Abstract
Background
Surgical patients are getting older with increasing comorbidity. Acute kidney injury (AKI) is a commonly underestimated perioperative complication. 2–18% of hospitalized patients and 22–57% of patients in the intensive care unit develop AKI. Even though it has a major impact on patients’ outcomes, it goes unrecognized in 57–75.6% of cases.
Methods
This review is based on pertinent papers retrieved by a selective search in PubMed and the Cochrane Library employing the searching terms “acute kidney injury,” “biomarker,” “perioperative,” “renal function,” and “KDIGO.”
Results
The pathophysiology of AKI is complex. Conventional biomarkers are either not specific enough (urine output) or not sensitive enough (serum creatinine) for timely diagnosis. In view of the pathophysiology of the condition and the limited treatment options for it, the early detection of subclinical AKI (kidney damage without functional impairment) would seem to be a reasonable first step toward the prevention of worsening or permanent renal injury. New biomarkers of damage enable the early initiation of nephroprotective interventions. According to the “Kidney Disease: Improving Global Outcomes” (KDIGO) statement, a multimodal treatment approach is needed, including, among other things, optimization of hemodynamics and the discontinuation of nephrotoxic drugs.
Conclusion
It is essential to identify patients at risk and sensitize the treating personnel to the implementation of the guidelines. The incorporation of new biomarkers into routine clinical practice is also reasonable and necessary. Future clinical trials must show in what form these biomarkers should be used (singly or collectively).
The treatment of elderly multimorbid patients is assuming greater importance in surgical medicine. Avoidance of complications is important in the perioperative phase, and in this context the magnitude and significance of acute kidney injury (AKI) are often underestimated (1). Between 2% and 18% of all patients admitted to the hospital (2, 3) develop AKI during their hospital stay, as do 22% to 57% of intensive care patients (4, 5). This leads to an increase of 3% to 30% in mortality, more work for medical staff, and additional costs (6– 8). The mortality among patients who become dependent on dialysis during their hospital stay is drastically increased and lies between 14% and 41% (9). Despite all this, AKI seems not to receive adequate recognition or attention: in Germany and elsewhere, 57% to 75.6% of cases go undetected, undiagnosed, and/or undocumented (10– 13). As well as leading to significant underestimation of prevalence, this implies a lack of knowledge and awareness. AKI is an independent risk factor for the occurrence of cardiovascular/cerebrovascular complications and the development of chronic renal insufficiency (4, 14, 15). It is therefore morally, medically, and economically imperative to broaden the awareness of AKI.
Methods
Prevalence.
Between 2% and 18% of patients admitted to the hospital and 22% to 57% of intensive care patients develop AKI during their hospital stay.
The databases PubMed and Cochrane Library were selectively searched for records featuring the terms “acute kidney injury,” “biomarker,” “perioperative,” “renal function,” or “KDIGO,” with the emphasis on studies published in the past 15 years.
Independent risk factor.
Acute kidney injury is an independent risk factor for the occurrence of cardiovascular/cerebrovascular complications and the development of chronic renal insufficiency.
Learning goals
After finishing this article, the reader should be:
Aware that an unrecognized and untreated AKI and its consequences are significant
Familiar with the multimodal approach for prevention and treatment of AKI
Thoroughly acquainted with the complex pathomechanism of AKI.
Definition
Acute kidney injury is diagnosed and classified according to established criteria (the three-stage KDIGO [Kidney Disease: Improving Global Outcome] classification of severity) (16). The KDIGO staging is based on the determination of two readily available parameters, serum creatinine concentration and urine output (table). For more detail, please see the recently published review article by Alscher et al. (17).
Table. KDIGO: AKI classification and criteria.
| Severity | Serum creatinine concentration | Urine excretion |
| 1 | 1.5- to 1.9-fold within 7 d or ≥ 0.3 mg/dL within 48 h |
<0.5 ml/kg/h over a period >6 h |
| 2 | 2.0- to 2.9-fold | <0.5 ml/kg/h for >12 h |
| 3 | ≥ 3-fold or serum creatinine ≥ 4 mg/dL with an acute increase ≥ 0.5 mg/dL | <0.3 ml/kg/h for >24 h or anuria >12 h |
AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes
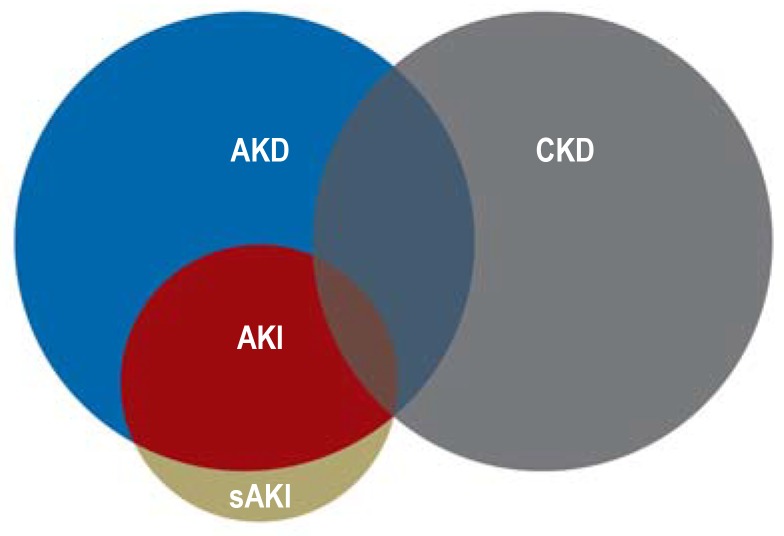
AKI is a broad clinical syndrome that embraces various etiologies. It affects both renal structure and renal function, and is characterized by abrupt deterioration of the latter. AKI thus includes renal failure but encompasses more than that. Kidney injury unaccompanied by loss of function (subclinical AKI) may also occur, and AKI may be reversible (figure 1) (16).
Figure 1.
Schematic depiction of the relationships between acute kidney disease/disorder (AKD), acute kidney injury (AKI), subclinical AKI (sAKI), and chronic kidney disease (CKD)
Physiology and pathophysiology
In the situation of normotension, perfusion of the kidney is maintained by renal autoregulation. The goal is to ensure a sufficient glomerular filtration rate by means of “redistribution” despite fluctuations in renal blood flow.
Definition.
The diagnosis of acute kidney injury is based on determination of two readily available parameters, serum creatinine concentration and urine output.
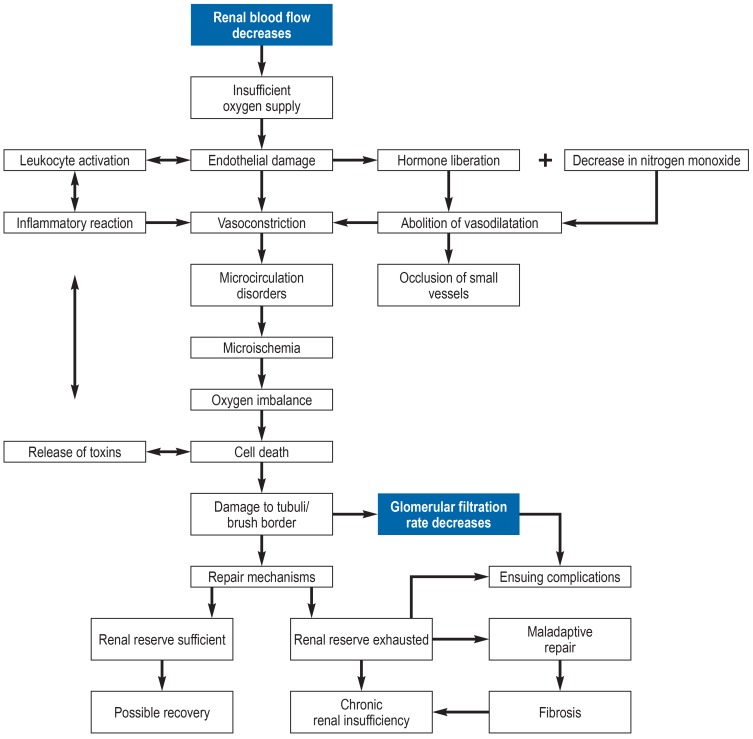
In the perioperative period various factors can act to reduce the renal blood flow, thus leading to renal hypotension and consequently an insufficient supply of oxygen (figure 2). At this point two potent renal vasoconstrictors can be mentioned: antidiuretic hormone (ADH), which is released in greater quantities at times of stress, and angiotensin II (18, 19). Regional changes in renal blood flow arise, damaging the endothelium and triggering an inflammatory reaction (20). Disruption of the microcirculation (20, 21) and occlusion of small-caliber vessels lead to local microischemia.
Figure 2.
Flow chart of the pathophysiology of acute kidney injury
Owing to its low oxygenation and the nature of its vascular architecture, the outer medulla of the kidney is very sensitive to changes in perfusion and oxygen supply (22). Furthermore, the blood supply is compromised by locally occurring edema. The imbalance between production of and demand for oxygen and nutrients harms the tubular epithelial cells. Cytokines are released, again attracting inflammatory cells and damaging tubular cells. A vicious circle arises and harmful waste products accumulate. In parallel, repair mechanisms are initiated (21). Mild kidney injury can be compensated and noticeable loss of function avoided. If this renal reserve is exhausted, long-term kidney disease may result.
Urine and serum creatinine: functional biomarkers
Physiology and pathophysiology.
In the situation of normotension, perfusion of the kidney is maintained by renal autoregulation. The goal is to ensure a sufficient glomerular filtration rate by means of “redistribution” despite fluctuations in renal blood flow.
Kidney function is evaluated by determination of the amount of urine excreted and the serum concentration of creatinine. Urine and serum creatinine serve as functional biomarkers, but are of limited value. Around 400–500 mL of urine has to be excreted each day to eliminate nitrogen-containing waste products. Perioperative factors (figure 2) often lead to oliguria (urine <0.5 mL/kg/h). One way of determining a patient’s precise glomerular filtration rate would be to measure the amount of urine excreted each hour (or 24 h). This approach is, however, cumbersome, time-consuming, and error-prone (e.g., distortion by medication), especially in patients with pre-existing AKI. Other factors also contribute to oliguria, such as stress-related release of ADH, which inhibits the excretion of free water. In the past, a perioperative decrease in urine output was frequently treated with volume substitution, which resulted in hypervolemia and tissue edema (see “Hemodynamics” below). Because the kidney itself is surrounded by a tissue capsule, edema can contribute to elevated pressure within the capsule and thus to reduced organ perfusion. A general increase in abdominal pressure, e.g., during laparoscopic surgery or in the presence of abdominal compartment syndrome, also lowers the amount of urine produced. Studies have demonstrated that an decreased urine output is associated with a poorer outcome of treatment in both adults (23) and children (24). However, a decreased amount of urine does not necessarily indicate a renal function disorder. (Short) episodes of oliguria are not always associated with AKI as defined in the current KDIGO criteria (25– 27).
Perioperative urine output is affected by many different factors and thus possesses only low specificity. Transient or less pronounced changes go undetected, although the kidney may already have been injured.
Limitations also apply to serum creatinine. It is the end product of the creatine phosphate metabolic pathway, which primarily serves to supply energy to muscle, liver, and brain. The concentration of creatinine in serum is determined principally by muscle mass, nutrition, activity, and hemodilution. Cephalosporins and barbiturates can also increase the serum concentration. The serum creatinine concentration and the glomerular filtration rate have an exponentially antiproportional relationship to one another: even only slight elevations of serum creatinine cause a reduction of = 50% in renal function, which is associated with a poorer prognosis (28, 29). The relationship is hyperbolic: even when 50% of functional kidney tissue is lost, there is “only” a doubling of serum creatinine concentration, which thus possesses low sensitivity. Moreover, the concentration gives no indication of the renal reserve (30).
Particularly in elderly and muscle-deficient patients, the serum creatinine concentration can be near normal despite a marked reduction in the glomerular filtration rate. In this situation, determination of serum creatinine may lead to significant overestimation of renal function (31). The rise in serum creatinine level and the decrease in urine output are not early enough indicators, and their sensitivity and specificity are too low for timely diagnosis of AKI (32). On pathophysiological grounds, however, it can be assumed that early intervention is a crucial factor for success of treatment. This justifies the search for new biomarkers that not only show deterioration of renal function but also permit earlier identification of structural damage to the kidney (32).
New injury markers
Biomarker “urine output”.
Perioperative urine output is affected by many different factors and thus possesses only low specificity. Transient or less pronounced changes go undetected, although the kidney may already have been injured.
Investigations have demonstrated that various new renal biomarkers show the presence of kidney injury (33– 35). The goal is to identify and treat subclinical AKI. Studies have emphasized that many new biomarkers are indeed superior to the conventional markers in early diagnosis, determination of prognosis, and estimation of long-term mortality. Perioperative increases in concentration were significantly associated with the occurrence of postoperative AKI (36– 40, e1– e3). For example, the product of TIMP-2 and IGFBP7 predicted moderate to severe AKI with 92% accuracy. In contrast to the aforementioned glomerular processes, the new injury markers are released in the tubules—the most likely site of injury. They permit conclusions regarding various injury mechanisms and aspects of renal function (36– 38). These markers include NGAL, TIMP-2, IGFBP7, and cystatin C, all of which can be determined more and more economically via routine laboratory procedures and point-of-care systems. A more detailed account would exceed the scope of this article.
The concept of angina renalis,subclinical AKI,and biomarker-guided treatment
New injury markers.
Studies have emphasized that many new biomarkers are superior to the conventional markers in early diagnosis, determination of prognosis, and estimation of long-term mortality.
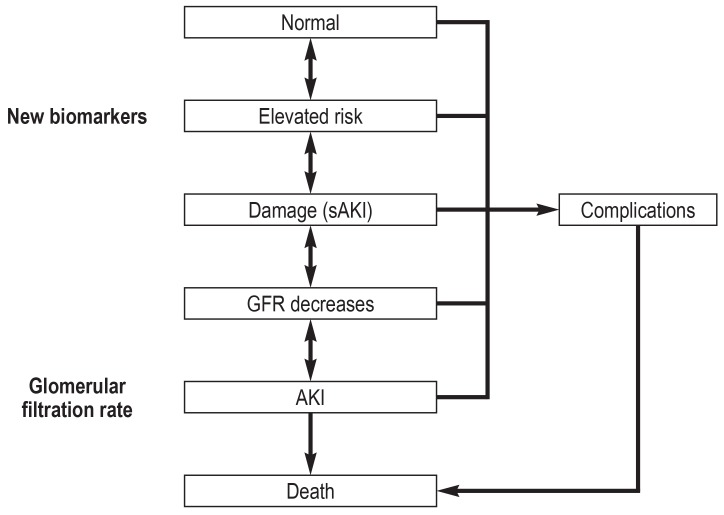
Sensitive biomarkers such as troponin have helped to improve the early detection and treatment of ischemic myocardial insults. The year 2010 saw the introduction of the concept of “angina renalis.” In contrast to acute coronary syndrome, AKI is asymptomatic, more complex in origin, and diagnosis using conventional functional markers is delayed, postponing the initiation of treatment. For these reasons, the two clinical presentations are difficult to compare. The concept of angina renalis is intended to achieve early renal risk stratification with the aid of biomarkers. This is the context in which the term “subclinical AKI” was coined to describe a biomarker-positive yet (still) serum creatinine-normal status—kidney injury without functional impairment (e4) (figure 1). Biomarker-positive patients seem already to be at higher risk of complications (renal replacement therapy: odds ratio [OR] 16.4, 95% confidence interval [3.6; 76.9], p = 0.001; hospital mortality: OR 2.8 [1.9; 4.1], p = 0.001) (e5, e6). Although an increase in the biomarkers predicts AKI and a subsequent rise in serum creatinine concentration, the (current) situation is that, by definition, AKI can be diagnosed only on the basis of changes in the serum concentration of creatinine or in the amount of urine produced. According to a consensus definition published in 2017, AKI can also be identified on the basis of a biomarker increase without a change in serum creatinine (e7). The early release of biomarkers in the injured renal tubuli could open a time window to prevent further damage and thus avoid impairment of renal function (figure 3) (e8). The additional implementation of clinical risk scores (e.g., the renal risk index) and consideration of the clinical context increase the informational worth and improve the negative predictive value (e9– e11). In patients with pre-existing AKI, the furosemide stress test can be carried out to estimate the severity of the AKI. This is useful for further stratification of the risk (AKI progression, need for renal replacement therapy, mortality) (e12, e13).
Figure 3.
Schematic model of acute kidney injury
In subclinical acute kidney injury (sAKI) there is a biomarker-positive status without loss of function. If the damage progresses, the glomerular filtration rate (GFR) decreases further and acute kidney injury (AKI) arises. While the GFR goes down, the concentrations of the biomarkers increase
The concept of “angina renalis”.
The “angina renalis” concept is intended to achieve early renal risk stratification with the aid of biomarkers. This is the context in which the term “subclinical AKI” was coined.
Further risk stratification.
In patients with pre-existing AKI, the furosemide stress test can be carried out to estimate the severity of the AKI. This is useful for further stratification of the risk.
The new biomarkers represent an important means of detecting AKI and planning the appropriate treatment. They should therefore be implemented in routine clinical practice, provided the local health care system has the necessary resources—ideally together with the KDIGO bundle.
Treatment options in the perioperative phase
It was long assumed that AKI was neither preventable nor treatable. The findings of more recent studies led to a paradigm shift.
The KDIGO bundle
The KDIGO bundle is a multimodal approach and recommends prophylactic measures to be carried out in patients at high risk of AKI. These comprise avoidance of nephrotoxic substances, optimization of volume status and perfusion pressure, maintenance of normoglycemia, regular monitoring of serum creatinine and urine excretion, and, if required, extension of hemodynamic monitoring (box) (e14).
BOX. KDIGO bundle recommendations by severity of AKI.
-
High risk
Discontinue all nephrotoxic medications, if possible
Optimize volume status and perfusion pressure
Consider hemodynamic monitoring
Monitor serum creatinine and urine output
Avoid hyperglycemia
Do not use contrast medium
Severity
-
KDIGO-AKI 1
Consider non-invasive diagnostics
Consider invasive diagnostics
-
KDIGO-AKI 2
Consider dose adjustment of medications
Consider renal replacement therapy
Consider management in intensive care unit
-
KDIGO-AKI 3
Avoid subclavian catheterization
Treatment measures become more important from top to bottom AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes
The KDIGO bundle.
The KDIGO bundle comprises avoidance of nephrotoxic substances, optimization of volume status and perfusion pressure, maintenance of normoglycemia, and regular monitoring of serum creatinine and urine output.
Two recently published randomized controlled clinical trials illustrated that biomarker-guided implementation of the KDIGO bundle, compared with standard treatment, led to a significant reduction in the occurrence of AKI in both heart surgery patients and general surgery patients (Prevention of Acute Kidney Injury [PrevAKI]: 55.1% versus 71.1%, p = 0.004; Biomarker-guided Intervention to Prevent Acute Kidney Injury [BigpAK]: 27.1% versus 48.0%, p = 0.03) (e15, e16).
In this context, one must also consider the treating medical personnel. Investigations into the implementation of guideline recommendations showed clearly that although the measures ought to be known, they were properly applied in only 24% of cases. As one complex example, the sepsis bundle achieved an acceptance rate of only 34%, while the glucose monitoring component reached acceptance of 45% (e17). Another study on the implementation of recommendations with regard to AKI found a similarly poor rate: the AKI care bundle was completed in only 26% of patients within 24 hours (e18). Steps must definitively be taken in respect of training, information, and exemplary action.
Hemodynamics.
Renal underperfusion with the consequent shortage of oxygen and inflammatory reactions are thought to be two of the main factors responsible for the development of acute kidney injury.
Hemodynamics
Renal underperfusion with the consequent underoxygenation and inflammatory reactions are thought to be two of the main factors responsible for the development of AKI. AKI is associated with intraoperative hypotension (e19, e20). Moreover, persistent hypotensive episodes lead to impaired autoregulation (e21).
Effect of hemodynamic monitoring.
Perioperative hemodynamic monitoring, by whatever method, results in reduced occurrence of AKI.
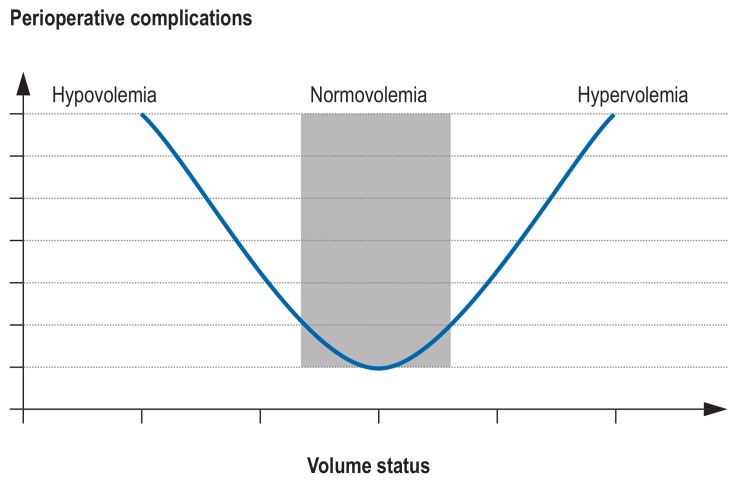
Perioperative hemodynamic monitoring, by whatever method, results in reduced occurrence of AKI (e22– e25). In a large retrospective cohort study of 5127 non-cardiosurgical patients, AKI was found whenever the intraoperative mean arterial pressure was <60 mm Hg for = 20 min or <55 mm Hg for = 10 min (adjusted OR 2.34 [1.35; 4.05], p <0.05 (e19). Hypotensive phases should therefore be kept as brief as possible (e19). Nevertheless, there is no universal optimal blood pressure, as the blood pressure depends on the patient’s medical history and current status. The INPRESS study (Intraoperative Norepinephrine to Control Arterial Pressure) investigated whether a personalized blood pressure regimen influenced the postoperative function of various organ systems in patients with an elevated risk of AKI. Patients whose systolic blood pressure was kept within 10% above or below the systolic resting pressure had a lower risk of postoperative organ dysfunction (relative risk 0.73 [0.56; 0.94], p = 0.02; absolute risk difference –14% [-25; -2]). Renal function disorders occurred less often in the individualized group (32.7%) than in the standard control group (49%; absolute risk difference –16% [-27; -5], adjusted relative risk 0.70 [0.53; 0.92], p = 0.01) (e26). Arterial hypotension is a common consequence of hypovolemia. In a recently published study, 3000 abdominal surgery patients were randomized into a liberal volume group and a restricted volume group. Those in the restrictive arm of the study were more likely to develop AKI after their operation (8.6% versus 5.0%, p <0.001) and more likely to need renal replacement (0.9% versus 0.3%, p = 0.048) (e27). By contrast, numerous studies have found that volume overload worsens renal function and increases overall mortality (e28, e29). In this context, distant organ damage deserves mention: AKI can also have extrarenal effects, e.g., hyperpermeability, that would aggravate volume overload (e.g., pulmonary edema) (e30– e32). Therefore it seams resonable to take an intermediate approach to ensure a balanced volume status (figure 4).
FIGURE 4.
U-shaped relationship between perioperative complications and the patient’s volume status
Just as important is the choice of substance for substitution. Isotonic saline solution 0.9% contains an unphysiologically high amount of chloride, leading to hyperchloremic acidosis and to renal vasoconstriction with reduced renal blood flow and a decreased glomerular filtration rate (e33– e36) as well as elevated incidence of AKI (e37). A chloride-restricted infusion regimen is associated with reduced incidence of moderate and severe AKI (e38– e40). Balanced crystalloid solutions should therefore be preferred (e41).
Most guidelines recommend norepinephrine as the first-line blood pressure–supporting medication. Although it is not yet known which vasopressor drug exerts the greatest protective effect against the development of AKI, norepinephrine raises both the global and medullary blood pressure and thus increases diuresis.
Recent findings.
In a recently published study, 3000 abdominal surgery patients were randomized into a liberal volume group and a restricted volume group. Those in the restrictive arm of the study were more likely to develop AKI after their operation and more likely to need renal replacement.
Diminished cardiac ejection fraction and right ventricular dysfunction should be considered in this context. If the right ventricle is compromised, venous outflow is decreased and blood backs up all the way to the kidney. Together with development of interstitial capsular edema there is increased organ resistance, potentially leading to a significant reduction in renal blood flow and thus to a higher risk of AKI (e42). This may be indicated by elevated central venous pressure, a “drainage marker” that may be associated not only with an increased risk of kidney injury but also with greater severity of kidney injury (e43– e45).
In this context, maintenance of the cardiac index makes sense (e46). From the pathophysiological viewpoint the administration of vasopressors would be indicated in these phases, with or without inotropics and accompanied by extended hemodynamic monitoring. If nothing else, renal oxygen deficiency may be caused by a lack of oxygen carriers. Preoperative anemia with hemoglobin <8 mg/dL is associated with an up to fourfold risk of AKI (e47, e48). It must be remembered that blood transfusions constitute an independent risk for AKI (e49– e51).
Pharmacological recommendations/treatment
There is currently no pharmacological option for the prevention and treatment of AKI. For some years, dexmedetomidine, a highly selective α2-agonist with pleiotropic effects (e.g. sedative analgesic and anxiolytic effects decreased endogenous norepinephrine release, improvement of hemodynamic stability, and the balancing of myocardial oxygen supply and demand), has been gaining in popularity. In heart surgery, administration of dexmedetomidine led to a significant reduction in the occurrence of AKI in patients whose renal function was normal or only slightly impaired before operation (e52– e54). The incidence of AKI in the first 48 h after surgery was significantly lower in the intervention group than in the control group (14% [n = 14] versus 33% [n = 33]) (e55). Dexmedetomidine seems to bring about a dose-dependent reduction of the increase in neutrophil gelatinase-associated lipocalin (e56), and may therefore have a nephroprotective action. However, further randomized controlled trials are required before dexmedetomidine can be definitively recommended for use in treatment.
Central venous pressure as “drainage marker”.
Elevated central venous pressure is a “drainage marker” that may be associated not only with an increased risk of kidney injury but also with greater severity of kidney injury.
Some studies have shown that volatile anesthetics possess nephroprotective and anti-inflammatory properties, although they may induce reductions in renal blood flow and glomerular filtration rate (e57, e58). Volatile anesthetics are believed to ameliorate the negative effects of renal reperfusion by activation of anti-inflammatory and inhibition of pro-inflammatory mediators. However, the research data are too sparse for a definite recommendation (e59). Several studies have been initiated to compare inhalational and intravenous anesthetics.
It used to be thought that statins reduce the incidence of AKI, but recently published studies do not support this assumption (e60, e61). The data are too sparse for a general recommendation to use statins for the prevention of AKI (e62).
An initially postulated nephroprotective effect of sodium bicarbonate (e63) by alkalization of the urine was not confirmed in subsequent randomized controlled trials (e64, e65).
Renal replacement therapy
Renal replacement therapy.
The current KDIGO guidelines recommend starting renal replacement therapy in the presence of life-threatening complications (absolute indication), including diuretic-resistant volume overload and significant metabolic/electrolyte disorders.
The central questions in treating a patient with severe AKI are whether and when renal replacement therapy should be initiated. The current KDIGO guidelines recommend starting renal replacement therapy in the presence of life-threatening complications (absolute indication), including diuretic-resistant volume overload and significant metabolic/electrolyte disorders (e14). However, the majority of patients develop severe AKI without life-threatening complications, so the “whether and when” decision about renal replacement therapy lies in the hands of the intensivist or nephrologist. If the treatment is started too late, complications (e.g., volume overload) possibly associated with elevated mortality may already have set in; if too early, an invasive procedure may be carried out unnecessarily. The right time to begin renal replacement therapy in AKI patients with no uremic symptoms, electrolyte imbalance, or volume overload is a subject of heated debate—not least owing to the differing definitions of “early” and “late” initiation of treatment across studies (e66). For example, one randomized controlled trial found no difference between early and late renal replacement therapy with regard to 60-day mortality in patients with KDIGO stage 3 AKI. Forty-nine percent of the patients who received renal replacement therapy in the late group did not need it. There were more catheter infections in the early renal replacement therapy group. In contrast, it was shown that 90-day mortality was lower after early than after late commencement of renal replacement therapy in patients with KDIGO stage 2 AKI (e67). Further studies are in progress to supplement the as yet inconclusive data.
Renal replacement therapy.
The majority of patients with severe AKI do not develop life-threatening complications, so the “whether and when” decision about renal replacement therapy lies in the hands of the intensivist or nephrologist.
Further information on CME.
Participation in the CME certification program is possible only over the Internet: cme.aerzteblatt.de. This unit can be accessed until 1 March 2020. Submissions by letter, e-mail or fax cannot be considered.
-
The following CME units can still be accessed for credit:
“Medical eligibility for contraception in women at increased risk” (issue 45/2019) until 2 February 2020
“The diagnosis and treatment of idiopathic facial paresis (Bell´s palsy)” (issue 41/2019) until 5 January 2020
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN), which is found on the CME card (8027XXXXXXXXXXX). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or else entered in “Meine Daten,” and the participant must agree to communication of the results.
CME credit for this unit can be obtained via cme.aerzteblatt.de until 1 March 2020. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which of the following statements applies most accurately to acute kidney injury (AKI)?
According to current knowledge, AKI exerts no influence on patients’ recovery.
The occurrence of AKI leads to a pronounced increase in mortality.
Owing to improved medical care, intensive care patients have only a slight risk of developing AKI.
There is widespread high awareness of the magnitude and importance of AKI.
AKI is not an independent risk factor.
Question 2
For which of these complications is acute kidney injury an independent risk factor?
Oropharyngeal complications
Orthostatic complications
Cerebrovascular complications
Gastrointestinal complications
Pulmonary complications
Question 3
What proportion of intensive care patients develop acute kidney injury during their hospital stay?
1–37%
11–47%
22–57%
33–67%
44–77%
Question 4
What parameters are used to evaluate renal function?
Erythrocyte sedimentation rate and protein–creatinine ratio
Creatinine concentration and C-reactive protein
Urine output and creatine phosphate metabolism
Serum creatinine concentration and urine output
Hematocrit level and spontaneous urine
Question 5
What is the advantage of using new injury markers?
They can be used by the patients themselves.
They yield information on renal damage.
They color the urine green and thus enable identification of kidney injury at the bedside.
They are more economical than conventional procedures.
They help to predict wound healing and discharge from the hospital.
Question 6
Which of the following describes “subclinical AKI”?
Acute kidney injury with no further clinical relevance
A biomarker-positive status in the presence of a normal serum creatinine concentration
Acute kidney injury with a moderately reduced serum creatinine concentration
A serum creatinine–positive but biomarker-negative status
Oliguria
Question 7
What factors play a key role in the development of AKI?
Renal oxygen shortage and inflammation
Hypertension and decreased erythrocyte sedimentation rate
Adrenal cortex insufficiency and elevated C-reactive protein
Electrolyte disorders and eosinophilia
Leukopenia and oxygen shortage
Question 8
Vasopressors have a protective effect against the development of acute kidney injury. What must be remembered when norepinephrine is used?
It may not be used in acute hypotension.
Chronic skin disease must be excluded before its use.
Its use causes volume overload, which protects the kidney from being damaged by “running dry.”
It increases global and medullary blood pressure and thus diuresis.
It has no consequences for osmoregulation.
Question 9
What parameters have a decisive influence on serum creatinine concentration?
Peripheral arterial occlusive disease and metabolic syndrome
Antiarrhythmics and antidepressants
Body fat mass and female sex
Statins and acetylsalicylic acid
Muscle mass and hemodilution
Question 10
How high is the mortality among patients who become dependent on dialysis during their hospital stay?
14–41%
24–51%
34–61%
44–71%
54–81%
► Participation is possible only via the Internet: cme.aerzteblatt.de
Acknowledgments
Translated from the original German by David Roseveare
Footnotes
Conflict of interest statement
Prof. Zarbock has received lecture fees and reimbursement of travel and accommodation costs from Fresenius, Astute Medical, Bio Merieux, Braun, Baxter, AM Pharma, Ratiopharm, Amomed, and Edwards; payments for conducting commissioned clinical studies from Biotest, Fresenius, La Jolla Pharmaceuticals, Astute Medical, and Astellas; and funding for a research project of his own initiation from Baxter, Fresenius, Astute Medical, Astellas, and Bio Merieux.
Dr. Meersch has received lecture fees and reimbursement of travel and accommodation costs from Astute Medical.
Dr. Weiss and Prof. Pavenstädt declare that no conflict of interest exists.
References
- 1.Li PK, Burdmann EA, Mehta RL. World Kidney Day Steering Committee 2013: Acute kidney injury: global health alert. Kidney Int. 2013;83:372–376. doi: 10.1038/ki.2012.427. [DOI] [PubMed] [Google Scholar]
- 2.Lewington AJ, Cerdá J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457–467. doi: 10.1038/ki.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 4.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 5.Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87:46–61. doi: 10.1038/ki.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu HC, Lee LC, Wang WJ. Incidence and mortality of postoperative acute kidney injury in non-dialysis patients: comparison between the AKIN and KDIGO criteria. Ren Fail. 2016;38:330–339. doi: 10.3109/0886022X.2015.1128790. [DOI] [PubMed] [Google Scholar]
- 7.Petäjä L, Vaara S, Liuhanen S, et al. Acute kidney injury after cardiac surgery by complete KDIGO criteria predicts increased mortality. J Cardiothorac Vasc Anesth. 2017;31:827–836. doi: 10.1053/j.jvca.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21:345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu VC, Shiao CC, Chang CH, et al. Long-term outcomes after dialysis-requiring acute kidney injury. Biomed Res Int. 2014;2014 doi: 10.1155/2014/365186. 365186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khadzhynov D, Schmidt D, Hardt J, et al. The incidence of acute kidney injury and associated hospital mortality—a retrospective cohort study of over 100 000 patients at Berlin‘s Charité hospital. Dtsch Arztebl Int. 2019;116:397–404. doi: 10.3238/arztebl.2019.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson FP, Bansal AD, Jasti SK, et al. The impact of documentation of severe acute kidney injury on mortality. Clin Nephrol. 2013;80:417–425. doi: 10.5414/CN108072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Xing G, Wang L, et al. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386:1465–1471. doi: 10.1016/S0140-6736(15)00344-X. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell T, Feher E, Mitchell G, Chakera A. Acute kidney injury is under-recognised and under-reported in hospitalised patients in Australia. Intern Med J. 2017;47:1451–1454. doi: 10.1111/imj.13639. [DOI] [PubMed] [Google Scholar]
- 14.Hansen MK, Gammelager H, Mikkelsen MM, et al. Post-operative acute kidney injury and five-year risk of death, myocardial infarction, and stroke among elective cardiac surgical patients: a cohort study. Crit Care. 2013;17 doi: 10.1186/cc13158. R292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu VC, Wu PC, Wu CH, et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000933. 10.1161/JAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KDIGO. Clinical practice guideline for acute kidney injury. Kidney Int. 2012;3 [Google Scholar]
- 17.Alscher MD, Erley C, Kuhlmann MK. Acute renal failure of nosocomial origin. Dtsch Arztebl Int. 2019;116:149–158. doi: 10.3238/arztebl.2019.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdulla MH, Sattar MA, Abdullah NA, et al. Effect of renal sympathetic nerve on adrenergically and angiotensin II-induced renal vasoconstriction in normal Wistar-Kyoto rats. Ups J Med Sci. 2011;116:18–25. doi: 10.3109/03009734.2010.526723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinh QN, Drummond GR, Kemp-Harper BK, et al. Pressor response to angiotensin II is enhanced in aged mice and associated with inflammation, vasoconstriction and oxidative stress. Aging. 2017;9:1595–1606. doi: 10.18632/aging.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 22.Karlberg L, Norlén BJ, Ojteg G, Wolgast M. Impaired medullary circulation in postischemic acute renal failure. Acta Physiol Scand. 1983;118:11–17. doi: 10.1111/j.1748-1716.1983.tb07234.x. [DOI] [PubMed] [Google Scholar]
- 23.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26:2231–2238. doi: 10.1681/ASN.2014070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. AWARE Investigators: Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leedahl DD, Frazee EN, Schramm GE, et al. Derivation of urine output thresholds that identify a very high risk of AKI in patients with septic shock. Clin J Am Soc Nephrol. 2014;9:1168–1174. doi: 10.2215/CJN.09360913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans health administration data. Am J Kidney Dis. 2016;67:872–880. doi: 10.1053/j.ajkd.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Connor ME, Kirwan CJ, Pearse RM, Prowle JR. Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med. 2016;42:521–530. doi: 10.1007/s00134-015-4157-7. [DOI] [PubMed] [Google Scholar]
- 28.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 29.Praught ML, Shlipak MG. Are small changes in serum creatinine an important risk factor? Curr Opin Nephrol Hypertens. 2005;14:265–270. doi: 10.1097/01.mnh.0000165894.90748.72. [DOI] [PubMed] [Google Scholar]
- 30.Molitoris BA, Reilly E. Quantifying glomerular filtration rates in acute kidney injury: A requirement for translational success. Semin Nephrol. 2016;36:31–41. doi: 10.1016/j.semnephrol.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prowle JR, Kolic I, Purdell-Lewis J, et al. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol. 2014;9:1015–1023. doi: 10.2215/CJN.11141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schetz M, Schortgen F. Ten shortcomings of the current definition of AKI. Intensive Care Med. 2017;43:911–913. doi: 10.1007/s00134-017-4715-2. [DOI] [PubMed] [Google Scholar]
- 33.Heimbürger O, Stenvinkel P, Bárány P. The enigma of decreased creatinine generation in acute kidney injury. Nephrol Dial Transplant. 2012;27:3973–3974. doi: 10.1093/ndt/gfs459. [DOI] [PubMed] [Google Scholar]
- 34.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bihorac A, Kellum JA. Acute kidney injury in 2014: a step towards understanding mechanisms of renal repair. Nat Rev Nephrol. 2015;11:74–75. doi: 10.1038/nrneph.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093460. e93460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17 doi: 10.1186/cc12503. R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cummings JJ, Shaw AD, Shi J. Intraoperative prediction of cardiac surgery-associated acute kidney injury using urinary biomarkers of cell cycle arrest. J Thorac Cardiovasc Surg. 2019;157:1545–1553e5. doi: 10.1016/j.jtcvs.2018.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aregger F, Uehlinger DE, Witowski J, et al. Identification of IGFBP-7 by urinary proteomics as a novel prognostic marker in early acute kidney injury. Kidney Int. 2014;85:909–919. doi: 10.1038/ki.2013.363. [DOI] [PubMed] [Google Scholar]
- E1.Arthur JM, Hill EG, Alge JL, et al. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014;85:431–438. doi: 10.1038/ki.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23:905–914. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Cummings JJ, Shaw AD, Shi J. Intraoperative prediction of cardiac surgery-associated acute kidney injury using urinary biomarkers of cell cycle arrest. J Thorac Cardiovasc Surg. 2019;157:1545–1553e5. doi: 10.1016/j.jtcvs.2018.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Haase M, Kellum JA, Ronco C. Subclinical AKI-an emerging syndrome with important consequences. Nat Rev Nephrol. 2012;8:735–739. doi: 10.1038/nrneph.2012.197. [DOI] [PubMed] [Google Scholar]
- E5.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- E8.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Vijayan A, Faubel S, Askenazi DJ, et al. Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis. 2016;68:19–28. doi: 10.1053/j.ajkd.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Basu RK, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol. 2014;9:654–662. doi: 10.2215/CJN.09720913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Goldstein SL, Chawla LS. Renal angina. Clin J Am Soc Nephrol. 2010;5:943–949. doi: 10.2215/CJN.07201009. [DOI] [PubMed] [Google Scholar]
- E12.Koyner JL, Davison DL, Brasha-Mitchell E, et al. Furosemide stress test and biomarkers for the prediction of AKI severity. J Am Soc Nephrol. 2015;26:2023–2031. doi: 10.1681/ASN.2014060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Chawla LS, Davison DL, Brasha-Mitchell E, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17 doi: 10.1186/cc13015. R207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.KDIGO. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- E15.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E16.Göcze I, Jauch D, Götz M, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK study. Ann Surg. 2018;267:1013–1020. doi: 10.1097/SLA.0000000000002485. [DOI] [PubMed] [Google Scholar]
- E17.Leone M, Ragonnet B, Alonso S, et al. Variable compliance with clinical practice guidelines identified in a 1-day audit at 66 French adult intensive care units. Crit Care Med. 2012;40:3189–3195. doi: 10.1097/CCM.0b013e31826571f2. [DOI] [PubMed] [Google Scholar]
- E18.Kolhe NV, Reilly T, Leung J, et al. A simple care bundle for use in acute kidney injury: a propensity score-matched cohort study. Nephrol Dial Transplant. 2016;31:1846–1854. doi: 10.1093/ndt/gfw087. [DOI] [PubMed] [Google Scholar]
- E19.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective non-cardiac surgery. Anesthesiology. 2015;123:515–523. doi: 10.1097/ALN.0000000000000765. [DOI] [PubMed] [Google Scholar]
- E20.Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–515. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- E21.Dünser MW, Takala J, Ulmer H, et al. Arterial blood pressure during early sepsis and outcome. Intensive Care Med. 2009;35:1225–1233. doi: 10.1007/s00134-009-1427-2. [DOI] [PubMed] [Google Scholar]
- E22.Pearse RM, Harrison DA, MacDonald N, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311:2181–2190. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- E23.Grocott MP, Dushianthan A, Hamilton MA, Mythen MG, Harrison D, Rowan K. Optimisation Systematic Review Steering Group. Perioperative increase in global blood flow to explicit defined goals and outcomes after surgery: a Cochrane Systematic Review. Br J Anaesth. 2013;111:535–548. doi: 10.1093/bja/aet155. [DOI] [PubMed] [Google Scholar]
- E24.Benes J, Chytra I, Altmann P, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care. 2010;14 doi: 10.1186/cc9070. R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E25.Brienza N, Giglio MT, Marucci M, Fiore T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 2009;37:2079–2090. doi: 10.1097/CCM.0b013e3181a00a43. [DOI] [PubMed] [Google Scholar]
- E26.Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: A randomized clinical trial. JAMA. 2017;318:1346–1357. doi: 10.1001/jama.2017.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263–2274. doi: 10.1056/NEJMoa1801601. [DOI] [PubMed] [Google Scholar]
- E28.Haase-Fielitz A, Haase M, Bellomo R, et al. Perioperative hemodynamic instability and fluid overload are associated with increasing acute kidney injury severity and worse outcome after cardiac surgery. Blood Purif. 2017;43:298–308. doi: 10.1159/000455061. [DOI] [PubMed] [Google Scholar]
- E29.Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2013;10:37–47. doi: 10.1038/nrneph.2013.232. [DOI] [PubMed] [Google Scholar]
- E30.Basu RK, Wheeler DS. Kidney-lung cross-talk and acute kidney injury. Pediatr Nephrol. 2013;28:2239–2248. doi: 10.1007/s00467-012-2386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E31.Grams ME, Rabb H. The distant organ effects of acute kidney injury. Kidney Int. 2012;81:942–948. doi: 10.1038/ki.2011.241. [DOI] [PubMed] [Google Scholar]
- E32.Feltes CM, Hassoun HT, Lie ML, Cheadle C, Rabb H. Pulmonary endothelial cell activation during experimental acute kidney injury. Shock. 2011;36:170–176. doi: 10.1097/SHK.0b013e3182169c76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E33.Bullivant EM, Wilcox CS, Welch WJ. Intrarenal vasoconstriction during hyperchloremia: role of thromboxane. Am J Physiol. 1989;256:152–157. doi: 10.1152/ajprenal.1989.256.1.F152. [DOI] [PubMed] [Google Scholar]
- E34.Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 09% saline and plasma-lyte 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- E35.Zhou F, Peng ZY, Bishop JV, et al. Effects of fluid resuscitation with 09% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis. Crit Care Med. 2014;42:e270–e278. doi: 10.1097/CCM.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E36.Li H, Sun SR, Yap JQ, et al. 09% saline is neither normal nor physiological. J Zhejiang Univ Sci B. 2016;17:181–187. doi: 10.1631/jzus.B1500201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E37.McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg. 2013;117:412–421. doi: 10.1213/ANE.0b013e318293d81e. [DOI] [PubMed] [Google Scholar]
- E38.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- E39.Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E40.Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378:819–828. doi: 10.1056/NEJMoa1711586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E41.Kümpers P. Volumensubstitution mit NaCl 0,9 % Internist. 2015;56:773–778. doi: 10.1007/s00108-015-3676-1. [DOI] [PubMed] [Google Scholar]
- E42.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function inadvanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E43.Tarvasmäki T, Haapio M, Mebazaa A, et al. Acute kidney injury in cardiogenic shock: definitions, incidence, haemodynamic alterations, and mortality. Eur J Heart Fail. 2018;20:572–581. doi: 10.1002/ejhf.958. [DOI] [PubMed] [Google Scholar]
- E44.Chen X, Wang X, Honore PM, et al. Renal failure in critically ill patients, beware of applying (central venous) pressure on the kidney. Ann Intensive Care. 2018;8 doi: 10.1186/s13613-018-0439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E45.Damman K, van Deursen VM, Navis G, et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- E46.Westaby S, Balacumaraswami L, Sayeed R. Maximizing survival potential in very high risk cardiac surgery. Heart Fail Clin. 2007;3:159–180. doi: 10.1016/j.hfc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- E47.Fowler AJ, Ahmad T, Phull MK, Allard S, Gillies MA, Pearse RM. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg. 2015;102:1314–1324. doi: 10.1002/bjs.9861. [DOI] [PubMed] [Google Scholar]
- E48.Walsh M, Garg AX, Devereaux PJ, et al. The association between perioperative hemoglobin and acute kidney injury in patients having noncardiac surgery. Anesth Analg. 2013;117:924–931. doi: 10.1213/ANE.0b013e3182a1ec84. [DOI] [PubMed] [Google Scholar]
- E49.Karkouti K, Stukel TA, Beattie WS, et al. Relationship of erythrocyte transfusion with short- and long-term mortality in a population-based surgical cohort. Anesthesiology. 2012;117:1175–1183. doi: 10.1097/ALN.0b013e318271604e. [DOI] [PubMed] [Google Scholar]
- E50.Karkouti K, Grocott HP, Hall R, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth. 2015;62:377–384. doi: 10.1007/s12630-014-0302-y. [DOI] [PubMed] [Google Scholar]
- E51.Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant. 2012;27:153–160. doi: 10.1093/ndt/gfr275. [DOI] [PubMed] [Google Scholar]
- E52.Ji F, Li Z, Young JN, Yeranossian A, Liu H. Post-bypass dexmedetomidine use and postoperative acute kidney injury in patients undergoing cardiac surgery with cardiopulmonary bypass. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077446. e77446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E53.Xue F, Zhang W, Chu HC. Assessing perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney Int. 2016;89 doi: 10.1016/j.kint.2015.12.053. [DOI] [PubMed] [Google Scholar]
- E54.Kwiatkowski DM, Axelrod DM, Sutherland SM, Tesoro TM, Krawczeski CD. Dexmedetomidine is associated with lower incidence of acute kidney injury after congenital heart surgery. Pediatr Crit Care Med. 2016;17:128–134. doi: 10.1097/PCC.0000000000000611. [DOI] [PubMed] [Google Scholar]
- E55.Cho JS, Shim JK, Soh S, Kim MK, Kwak YL. Perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney Int. 2016;89:693–700. doi: 10.1038/ki.2015.306. [DOI] [PubMed] [Google Scholar]
- E56.Balkanay OO, Goksedef D, Omeroglu SN, Ipek G. The doserelated effects of dexmedetomidine on renal functions and serum neutrophil gelatinase-associated lipocalin values after coronary artery bypass grafting: a randomized, triple-blind, placebo-controlled study. Interact Cardiovasc Thorac Surg. 2015;20:209–214. doi: 10.1093/icvts/ivu367. [DOI] [PubMed] [Google Scholar]
- E57.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101:1313–1324. doi: 10.1097/00000542-200412000-00011. [DOI] [PubMed] [Google Scholar]
- E58.Hashiguchi H, Morooka H, Miyoshi H, Matsumoto M, Koji T, Sumikawa K. Isoflurane protects renal function against ischemia and reperfusion through inhibition of protein kinases, JNK and ERK. Anesth Analg. 2005;101:1584–1589. doi: 10.1213/01.ANE.0000184044.51749.B8. [DOI] [PubMed] [Google Scholar]
- E59.Fukazawa K, Lee TH. Volatile Anesthetics and AKI: Risks, Mechanisms, and a Potential Therapeutic Window. J Am Soc Nephrol. 2014;25:884–892. doi: 10.1681/ASN.2013111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E60.Murugan R, Weissfeld L, Yende S, et al. Association of statin use with risk and outcome of acute kidney injury in community-acquired pneumonia. Clin J Am Soc Nephrol. 2012;7:895–905. doi: 10.2215/CJN.07100711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E61.Billings FT, Hendricks PA, Schildcrout JS, et al. High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA. 2016;315:877–888. doi: 10.1001/jama.2016.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E62.Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20:67–75. doi: 10.1053/j.ackd.2012.10.003. [DOI] [PubMed] [Google Scholar]
- E63.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- E64.Haase M, Haase-Fielitz A, Plass M, et al. Prophylactic perioperative sodium bicarbonate to prevent acute kidney injury following open heart surgery: a multicenter double-blinded randomized controlled trial. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001426. e1001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E65.McGuinness SP, Parke RL, Bellomo R, Van Haren FM, Bailey M. Sodium bicarbonate infusion to reduce cardiac surgery-associated acute kidney injury: a phase II multicenter double-blind randomized controlled trial. Crit Care Med. 2013;41:1599–1607. doi: 10.1097/CCM.0b013e31828a3f99. [DOI] [PubMed] [Google Scholar]
- E66.Wierstra BT, Kadri S, Alomar S, Burbano X, Barrisford GW, Kao RL. The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Crit Care. 2016;20 doi: 10.1186/s13054-016-1291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E67.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315:2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]