Abstract
Background
Hypertriglyceridemia affects 15–20% of the adult population and is associated with overweight, metabolic syndrome, and diabetes mellitus. It is often discovered incidentally.
Methods
This review is based on pertinent publications retrieved by a selective literature search, including current guidelines on hypertriglyceridemia.
Results
Elevated triglyceride (TG) levels are causally linked to cardiovascular disease; TG levels above 1000 mg/dL (11.4 mmol/L) can induce acute pancreatitis. The individual risk of cardiovascular disease and of pancreatitis must be estimated in order to decide whether, and how, hypertriglyceridemia should be treated. Lifestyle modifications (cessation of alcohol consumption, reduced intake of rapidly metabolized carbohydrates), weight loss, and blood sugar control are the most effective ways to lower TG levels. The need to lower the low-density lipoprotein (LDL) concentration must be determined on the basis of the cardiovascular risk, independently of the success of the lifestyle changes. Few patients need specific drug treatment to lower the TG level. Fibrates can lower TG concentrations, but their efficacy in combination with statins has not been clearly shown in endpoint studies. A daily dose of 2–4 g omega-3 fatty acids can also lower TG levels. To date, only a single large-scale randomized, blinded trial has shown the efficacy of 4 g of eicosapentaenoic acid ethyl ester per day in lowering the risk in high-risk patients (number needed to treat = 21). Patients with the very rare purely genetic types of hypertriglyceridemia (familial chylomicronemia syndrome) should be treated in specialized outpatient clinics.
Conclusion
Hypertriglyceridemia is causally linked to cardiovascular disease and pancreatitis. Lifestyle modifications play a paramount role in its treatment.
Approximately 15% to 20% of patients visiting a medical practice are diagnosed with hypertriglyceridemia—frequently as an incidental finding (1). Given the increases in the prevalence of diabetes, metabolic syndrome, and obesity, the prevalence of hypertriglyceridemia is likely to increase too. The severity of hypertriglyceridemia (table 1) varies widely, and to date no uniform classification of the condition has been established. To further complicate the matter, triglyceride (TG) levels can show intraindividual fluctuation. Most affected persons (80–90%) have moderately increased TG levels, i.e., between 150 mg/dL (1.7 mmol/L) and 400 mg/dL (4.6 mmol/L). In a small proportion of patients (approximately 15%), TG levels range between 400 mg/dL and 1000 mg/dL (4.6–11.4 mmol/L); occasionally, significantly higher levels are found (e1). In very rare cases, TG levels above 15 000 mg/dL (170 mmol/L) have been identified.
Table 1. Classification of hypertriglyceridemia based on fasting triglyceride levels.
| Designation | Triglyceride levels | Clinical significance | Remarks |
| Normal finding | <150 mg/dl (<1.7 mmol/l) |
The threshold level of 150 mg/dL is now accepted by all medical societies (2– 5). | |
| Moderate hypertriglyceridemia | 150–1000 mg/dL (1.7–11.4 mmol/L) |
– Increased risk of cardiovascular events – Slightly increased risk of acute pancreatitis (dose-dependent) | Different medical societies define moderate hypertriglyceridemia differently: – Lower threshold: 150 mg/dL (175 mg/dL); – Upper threshold: between 500 mg/dL (5.6 mmol/L) (3) and 1000 mg/dL (11,4 mmol/L); 885 mg/dL (10 mmol/L) is also commonly reported (2, 3, 5). |
| Severe hypertriglyceridemia | >1000 mg/dL (>11.4 mmol/L) |
– Increased risk of cardiovascular events – Significantly increased risk of acute pancreatitis (dose-dependent) | See remarks on “moderate hypertriglyceridemia“ |
When interpreting TG concentrations, one should be aware that these threshold values apply to fasting TG levels. Since circulating TG levels after a meal can vary in both magnitude and duration, no postprandial thresholds have been established. TG levels typically peak 4 to 6 h after fat intake (e2).
Furthermore, there is currently no established “fat tolerance test”—by analogy with the glucose tolerance test—that would allow for standardized assessment of postprandial TG response. In metabolically healthy individuals, TG levels rarely increase above 400 mg/dL (4.6 mmol/L), even after a meal rich in fat (e2).
Hypertriglyceridemia is closely associated with the presence of obesity, metabolic syndrome, and diabetes mellitus. For instance, up to 50% of patients with type 2 diabetes have concomitant hypertriglyceridemia (6). Independent of this, there is frequently a genetic predisposition, leading—in combination with lifestyle factors—to hypertriglyceridemia. This predisposition is usually polygenic and can include a wide range of serum TG. The spectrum ranges from a disposition resulting in hypertriglyceridemia only in the presence of considerable overweight and/or excessive alcohol consumption to very rare serious mutations (e.g., lipoprotein lipase and apolipoproteins A5, CII and CIII) that may lead to extremely severe hypertriglyceridemia in childhood, even in the absence of additional factors (familial chylomicronemia syndrome) (4, e3).
From a clinical perspective, hypertriglyceridemia is relevant in two respects:
First, patients with hypertriglyceridemia are at a higher risk of atherosclerosis and its late complications, with a causal, dose-dependent association for TG concentrations up to approximately 1000 mg/dL (11.4 mmol/L) (7). At even higher TG levels, there is probably no further increase in the already elevated risk of atherosclerosis. The increased risk reflects the fact that lipoproteins rich in TG contain apolipoprotein B (apoB); based on currently available evidence, all apoB-containing lipoproteins have an atherogenic effect (8). Since in the presence of TG levels >1000 mg/dL primarily the loading with TG increases, but not the number of lipoproteins, atherogenicity does not further increase with very high levels of TG (4). Simply on grounds of their size, lipoproteins very rich in TG are presumably not able to penetrate the subendothelial space to initiate the development of atherosclerosis.
The majority of patients with hypertriglyceridemia also have lower high-density lipoprotein (HDL)-cholesterol levels, and for a long time it was assumed that the increased atherosclerosis rate was caused by reduced HDL-cholesterol levels (e4). However, it is now clear that the increased cardiovascular risk is mediated by elevated levels of triglyceride-rich (apolipoprotein-B-containing) lipoproteins (4, 9). Since triglyceride-rich lipoproteins contain not only TG and apoB but also variable amounts of cholesterol (remnant cholesterol, also known as very low-density lipoprotein [VLDL]-cholesterol), the concentration of VLDL/remnant cholesterol is also associated with atherosclerosis (9).
Second, patients with very high TG levels (typically >1000 mg/dL; approximately 10 mmol/L) can develop acute pancreatitis (so-called chylomicronemia syndrome). Interestingly, epidemiological data have shown that the risk of pancreatitis is increased even at lower TG levels, although still very low in absolute terms: for instance, 2.7 pancreatitis events per 10 000 person-years were found for TG levels <1 mmol/L and 5.5 pancreatitis events per 10 000 person-years for TG levels of 2.0–3.0 mmol/L (hazard ratio [HR] = 1.8) (10). It is generally assumed that TG levels above approximately 1000 mg/dL (10 mmol/L) significantly increase the likelihood of pancreatitis (4). The risk is particularly high in patients with familial chylomicronemia syndrome (FCS).
Diagnosis
Fasting versus non-fasting
It was long assumed that fasting blood lipid levels should be measured. This approach reflects the fact that postprandial changes in TG levels are difficult to interpret. However, recent data have shown that postprandial lipoproteins also have an atherogenic effect and thus can be used for risk assessment (10– 12). Furthermore, for a long time it had not been possible to measure LDL-cholesterol directly; instead, the Friedewald formula was used to estimate LDL-cholesterol levels (LDL-cholesterol = total cholesterol minus HDL-cholesterol minus triglycerides divided by 5 for mg/dL or by 2.2 for mmol/L) (13). A prerequisite for the use of this equation is that blood is collected from fasting patients. Today, however, LDL-cholesterol is usually measured directly. In keeping with current recommendations, blood should be collected from fasting patients if one of the criteria listed in Box 1 is met (14). In primary care, measuring non-fasting lipid levels is considered adequate for initial screening. Irrespective of this, it should be taken into account that day-by-day fluctuation in TG levels is more pronounced than for LDL-cholesterol.
BOX 1. Situations in which fasting triglyceride levels should be determined (14).
Non-fasting triglyceride levels >440 mg/dL (5 mmol/L)
Known hypertriglyceridemia
Following hypertriglyceridemia-associated pancreatitis
Before starting medications that may cause hypertriglyceridemia
Whenever other tests require fasting blood collection (e.g., determination of blood glucose or drug levels)
Parameters to be tested
Besides total cholesterol, TG, HDL-cholesterol, and LDL-cholesterol, the lipid profile should include the calculated non-HDL-cholesterol concentration (total cholesterol minus HDL-cholesterol). In addition to elevated TG, patients with hypertriglyceridemia typically have increased total cholesterol, decreased HDL-cholesterol, and normal to low LDL-cholesterol levels. The increase in total cholesterol is explained by the fact that all triglyceride-rich lipoproteins also contain cholesterol, which then raises the total cholesterol level. The parameter non-HDL-cholesterol specifies the amount of cholesterol associated with triglyceride-rich lipoproteins (VLDL-cholesterol or remnant cholesterol). The advantage of determining non-HDL-cholesterol is that the concentration of all atherogenic lipoproteins can be estimated by measuring one single parameter. The level of non-HDL-cholesterol is more closely correlated with adverse cardiovascular events than that of TG as it also includes LDL-cholesterol. Furthermore, atherogenicity increases with rising TG levels only if more lipoproteins are present, not when existing lipoproteins are loaded with additional TG (e5). Here, the concentration of remnant cholesterol is—like the concentration of apolipoprotein B—superior to TG concentration as a marker for the amount of abnormal lipoproteins (9).
Therefore, the current European guidelines for the management of dyslipidemias recommend non-HDL-cholesterol as a secondary target for lipid lowering (table 2) (5). In patients without hypertriglyceridemia, the non-HDL-cholesterol level exceeds the LDL-cholesterol level by no more than 30 mg/dL (0.8 mmol/L).
Table 2. Target lipid levels for cardiovascular disease prevention (5).
| Primary target level | Grade of recommendation/ level of evidence | Secondary targets | ||||
| Cardiovascular risk*1 | LDL-cholesterol | Non-HDL-cholesterol | apoB | |||
| mg/dL | mmol/L | mg/dL | mmol/L | mg/dL | ||
| Low*2 | <116 | <3.0 | IIb / A | |||
| Moderate*2 | <100 | <2.6 | IIa / A | <130 | <3.4 | <100 |
| High | <70 | <1.8 | I / A | <100 | <2.6 | <80 |
| Very high | <55 | <1.4 | I / A | <85 | <2.2 | <65 |
*1 Estimation of the cardiovascular risk based on clinical parameters and the European Society of Cardiology risk score (10-year risk of fatal cardiovascular disease); for example, “very high risk“ with evidence of atherosclerotic disease or score >20% or “high risk” with diabetes without evidence of end-organ damage.
*2 These targets may be considered.
Additional investigations (apolipoprotein B concentration, apolipoprotein E phenotype, genetic testing) are reserved for special situations (suspected FCS; evaluation of new treatment approaches), since in general their results have no impact on clinical decision making. Whenever a patient is diagnosed with hypertriglyceridemia, the first step is to rule out secondary causes (box 2). However, for patients to develop overt hypertriglyceridemia as the result of such secondary causes, an underlying genetic predisposition is usually required.
BOX 2. Secondary causes of hypertriglyceridemia.
Overweight/obesity
Metabolic syndrome
Diabetes mellitus
Increased alcohol consumption
Excessive calorie intake (as fat or rapidly metabolizable carbohydrates)
Hypothyroidism
Kidney disorders (especially nephrotic syndrome)
Paraproteinemia
Systemic lupus erythematosus
Anorexia nervosa
Glycogenoses
Sepsis
Pregnancy
Medications: steroids, estrogens, anabolics, tamoxifen, thiazides, non-cardioselective beta blockers, cyclophosphamide, cyclosporine, protease inhibitors, bile acid sequestrants, clozapine, atypical antipsychotics, antidepressants, etc.
Management
In the management of hypertriglyceridemia, therapeutic interventions (table 3) aim at reducing the risk of cardiovascular events and pancreatitis. Therefore, the European medical societies have specified target levels for lipids according to the overall risk (table 2) (5). For patients with hypertriglyceridemia, primarily the same standard target LDL-cholesterol levels apply as for persons without hypertriglyceridemia. Non-HDL-cholesterol and apoB represent secondary targets of lipid lowering, because the related evidence from randomized trials is weaker than that available for LDL-cholesterol. This reflects the fact that the design and statistical analysis of most large trials of lipid-lowering regimens focused on LDL-cholesterol.
Table 3. Interventions to treat hypertriglyceridemia.
| Intervention | Lowering of TG | Remarks | Evidence level |
| Lifestyle modification | Variable; up to 70% | – Alcohol abstinence and reduced intake of rapidly metabolizable carbohydrates have the greatest effect (e6, e7) – Increasing physical activity; the goal is >2.5 h (better 5 h) of aerobic exercise of moderate intensity spread over the week (e8) – Very variable effect: depends on the baseline condition and the underlying predisposition – Nutritional counseling should be offered to all patients | A |
| Weight loss | Variable; up to 70% | – Particularly effective in patients with abdominal obesity or with other factors related to metabolic syndrome (e9) – Variable effect: in some patients very pronounced lowering of TG levels after losing only a few kilograms of weight; in other patients minor effect despite significant weight loss | A |
| Blood glucose control | Variable; up to 70% | – In type 2 diabetes mellitus: in many cases significant improvement, but usually no return to normal blood glucose levels; largely independent of the antidiabetic agent used – In type 1 diabetes mellitus: hypertriglyceridemia usually only if blood glucose is uncontrolled; return to normal after control has been achieved | A |
| Administration of fibrates | 30–50% | – As monotherapy or in combination with non-statins (old studies): minor positive effect on cardiovascular endpoints (25, e13) – In combination with statins: no positive effects in endpoint studies, but potential benefits in subgroups (26, 27) – Consider use in patients with very high cardiovascular risk and persistent hypertriglyceridemia (5) – Try fibrates in patients with severe hypertriglyceridemia | A |
| Administration of omega-3 fatty acids | 30–50% | – Low-dose (1–2 g daily) omega-3 fatty acids show no clinical benefits (31) – High-dose (3–4 g daily) omega-3 fatty acids should be considered in patients with high cardiovascular risk and persistent hypertriglyceridemia (4) – Try omega-3 fatty acids in patients with severe hypertriglyceridemia – In one study (REDUCE-IT), treatment with eicosapentaenoic acid ethyl ester at a dose of 4 g daily showed significant clinical benefits in high-risk patients with statin therapy; the mechanism of action is unclear (32) | A |
| Administration of MCT fats | Variable | – As a replacement for other fats; hardly any effect on fasting lipids, but no postprandial TG increase; in the medium term, in most patients improved fasting TG levels (because postprandial lipoproteins can also be detected in fasting blood specimens of patients with severe hypertriglyceridemia) – Consider use in patients with high uncontrolled TG levels (4) | B |
| Administration of statins | 10–20% | – Used to reduce cardiovascular risk depending on overall risk and LDL-cholesterol, minor direct effect on TG (21) | A |
| Administration of ezetimibe | 5–10% | – Used to reduce cardiovascular risk in addition to statins depending on overall risk and LDL-cholesterol; no direct effect on TG (e10) | B |
| Administration of PCSK9 inhibitors | 10–20% | – Used to reduce cardiovascular risk in addition to maximum oral treatments depending on overall risk and LDL-cholesterol, minor direct effect on TG (e11, e12) | A |
| Administration of bile acid sequestrants | Increase | – Contraindicated; bile acid sequestrants can increase TG levels in patients with hypertriglyceridemia | B |
LDL, Low-density lipoprotein; MCT, medium-chain triglycerides; PCSK9, proprotein convertase subtilisin/kexin type 9; TG, triglyceride(s)
The recommendations of the UK National Institute for Health and Care Excellence (NICE) follow a similar line of reasoning: For patients with hypertriglyceridemia, NICE recommends optimization of the cardiovascular risk profile on the basis of non-HDL-cholesterol, but does not mention concrete target levels (15). Likewise, the German College of General Practitioners and Family Physicians (DEGAM, Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin) points out that the cardiovascular risk is increased in patients with both hypertriglyceridemia and combined hyperlipoproteinemia (16).
All of the recommendations issued by medical societies focus on the assessment of the overall risk when making the decision on whether lowering lipid levels is advisable. However, there are differences in the approach to lipid reduction: The European and now also the US medical societies define precise target LDL levels, whereas other medical societies do not state specific targets. We support the use of target levels as these facilitate the communication with patients and with other healthcare providers, making this the best approach to ensure that patients are treated according to their respective risk. Another difference concerns age limits. While the US recommendations focus on the age group from 40 to 75 years (for which there is evidence from studies), the European guidelines do not limit their recommendations to this age group and extrapolate the evidence for those age groups for which no or only limited data are available.
Lifestyle modifications
Lifestyle modifications are of paramount importance when treating patients with hypertriglyceridemia (e6– e9). The key measures are to avoid alcohol and to significantly reduce the intake of rapidly metabolizable carbohydrates, especially drinks containing sugar (17). In addition, the intake of animal fats should be restricted. However, there are as yet no cardiovascular endpoint studies demonstrating the benefits of lifestyle modifications. Given the central role of lifestyle changes, patients should be offered the opportunity to receive nutritional counseling. Equally important is an increase in physical activity to a level of 2.5 to 5 h per week of moderate-intensity aerobic exercise (18).
In overweight patients, the goal is to bring about weight loss and in patients with diabetes mellitus, to achieve good blood sugar control. In addition, it is important to take into account that the response to lifestyle modifications varies considerably between individuals.
TG-lowering pharmacotherapy should be started only after lifestyle modifications have been implemented and control of diabetes has been achieved. Overall, in our experience, only a small proportion of patients with hypertriglyceridemia (approximately 10%) require specific drug treatment to lower TG levels.
Drug treatment of hypertriglyceridemia
The primary goal of pharmacotherapy is to reduce the incidence of cardiovascular events. Since studies have yielded unequivocal evidence that this reduction is achieved by lowering LDL-cholesterol levels, the first step in the management of patients with hypertriglyceridemia is to attempt to achieve the target LDL-cholesterol level (19). For this purpose, the strategies available for lowering LDL-cholesterol levels (lifestyle modifications, statins, ezetimibe, PCSK9 inhibitors) should be used, taking into account the overall risk (20, 21, e10– e12). While the above-mentioned medications do not lower TG levels, they are indicated for the reduction of the cardiovascular risk. Bile acid sequestrants should not be used, as they can amplify any existing hypertriglyceridemia; moreover, there is no evidence that cardiovascular events are reduced by a combination of bile acid sequestrants and statins (22).
Once the target LDL-cholesterol level has been achieved, and taking into account the overall risk, the decision has to be made whether a specific treatment for hypertriglyceridemia is required to achieve the non-HDL-cholesterol target (secondary target). This decision is primarily based on the extent of hypertriglyceridemia and the absolute risk. Drug treatment should always be considered if the TG level is above 400 mg/dL (4.7 mmol/L), on the assumption that much higher levels (at times >1000 mg/dL, 11.4 mmol/L) will be reached intermittently (for example, after a meal or with alcohol consumption).
Fibrates
Fibrates can variably reduce TG levels by 20% to 70% (23, 24). Some studies from the “pre-statin era” showed that fibrate treatment also results in a reduction of the cardiovascular risk (25, e13). In the Helsinki Heart Study of 4081 men, gemfibrozil treatment led to a relative risk reduction for cardiovascular endpoints by 37% (absolute risk reduction: 14.1%) and the VA-HIT study of 2531 men found a relative risk reduction of 22% (absolute risk reduction: 4.4%) in those treated with gemfibrozil (25, e13). By contrast, studies evaluating fibrates in combination with statins found no additional benefit (26). A Cochrane analysis also concluded that fibrates should be used very restrictevly (24). Because of its very high potential for interactions with other medications, especially statins, gemfibrozil should be used only in exceptional cases and then always by pharmacologically experienced physicians.
Nevertheless, in patients with hypertriglyceridemia and a very high risk (for example progression of atherosclerotic disease despite achieving target LDL levels) the use of fibrates may be considered, because analyses in subgroups of the above-mentioned studies hinted at potential benefits in this constellation (24, 27). Special mention should be made of ongoing studies evaluating whether patients with high risk and elevated TG levels may benefit from fibrates in combination with statins (28).
In patients with excessively high TG levels (>1000 mg/dL, approximately 10 mmol/L; pancreatitis prevention), it should be established on an individual basis whether fibrates are effective. After about 4 to 6 weeks, during which lifestyle modifications are rigorously maintained, patients should be reevaluated. If no clinically relevant effect (reduction >30%) is found during reevaluation, the medication should be discontinued. The available evidence does not indicate that fibrates can reduce the risk of pancreatitis.
Omega-3 fatty acids
High doses of omega-3 fatty acids (>1.5–2 g icosapent ethyl plus 1.2–1.5 g doconexent ethyl daily) have a triglyceride-lowering effect (about 25–30%). However, the evidence from studies evaluating the use of low-dose omega-3 fatty acids (1 g daily) to prevent cardiovascular events is neutral (29– 31). Thus, there is no reason to initiate treatment with low doses of omega-3 fatty acids.
By contrast, the recently published REDUCE-IT study has shown that with a considerably higher dose (4 g daily) of a specific omega-3 fatty acid (icosapent ethyl = eicosapentaenoic acid [EPA] ethyl ester) the incidence of cardiovascular events was greatly reduced (32). In this study of 8179 patients (high risk, receiving statin treatment), relative risk reduction by 25% and absolute risk reduction by 4.8% were achieved (number needed to treat [NNT] = 21 over a period of 4.9 years). It remains unclear by what mechanism this positive effect was brought about and whether the difference between the results of this study and the results of other analyses on omega-3 fatty acids was due to the choice of a different patient population, the administration of a higher dose, the use of potentially harmful mineral oil as a comparator, or the use of a specific omega-3 fatty acid. It is also striking that patients benefited from the treatment regardless of their baseline TG levels. In this respect, it is worth mentioning that another study evaluating a higher dose of omega-3 fatty acids is currently being conducted; after completion of that study (expected for 2021), it will be possible to define more closely which patient population actually benefits from such an intervention (33).
As for fibrates, treatment with omega-3 fatty acids should be tried in patients with excessively high TG levels treatment with this substances in the attempt to determine which strategies, including combinations, lead to the lowest TG levels.
New treatment approaches
For patients with very rare, severe, hereditary forms of hypertriglyceridemia (e.g., FCS caused by a lipoprotein lipase defect), novel treatments are in clinical development (34). The Apo C3 antisense oligonucleotide volanesorsen has recently been approved in Germany specifically for the treatment of patients with FCS (35). The aim in these patients, who have often already experienced recurrent episodes of pancreatitis (chylomicronemia syndrome) in childhood, is to lower TG levels to a range where pancreatitis is unlikely to occur.
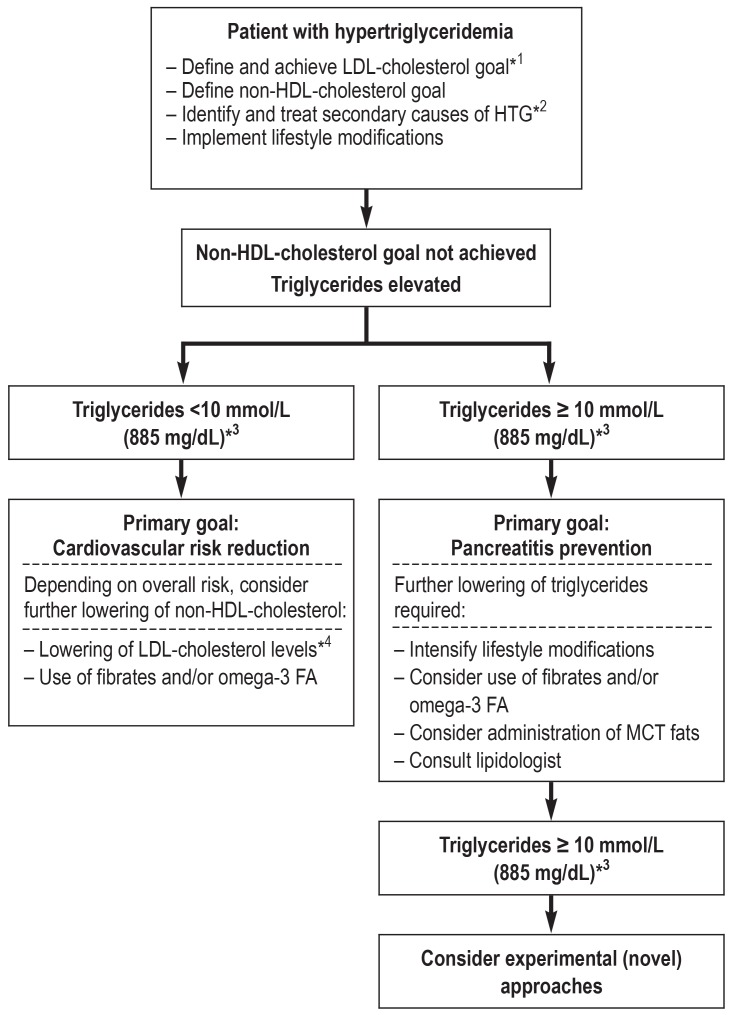
Management
One possible management approach is outlined in the Figure. However, it should be noted that there is no definite recommendation with regard to the sequence in which fibrates and/or omega-3 fatty acids should be used.
Figure.
Possible treatment algorithm for patients with HTG.
It should be taken into account that the goals “cardiovascular risk reduction” and “pancreatitis prevention” cannot be clearly separated. The overall risk is based on the cardiovascular risk and can be assessed using the ESC risk score (5).
*1 Target LDL levels according to ESC and EAS are listed in Table 2
*2 Box 2 provides an overview of secondary causes
*3 No clear definition of the upper threshold level (table 1)
*4 By further lowering of the LDL-cholesterol level, the non-HDL-cholesterol level can also be decreased
EAS, European Atherosclerosis Society; ESC, European Society of Cardiology;
HDL, high-density lipoprotein; HTG, hypertriglyceridemia; LDL, low-density lipoprotein;
MCT, medium-chain triglycerides; omega-3 FA, omega-3 fatty acids
Acute pancreatitis associated with significant hypertriglyceridemia: a special situation
Treatment of acute pancreatitis in patients with massive hypertriglyceridemia (>1000 mg/dL or 11.4 mmol/L) comprises strict fasting and intravenous fluid replacement as basic measures. Whether plasmapheresis is indicated has to be decided on a case-by-case basis. With this approach TG levels can be lowered rapidly, which may interrupt the underlying pathomechanism so that the pancreatitis takes a milder course (36). However, there are no randomized studies on this topic (evidence level C). Moreover, a recent analysis showed that TG levels decline quickly (24–36 h) without plasmapheresis (37). Furthermore, patients with acute pancreatitis frequently present with various triggering factors (e.g., concurrent alcohol consumption), making it difficult to distinguish the role played by hypertriglyceridemia from those of other factors. In addition, plasmapheresis is not suitable as a long-term treatment in patients with hypertriglyceridemia because TG levels quickly rebound after hemofiltration.
Patients with FCS in whom hypertriglyceridemia is apparently a key factor in the pathogenesis of acute pancreatitis should immediately be treated with plasmapheresis. In the long term, a diet change from standard fats (saturated fatty acids, monounsaturated fatty acids) to medium-chain TG (MCTs) may be beneficial, as MCTs are metabolized independent of chylomicrons and do not result in an increase in TG levels after a meal (38). This is all the more relevant when women with hereditary disorders such as FCS become pregnant, as estrogens can trigger a significant increase in TG levels (39).
Patients with complicated hypertriglyceridemia, e.g., with recurrent episodes of pancreatitis, should be treated in specialized lipidological centers where it can be evaluated whether there is an indication for the use of novel treatment approaches (40).
Key Messages.
Hypertriglyceridemia can be divided into moderate (TG levels from 150 mg/dL to about 1000 mg/dL) and severe (TG levels >1000 mg/dL) forms.
In patients with moderate hypertriglyceridemia the focus is on the increased cardiovascular risk, in severe hypertriglyceridemia, on the increased risk of pancreatitis.
Lifestyle factors and comorbidities (diabetes mellitus) contribute to the development of hypertriglyceridemia in patients with an underlying genetic predisposition.
Lifestyle modifications (avoiding alcohol; reducing rapidly metabolizable carbohydrates; weight loss; physical activity) are the mainstays of treatment.
The use of medications depends on an individual’s risks for cardiovascular disease and pancreatitis.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
Prof. Parhofer has received lecture fees, consulting (advisory board) fees, and fees for Data Monitoring Committee (DMC) responsibilities and/or research support from the following companies: Aegerion, Akcea, Amarin, Amgen, Amryt, Berlin-Chemie, Boehringer-Ingelheim, Daiichi Sankyo, MSD, Novartis, Pfizer, Regeneron, and Sanofi.
Prof. Laufs has received lecture or consulting fees from the following companies: Amgen, Boehringer-Ingelheim, Daiichi Sankyo, Novartis, Pfizer, Sanofi, and Servier.
References
- 1.Jaross W, Assmann G, Bergmann S, et al. Comparison of risk factors for coronary heart disease in Dresden and Munster. Results of the DRECAN (Dresden Cardiovascular Risk and Nutrition) study and the PROCAM (Prospective Cardiovascular Munster) Study. Eur J Epidemiol. 1994;10:307–315. doi: 10.1007/BF01719355. [DOI] [PubMed] [Google Scholar]
- 2.Arca M, Borghi C, Pontremoli R, et al. Hypertriglyceridemia and omega-3 fatty acids: Their often overlooked role in cardiovascular disease prevention. Nutr Metab Cardiovasc Dis. 2018;28:197–205. doi: 10.1016/j.numecd.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines Circulation. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Hegele RA, Ginsberg HN, Chapman MJ, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2014;2:655–666. doi: 10.1016/S2213-8587(13)70191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019 doi: 10.1093/eurheartj/ehz455. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 6.Leiter LA, Lundman P, da Silva PM, et al. Persistent lipid abnormalities in statin-treated patients with diabetes mellitus in Europe and Canada: results of the Dyslipidaemia International Study. Diabet Med. 2011;28:1343–1351. doi: 10.1111/j.1464-5491.2011.03360.x. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen SB, Langsted A, Nordestgaard BG. Nonfasting Mild-to-Moderate Hypertriglyceridemia and Risk of Acute Pancreatitis. JAMA Intern Med. 2016;176:1834–1842. doi: 10.1001/jamainternmed.2016.6875. [DOI] [PubMed] [Google Scholar]
- 8.Ference BA, Kastelein JJP, Ray KK, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321:364–373. doi: 10.1001/jama.2018.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varbo A, Benn M, Tybjaerg-Hansen A, et al. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;6:427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 10.Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 11.Tushuizen ME, Diamant M, Heine RJ. Postprandial dysmetabolism and cardiovascular disease in type 2 diabetes. Postgrad Med J. 2005;81:1–6. doi: 10.1136/pgmj.2004.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Nordestgaard BG, Langsted A, Mora S, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. 2016;37:1944–1958. doi: 10.1093/eurheartj/ehw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NICE. National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. https://www.nice.org.uk/guidance/cg181/resources/cardiovascular-disease-risk-assessment-and-reduction-including-lipid-modification-pdf-35109807660997 (last accessed on 17 October 2019) [Google Scholar]
- 16.DEGAM. Hausärztliche Risikoberatung zur kardiovaskulären Prävention. S3-Leitlinie. AWMF-Register-Nr. 053-024 DEGAM-Leitlinie Nr. 19. https://www.degam.de/files/Inhalte/Leitlinien-Inhalte/Dokumente/DEGAM-S3-Leitlinien/053-024_Risikoberatung%20kardiovaskul.%20Praevention/053-024l_Hausa¨rztliche_Risikoberatung_kardivaskula¨re_Praevention_29-08-2018.pdf (last accessed on 17 October 2019) [Google Scholar]
- 17.Universitätsklinikum Leipzig — AöR. Merkblatt zur Ernährung für Patienten mit Hypertriglyzeridämie: https://www.uniklinikum-leipzig.de/einrichtungen/kardiologie/Freigegebene%20Dokumente/Merkblatt_Hypertriglyzeridämie.pdf (last accessed on 17 October 2019) [Google Scholar]
- 18.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parhofer KG. The treatment of disorders of lipid metabolism. Dtsch Arztebl Int. 2016;113:261–268. doi: 10.3238/arztebl.2016.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baigent C, Blackwell L, et al. Cholesterol Treatment Trialists (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ast M, Frishman WH. Bile acid sequestrants. J Clin Pharmacol. 1990;30:99–106. doi: 10.1002/j.1552-4604.1990.tb03447.x. [DOI] [PubMed] [Google Scholar]
- 23.Katsiki N, Nikolic D, Montalto G, et al. The role of fibrate treatment in dyslipidemia: an overview. Curr Pharm Des. 2013;19:3124–3131. doi: 10.2174/1381612811319170020. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Liu B, Tao W, et al. Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD009580.pub2. CD009580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 26.Ginsberg HN, Elam MB, Lovato LC, et al. ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375:1875–1884. doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 28.Araki E, Yamashita S, Arai H, et al. Effects of Pemafibrate, a novel selective PPARalpha modulator, on lipid and glucose metabolism in patients with type 2 Diabetes and hypertriglyceridemia: A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2018;1:538–546. doi: 10.2337/dc17-1589. [DOI] [PubMed] [Google Scholar]
- 29.[No authors listed] Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell‘Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 30.Aung T, Halsey J, Kromhout D, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: Meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018;3:225–234. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manson JE, Cook NR, Lee IM, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with Icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 33.Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41:1281–1288. doi: 10.1002/clc.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parhofer KG. New approaches to address dyslipidemia. Curr Opin Lipidol. 2017;28:452–457. doi: 10.1097/MOL.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 35.Witztum JL, Gaudet D, Freedman SD, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 2019;381:531–542. doi: 10.1056/NEJMoa1715944. [DOI] [PubMed] [Google Scholar]
- 36.Lennertz A, Parhofer KG, Samtleben W, Bosch T. Therapeutic plasma exchange in patients with chylomicronemia syndrome complicated by acute pancreatitis. Ther Apher. 1999;3:227–233. doi: 10.1046/j.1526-0968.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- 37.Berberich AJ, Ziada A, Zou GY, et al. Conservative management in hypertriglyceridemia-associated pancreatitis. J Intern Med. 2019 doi: 10.1111/joim.12925. doi: 10.1111/joim.12925. [DOI] [PubMed] [Google Scholar]
- 38.Mizushima T, Ochi K, Matsumura N, et al. Prevention of hyperlipidemic acute pancreatitis during pregnancy with medium-chain triglyceride nutritional support. Int J Pancreatol. 1998;23:187–192. doi: 10.1007/BF02788396. [DOI] [PubMed] [Google Scholar]
- 39.Bolla D, Schyrba V, Drack G, Schöning A, Stage A, Hornung R. Chylomicronemia syndrome in pregnancy: a case report of an acute necrotizing pancreatitis. Geburtshilfe Frauenheilkd. 2012;72:853–855. doi: 10.1055/s-0032-1315295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blom DJ, O‘Dea L, Digenio A, et al. Characterizing familial chylomicronemia syndrome: Baseline data of the APPROACH study. J Clin Lipidol. 2018;12:1234–1243e5. doi: 10.1016/j.jacl.2018.05.013. [DOI] [PubMed] [Google Scholar]
- E1.Retterstol K, Narverud I, Selmer R, et al. Severe hypertriglyceridemia in Norway: prevalence, clinical and genetic characteristics. Lipids Health Dis. 2017;16 doi: 10.1186/s12944-017-0511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Parhofer KG, Barrett PH, Schwandt P. Atorvastatin improves postprandial lipoprotein metabolism in normolipidemlic subjects. J Clin Endocrinol Metab. 2000;85:4224–4230. doi: 10.1210/jcem.85.11.6978. [DOI] [PubMed] [Google Scholar]
- E3.Stroes E, Moulin P, Parhofer KG, et al. Diagnostic algorithm for familial chylomicronemia syndrome. Atheroscler Suppl. 2017;23:1–7. doi: 10.1016/j.atherosclerosissup.2016.10.002. [DOI] [PubMed] [Google Scholar]
- E4.Angelantonio E, Sarwar N, et al. Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 2012;307:1302–1309. doi: 10.1001/jama.2012.366. [DOI] [PubMed] [Google Scholar]
- E6.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Santos FL, Esteves SS, da Costa Pereira A, Yancy WS Jr, Nunes JP. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13:1048–1066. doi: 10.1111/j.1467-789X.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- E8.Shaw K, Gennat H, O‘Rourke P, DelMar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003817.pub3. CD003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Zomer E, Gurusamy K, Leach R, et al. Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obes Rev. 2016;17:1001–1011. doi: 10.1111/obr.12433. [DOI] [PubMed] [Google Scholar]
- E10.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- E11.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- E12.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- E13.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]