitumor effects through antiangiogenesis and modulation of androgen milieu. However, further investigations in other ethnic groups will be required to verify these findings.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
We thank Anne M. O'Rourke, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2019.10.003.
Grant support
This work was supported by JSPS KAKENHI grant (17K11145).
Appendix A. Supplementary data
The following are the supplementary data to this article:
Fig. S1.
References
- 1.Shiota M., Eto M. Current status of primary pharmacotherapy and future perspectives toward upfront therapy for metastatic hormone-sensitive prostate cancer. Int J Urol. 2016;23:360–369. doi: 10.1111/iju.13091. [DOI] [PubMed] [Google Scholar]
- 2.Sweeney C.J., Chen Y.H., Carducci M., Liu G., Jarrard D.F., Eisenberger M. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James N.D., Sydes M.R., Clarke N.W., Mason M.D., Dearnaley D.P., Spears M.R. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fizazi K., Tran N., Fein L., Matsubara N., Rodriguez-Antolin A., Alekseev B.Y. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 5.James N.D., de Bono J.S., Spears M.R., Clarke N.W., Mason M.D., Dearnaley D.P. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuhn P., De Bono J.S., Fizazi K., Freedland S.J., Grilli M., Kantoff P.W. Update on systemic prostate cancer therapies: Management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019;75:88–99. doi: 10.1016/j.eururo.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Schofield J.D., Liu Y., Rao-Balakrishna P., Malik R.A., Soran H. Diabetes Dyslipidemia. Diabetes Ther. 2016;7:203–219. doi: 10.1007/s13300-016-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiota M., Yokomizo A., Takeuchi A., Imada K., Kiyoshima K., Inokuchi J. The feature of metabolic syndrome is a risk factor for biochemical recurrence after radical prostatectomy. J Surg Oncol. 2014;110:476–481. doi: 10.1002/jso.23677. [DOI] [PubMed] [Google Scholar]
- 9.Shiota M., Takeuchi A., Sugimoto M., Kashiwagi E., Dejima T., Kiyoshima K. The differential impact of body mass index and the feature of metabolic syndrome on oncological outcomes following different surgical procedures in Japanese men with prostate cancer. Ann Surg Oncol. 2017;24:1443–1450. doi: 10.1245/s10434-016-5705-2. [DOI] [PubMed] [Google Scholar]
- 10.Mitsuzuka K., Arai Y. Metabolic changes in patients with prostate cancer during androgen deprivation therapy. Int J Urol. 2018;25:45–53. doi: 10.1111/iju.13473. [DOI] [PubMed] [Google Scholar]
- 11.Fardet L., Fève B. Systemic glucocorticoid therapy: a review of its metabolic and cardiovascular adverse events. Drugs. 2014;74:1731–1745. doi: 10.1007/s40265-014-0282-9. [DOI] [PubMed] [Google Scholar]
- 12.Shevach J., Gallagher E.J., Kochukoshy T., Gresia V., Brar M., Galsky M.D. Concurrent diabetes mellitus may negatively influence clinical progression and response to androgen deprivation therapy in patients with advanced prostate cancer. Front Oncol. 2015;5:129. doi: 10.3389/fonc.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macleod L.C., Chery L.J., Hu E.Y., Zeliadt S.B., Holt S.K., Lin D.W. Metabolic syndrome, dyslipidemia and prostate cancer recurrence after primary surgery or radiation in a veterans cohort. Prostate Cancer Prostatic Dis. 2015;18:190–195. doi: 10.1038/pcan.2015.12. [DOI] [PubMed] [Google Scholar]
- 14.Zingales V., Distefano A., Raffaele M., Zanghi A., Barbagallo I., Vanella L. Metformin: a bridge between diabetes and prostate cancer. Front Oncol. 2017;7:243. doi: 10.3389/fonc.2017.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raval A.D., Thakker D., Negi H., Vyas A., Kaur H., Salkini M.W. Association between statins and clinical outcomes among men with prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2016;19:151–162. doi: 10.1038/pcan.2015.58. [DOI] [PubMed] [Google Scholar]
- 16.International Union Against Cancer . Urologic Tumors. Prostate. In: Sobin L.H., Wittekind C.H., editors. TNM Classification of Malignant Tumors. 5th edn. John Wiley & Sons; New York: 1997. pp. 170–173. [Google Scholar]
- 17.Scher H.I., Halabi S., Tannock I., Morris M., Sternberg C.N., Carducci M.A. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu M.B., Yang T., Hu J.M., Zhu W.H., Jiang H.W., Ding Q. Prognostic factors in Chinese patients with prostate cancer receiving primary androgen deprivation therapy: validation of Japan Cancer of the Prostate Risk Assessment (J-CAPRA) score and impacts of pre-existing obesity and diabetes mellitus. Int J Clin Oncol. 2018;23:591–598. doi: 10.1007/s10147-017-1236-5. [DOI] [PubMed] [Google Scholar]
- 19.Lutz S.Z., Hennenlotter J., Scharpf M.O., Sailer C., Fritsche L., Schmid V. Androgen receptor overexpression in prostate cancer in type 2 diabetes. Mol Metab. 2018;8:158–166. doi: 10.1016/j.molmet.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fokidis H.B., Yieng Chin M., Ho V.W., Adomat H.H., Soma K.K., Fazli L. A low carbohydrate, high protein diet suppresses intratumoral androgen synthesis and slows castration-resistant prostate tumor growth in mice. J Steroid Biochem Mol Biol. 2015;150:35–45. doi: 10.1016/j.jsbmb.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Rahman O. Impact of diabetes on the outcomes of patients with castration-resistant prostate cancer treated with docetaxel: a pooled analysis of three phase III studies. Clin Genitourin Cancer. 2019;17:e104–e112. doi: 10.1016/j.clgc.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Karantanos T., Karanika S., Gignac G. Uncontrolled diabetes predicts poor response to novel antiandrogens. Endocr Relat Cancer. 2016;23:691–698. doi: 10.1530/ERC-16-0222. [DOI] [PubMed] [Google Scholar]
- 23.Schnoeller T.J., Jentzmik F., Schrader A.J., Steinestel J. Influence of serum cholesterol level and statin treatment on prostate cancer aggressiveness. Oncotarget. 2017;8:47110–47120. doi: 10.18632/oncotarget.16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imada K., Shiota M., Kuroiwa K., Sugimoto M., Abe T., Kohashi K. FOXO3a expression regulated by ERK signaling is inversely correlated with Y-box binding protein-1 expression in prostate cancer. The Prostate. 2017;77:145–153. doi: 10.1002/pros.23254. [DOI] [PubMed] [Google Scholar]
- 25.Richards K.A., Liou J.I., Cryns V.L., Downs T.M., Abel E.J., Jarrard D.F. Metformin use is associated with improved survival for patients with advanced prostate cancer on androgen deprivation therapy. J Urol. 2018;200:1256–1263. doi: 10.1016/j.juro.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 26.Russo J.W., Gao C., Bhasin S.S., Voznesensky O.S., Calagua C., Arai S. Downregulation of dipeptidyl peptidase 4 accelerates progression to castration-resistant prostate cancer. Cancer Res. 2018;78:6354–6362. doi: 10.1158/0008-5472.CAN-18-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi C., Bin Li, Yang Y., Yang Y., Li J., Zhou Q. Glipizide suppresses prostate cancer progression in the TRAMP model by inhibiting angiogenesis. Sci Rep. 2016;6:27819. doi: 10.1038/srep27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y., Zheng X., Zhang H., Zhai J., Zhang L., Li C. In vitro inhibition of AKR1Cs by sulphonylureas and the structural basis. Chem Biol Interact. 2015;240:310–315. doi: 10.1016/j.cbi.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Häggström C., Van Hemelrijck M., Zethelius B., Robinson D., Grundmark B., Holmberg L. Prospective study of Type 2 diabetes mellitus, anti-diabetic drugs and risk of prostate cancer. Int J Cancer. 2017;140(3):611–617. doi: 10.1002/ijc.30480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.