Abstract
A type of high-risk human papillomavirus (HPV), HPV16 takes part in lung carcinogenesis. E6 and E7 are the major oncoproteins of high-risk HPV, and L1 is the major capsid protein. In this study, we detected their mRNA expressions and analyzed their relationship in the bronchial brushing cells of 211 patients with malignant lesions (squamous cell carcinoma of the lungs) and benign lesions (pneumonia and tuberculosis) by quantitative real-time PCR. HPV16 E6, E7, and L1 mRNA expressions in the malignant group were statistically higher than in benign group (P<0.05), and their mRNA expressions in the squamous cell carcinoma of the lung group were statistically higher than in pneumonia group (P<0.05). There was a negative correlation between L1 and E6 expression in the squamous cell carcinoma of the lungs group (Spearman correlation coefficient r=-0.498, P=0.000). An ROC curve shows that the combination of L1 and E6 is a significant predictor for the diagnosis of squamous cell carcinoma of the lungs (AUC: 0.878; Sensitivity: 96.00%; Specificity: 77.91%), which could make up for the deficiency of cytologic testing. The combined detection of HPV16 E6 and L1 mRNA expressions in bronchial brushing cells by quantitative real-time PCR has a great significance for the diagnosis of squamous cell carcinoma of the lungs, providing new therapeutic targets for the clinical treatment of squamous cell carcinoma of the lungs.
Keywords: HPV16 E6, HPV16 L1, bronchial brushing cells, squamous cell carcinoma of the lungs
Introduction
Lung cancer is one of the common malignancies in the world [1]. Squamous cell carcinoma is a common histological subtype of non-small-cell lung cancer [2]. However, there are no specific targeting genes for the treatment of squamous cell carcinoma of the lungs. A recent study indicates that human papillomavirus (HPV) infection may be an important role in the etiology of squamous cell carcinoma of the lungs [3].
As a kind of double-stranded circle DNA virus, HPV is associated with a variety of cancers [4], particularly a persistent infection of high-risk HPV [5]. Syrjanen firstly proposed the hypothesis that HPV infection might play an important role in lung cancer [6]. With the rapid development of molecular biology, a large number of epidemiological studies show that HPV16 (the high-risk type) infection plays a key role in lung cancer [7,8], and we have found that HPV16 expression in malignant lung lesions is significantly higher than in benign lung lesions [9].
The HPV genome is composed of an early region and a late region. The early region encodes the viral regulatory proteins (E1, E2, E3, E4, E5, E6, E7, and E8), and late region encodes the viral structural proteins (L1 and L2). E6 and E7 are multifunctional proteins, playing important roles in the transformation of HPV-infected cells. They can enhance the proliferation of HPV-infected cells [10], resulting in the inactivation of tumor suppressor genes p53 and Rb [11,12]. E6 and E7 are constantly expressed in HPV-infected cells [13], acting as tumor-specific antigens [14]. L1 expression is correlated with the severity of cervical intraepithelial neoplasia (the precursor of cervical cancer) [15]. A previous study indicated that the L1 protein could be detected in HPV infection lesions and cancer cells [16,17]. L1 has the ability to stimulate an effective cellular immune response [18]. A specific cellular immunity against HPV16 L1 inhibits and clears tumor cells expressing the antigen [19]. However, the regulatory mechanism among E6, E7, and L1 in HPV16-infected squamous cell carcinoma of the lungs is still uncertain.
In this study, we used quantitative real-time PCR to detect the mRNA expressions of HPV16 E6, E7, and L1 in the bronchial brushing cells of 211 patients with squamous cell carcinoma of the lungs, pneumonia, and tuberculosis. And we also analyzed the correlation among their mRNA expressions.
Materials and methods
Patient data
The samples used in this study were obtained in accordance with the Declaration of Helsinki and the Human Subject Research Protocols approved by the ethics committees of the First Affiliated Hospital of China Medical University (No. AF-SOP-07-1.0-01). We obtained written informed consent from the patients. A total of 211 cases of bronchial brushing cells from outpatients and hospitalized patients in the First Affiliated Hospital of China Medical University from June 2013 to June 2015 were collected, including 125 malignant samples (squamous cell carcinoma of the lungs) and 86 benign samples (pneumonia: 65 cases; tuberculosis: 21 cases). The cases of lung squamous cell carcinoma were selected as study subjects, and the cases of pneumonia and tuberculosis were selected as control subjects. All cases were diagnosed by cytologic testing and bronchial biopsy or confirmed by postoperative pathology.
Preparation of specimens
Two experienced bronchoscopists performed the bronchoscopies. The area of suspected malignancy was brushed three times; two smears were fixed in 95% alcohol and stained by Papanicolau’s method, and the third brushing was transferred to a small vial containing SurePathTM preservative fluid (BD Tripath, Burlington NC, USA) for the cytological diagnosis of the liquid-based Pap test. The remaining specimens in the small vial were tested for quantitative real-time PCR. The detailed procedures are shown in our recent research [20].
RNA extraction and cDNA synthesis
The remaining specimens in the small vial were added into centrifuge tubes and centrifuged at 2000 rpm for 5 min. Next the supernatant was discarded, and the sediment was mixed with Trizol, incubating for 5 min at room temperature. After adding chloroform, we centrifuged it at 12000 rpm for 15 min at 4°C. We transferred the upper layer to a new tube and added an equal amount of isopropanol. After centrifuging at 12000 rpm for 10 min, we discarded the supernatant and added 75% ethanol. After centrifuging at 8000 rpm for 5 min, we discarded the supernatant and put it in a vacuum for drying at room temperature for 5 min. Then the extracted RNA was incubated with DNase I (RNase-free DNase set, Qiagen) to eliminate any contamination by DNA. We used the Prime-Script™ 1st strand cDNA Synthesis Kit (TaKaRa, Dalian, China) for cDNA synthesis according to the manufacturer’s instructions.
Quantitative real-time PCR
We used SYBR Premix Ex Taq (Tli RNase H Plus, TaKaRa, Dalian, China) for quantitative real-time PCR (7900HT Fast Real-Time PCR) according to the manufacturer’s instructions. The results were normalized to the expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene. We analyzed the amplicon from the PCR reaction by its melting temperature in the dissociation curve. The relative quantification of the target genes (HPV16 E6, E7 and L1) was carried out using the ΔΔCT (threshold cycle) method [21]. GAPDH was selected as an internal reference for normalizing the relative gene expression. We repeated the experiments in triplicate. The sequences of primers are shown in Table 1.
Table 1.
The primer used in quantitative real-time PCR
| Gene | Primer sequences | Amplicon size (bp) | |
|---|---|---|---|
| L1 | Forward | 5’-GAGTAGGTGTTGAGGTAGGTCGT-3’ | 108 |
| Reverse | 5’-TTGCTGCATAAGCACTAGCATT-3’ | ||
| E6 | Forward | 5’-GTATGGAACAACATTAGAACAGCAA-3’ | 80 |
| Reverse | 5’-GTGGCTTTTGACAGTTAATACACC-3’ | ||
| E7 | Forward | 5’-GCATGGAGATACACCTACATTG-3’ | 292 |
| Reverse | 5’-TGGTTTCTGAGTAACAGATGG-3’ | ||
| GAPDH | Forward | 5’-TGCTGAGTAACCTTCGAACC-3’ | 136 |
| Reverse | 5’-ACTTGTCTCTCCCCGCAAAG-3’ |
Statistical analysis
Statistical analyses were performed using SPSS 17.0 (SPSS Inc, Chicago, IL). When the P value was less than 0.05, it was considered statistically significant. The results are expressed as mean ± SEM and statistically analyzed based on Student’s t-test. A Spearman correlation test was used to assay the correlations among the expression of these markers. The receiver operating characteristic (ROC) curve was used to evaluate the area under the curve (AUC), the sensitivity, and the specificity of the predictors, which was made and analyzed by MedCalc.
Results
The mRNA expressions of E6, E7, and L1 in bronchial brushing cells
The bronchial brushing cells of 211 patients were divided into squamous cell carcinoma of the lungs, tuberculosis, and pneumonia. We used quantitative real-time PCR to detect the mRNA expressions of HPV16 E6, E7, and L1 and then compared their ΔΔCT mean values. Their mRNA expressions in malignant bronchial brushing cells are significantly higher than in the benign bronchial brushing cells (P<0.05). Compared with the pneumonia group, the mRNA expressions of HPV16 E6, E7, and L1 in the malignant group (squamous cell carcinoma of the lungs) are statistically elevated (P<0.05). Compared with the tuberculosis group, the mRNA expression levels of HPV16 E6 in the squamous cell carcinoma of the lungs group are statistically elevated (P<0.05) (Table 2).
Table 2.
The detection of L1, E6 and E7 mRNA expressions in bronchial brushing cells of patients with malignant and benign lung lesions (Mean ± SEM) by quantitative real-time PCR
| Group | n | L1 | E6 | E7 |
|---|---|---|---|---|
| Malignant (SCC) | 125 | 0.0070±0.0006§,‡ | 0.0166±0.0010§,‡,* | 0.0079±0.0006§,‡ |
| Benign | 86 | 0.0021±0.0003§ | 0.0076±0.0013§ | 0.0055±0.0005§ |
| Pneu | 65 | 0.0012±0.0002‡ | 0.0069±0.0015‡ | 0.0049±0.0005‡ |
| Tuber | 21 | 0.0048±0.0010 | 0.0097±0.0029* | 0.0073±0.0008 |
SCC, squamous cell carcinoma; Pneu, pneumonia; Tuber, tuberculosis;
there was a statistical difference between the malignant group and the benign group (P<0.05);
there was a statistical difference between the SCC group and the pneumonia group (P<0.05);
there was a statistical difference between the SCC group and the tuberculosis group (P<0.05).
The correlation of HPV16 E6/E7 and L1 in the bronchial brushing cells of patients with squamous cell carcinoma of the lungs
There were 125 cases of bronchial brushing cells of patients with squamous cell carcinoma of the lungs. We analyzed the mRNA expressions of L1, E6 and E7 in this group. There is a significantly negative correlation between the mRNA expression of L1 and E6 (Spearman correlation coefficient r=-0.498, P=0.000; Figure 1A), but not between the mRNA expression of L1 and E7 (Spearman correlation coefficient r=-0.088, P=0.330; Figure 1B).
Figure 1.
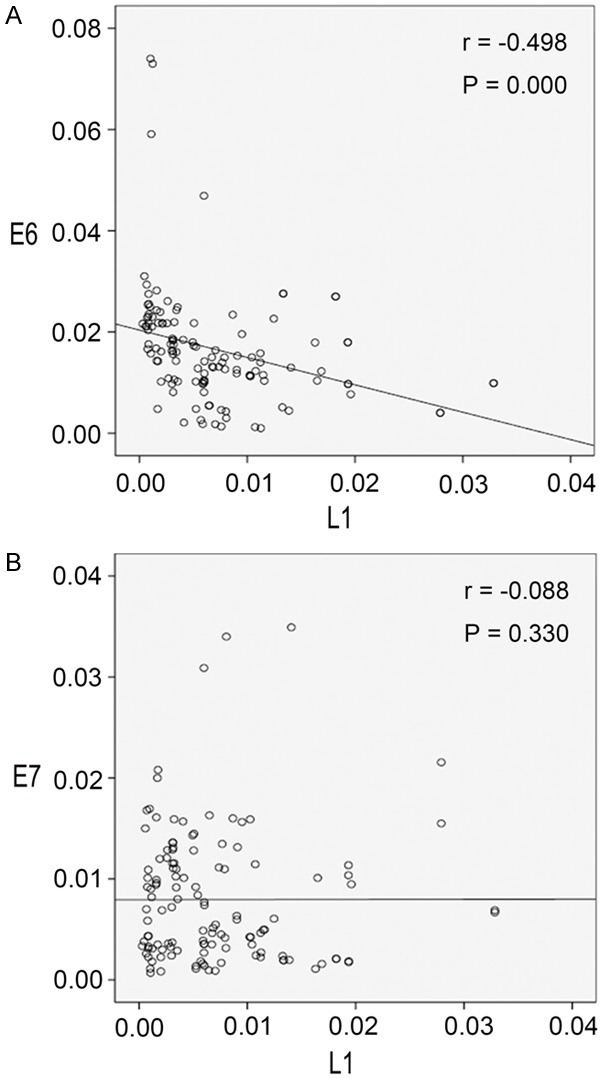
The correlation of HPV16 E6/E7 and L1 in the bronchial brushing cells of patients with squamous cell carcinoma of the lungs. A. Scatter plot of E6 and L1 in the bronchial brushing cells of patients with squamous cell carcinoma of the lungs (R Sq Linear =0.101); B. Scatter plot of E7 and L1 in bronchial brushing cells of patients with squamous cell carcinoma of the lungs (R Sq Linear =2.227E-6).
Diagnostic value of E6, E7, L1, and cytology in the diagnosis of bronchial brushing cells of patients with squamous cell carcinoma of the lungs
An ROC curve was used to determine the predictive values of E6, E7, and L1 for the diagnostic potential of bronchial brushing cells in squamous cell carcinoma of the lungs. The combined detection of E6 and L1 (AUC =0.878) was a better predictor than any one value alone or the combined detection of E7 and L1 (P<0.05, Figure 2; Table 3). Compared with the result of cytologic testing (Sensitivity: 60.80%; Specificity: 100%), the combined detection of E6 and L1 has a good sensitivity. They could complement each other.
Figure 2.
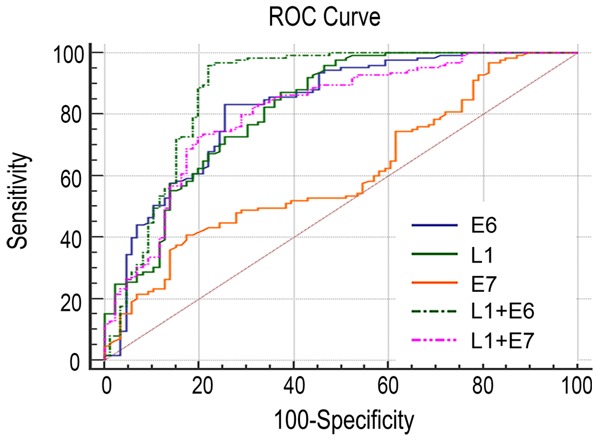
ROC curve analysis.
Table 3.
Diagnostic value of E6, E7, and L1 in the diagnosis of bronchial brushing cells of patients with squamous cell carcinoma of the lungs
| Variable | AUC | 95% CI | Youden index | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| L1 | 0.817 | 0.758-0.867 | 0.4999 | 87.20 | 62.79 |
| E6 | 0.822 | 0.764-0.871 | 0.5762 | 83.20 | 75.38 |
| E7 | 0.606 | 0.537-0.673 | 0.2336 | 40.80 | 82.56 |
| L1+E6 | 0.878 | 0.827-0.919 | 0.7391 | 96.00 | 77.91 |
| L1+E7 | 0.809 | 0.750-0.860 | 0.5303 | 72.80 | 80.23 |
Discussion
In recent years, lung cancer has had the highest morbidity and mortality among all malignant tumors. Many studies have shown that HPV infection is closely related to lung cancer [3]. HPV16, a high-risk type of HPV, is associated with the progression of lung cancer [8,9]. Our results show that the mRNA expressions of HPV16 E6/E7 and L1 in the bronchial brushing cells of malignant lesions are higher than in benign lesions. Therefore, infection control of the high-risk HPV virus may be a new treatment for lung cancer.
The carcinogenic effect of HPV is associated with the integration of HPV DNA. After integration, the E6 and E7 oncogenes take part in carcinogenesis by altering the cell cycle regulation so as to cause an unlimited proliferation of cancer cells [22-26]. The major late protein L1 is an important constituent part of the viral capsid. The L1 protein is detected only in its viral productive phase, reflecting the state of HPV replication. This indicates that the body is infected by HPV and the virus is at the stage of replication. L1 is the major target of cellular immunologic response. After the HPV genes are integrated into the host genes, the L1 protein is not expressed [27]. When L1 expression is lost, the body’s immune system cannot effectively recognize and eliminate the HPV infected cells, resulting in the progression of malignant transformation [28]. In this study, we found that there was a significant negative correlation between E6 and L1 in bronchial brushing cells of squamous cell carcinoma of the lungs. However, there is no significant correlation between the expression of HPV 16 E6/E7 and L1 in the group of patients who were diagnosed with squamous cell carcinoma of the lungs by histological biopsy with carcinoma cells not being found in the bronchial brushing specimens. It is probably because the brushed area of the bronchoscopic examination is paracancerous tissue in these patients. The ROC curve also showed that the combined detection of L1 and E6 was a good predictor. Therefore, the combined detection of L1 and E6 by quantitative real-time PCR is an effective auxiliary method for the diagnosis of squamous cell carcinoma of the lungs, which could make up for the deficiency of cytologic testing. We could speculate that L1 has an effect on E6 in squamous cell carcinoma of the lungs.
The detection of bronchial brushing specimens is generally useful in squamous cell carcinoma of the lungs [29,30]. In this study, we found that a significantly negative correlation between L1 and E6 mRNA expressions was present in lung squamous cell carcinoma. The number of lung adenocarcinoma cases detected by bronchial brushing was very limited and didn’t achieve statistical significance. Further research in this area is needed.
In this study, we found that the detection of HPV16 E6 and L1 mRNA expressions by quantitative real-time PCR had a great significance for cancer diagnosis in the bronchial brushing cells of patients with squamous cell carcinoma of the lungs, which provides new therapeutic targets for the clinical treatment of squamous cell carcinoma of the lungs.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81171650 and 81672082 to Guang-Ping Wu, No. 81602022 to Huan-Yu Zhao).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: Molecular subtypes and therapeutic opportunities. Clin Cancer Res. 2012;18:2443–51. doi: 10.1158/1078-0432.CCR-11-2370. [DOI] [PubMed] [Google Scholar]
- 3.Xiong WM, Xu QP, Li X, Xiao RD, Cai L, He F. The association between human papillomavirus infection and lung cancer: a system review and meta-analysis. Oncotarget. 2017;8:96419–32. doi: 10.18632/oncotarget.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naushad W, Surriya O, Sadia H. Prevalence of EBV, HPV and MMTV in Pakistani breast cancer patients: a possible etiological role of viruses in breast cancer. Infect Genet Evol. 2017;54:230–7. doi: 10.1016/j.meegid.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Tao G, Yaling G, Zhan G, Pu L, Miao H. Human papillomavirus genotype distribution among HPV-positive women in Sichuan province, Southwest China. Arch Virol. 2018;163:65–72. doi: 10.1007/s00705-017-3556-1. [DOI] [PubMed] [Google Scholar]
- 6.Syrjänen KJ. Condylomatous changes in neoplastic bronchial epithelium. Report of a case. Respiration. 1979;38:299–304. doi: 10.1159/000194095. [DOI] [PubMed] [Google Scholar]
- 7.Yang JH, Li XY, Wang X, Hou WJ, Qiu XS, Wang EH, Wu GP. Long-term persistent infection of HPV 16 E6 up-regulate SP1 and hTERT by inhibiting LKB1 in lung cancer cells. PLoS One. 2017;12:e0182775. doi: 10.1371/journal.pone.0182775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Shabbani N. Detection of human papilloma virus (type 16 and 18) in cytological samples in patients with lung cancer. An evaluation by chromogenic in situ hybridization technique. Redox Biol. 2015;5:416. doi: 10.1016/j.redox.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Fan R, Hou WJ, Zhao YJ, Liu SL, Qiu XS, Wang EH, Wu GP. Overexpression of HPV16 E6/E7 mediated HIF-1α upregulation of GLUT1 expression in lung cancer cells. Tumour Biol. 2016;37:4655–63. doi: 10.1007/s13277-015-4221-5. [DOI] [PubMed] [Google Scholar]
- 10.Choi YJ, Park JS. Clinical significance of human papillomavirus genotyping. J Gynecol Oncol. 2016;27:e21. doi: 10.3802/jgo.2016.27.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yim EK, Park JS. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005;37:319–24. doi: 10.4143/crt.2005.37.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 13.Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016;23:75. doi: 10.1186/s12929-016-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang A, Jeang J, Cheng K, Cheng T, Yang B, Wu TC, Hung CF. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev Vaccines. 2016;15:989–1007. doi: 10.1586/14760584.2016.1157477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu YW, Huang RL, Su PH, Chen YC, Wang HC, Liao CC, Lai HC. Genotype-specific methylation of HPV in cervical intraepithelial neoplasia. J Gynecol Oncol. 2017;28:e56. doi: 10.3802/jgo.2017.28.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Tian X, Liu F, Zhao Y, Sun M, Chen D, Lu C, Wang Z, Shi X, Zhang Q, Zhang D, Shen Z, Li F, Harris CC, Cai H, Ke Y. Detection of HPV DNA in esophageal cancer specimens from different regions and ethnic groups: a descriptive study. BMC Cancer. 2010;10:19. doi: 10.1186/1471-2407-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao W, Bian M, Ma L, Liu J, Chen Y, Yang B, Wu Q. Immunochemical analysis of human papillomavirus L1 capsid protein in liquid-based cytology samples from cervical lesions. Acta Cytol. 2010;54:661–7. doi: 10.1159/000325229. [DOI] [PubMed] [Google Scholar]
- 18.Ohlschläger P, Osen W, Dell K, Faath S, Garcea RL, Jochmus I, Müller M, Pawlita M, Schäfer K, Sehr P, Staib C, Sutter G, Gissmann L. Human papillomavirus type 16 L1 capsomeres induce L1-specific cytotoxic T lymphocytes and tumor regression in C57BL/6 mice. J Virol. 2003;77:4635–45. doi: 10.1128/JVI.77.8.4635-4645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li LL, Wang HR, Zhou ZY, Luo J, Xiao XQ, Wang XL, Li JT, Zhou YB, Zeng Y. One-prime multi-boost strategy immunization with recombinant DNA, adenovirus, and MVA vector vaccines expressing HPV16 L1 induces potent, sustained, and specific immune response in mice. Antiviral Res. 2016;128:20–7. doi: 10.1016/j.antiviral.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Li WN, Wang DF, Zhao YB, Qiu XS, Wang EH, Wu GP. Comparative analysis for diagnostic yield of small cell lung cancer by cytology and histology during the same bronchoscopic procedure. Clin Lung Cancer. 2017;18:e357–61. doi: 10.1016/j.cllc.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Bleda S, de Haro J, Varela C, Ferruelo A, Acin F. Elevated levels of triglycerides and vldl-cholesterol provoke activation of nlrp1 inflammasome in endothelial cells. Int J Cardiol. 2016;220:52–5. doi: 10.1016/j.ijcard.2016.06.193. [DOI] [PubMed] [Google Scholar]
- 22.Häfner N, Driesch C, Gajda M, Jansen L, Kirchmayr R, Runnebaum IB, Dürst M. Integration of the HPV16 genome does not invariably result in high levels of viral oncogene transcripts. Oncogene. 2008;27:1610–7. doi: 10.1038/sj.onc.1210791. [DOI] [PubMed] [Google Scholar]
- 23.Strickland SW, Vande Pol S. The human papillomavirus 16 E7 oncoprotein attenuates AKT signaling to promote internal ribosome entry site-dependent translation and expression of c-MYC. J Virol. 2016;90:5611–21. doi: 10.1128/JVI.00411-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tommasino M. The biology of beta human papillomaviruses. Virus Res. 2017;231:128–38. doi: 10.1016/j.virusres.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Hamid NA, Brown C, Gaston K. The regulation of cell proliferation by the papillomavirus early proteins. Cell Mol Life Sci. 2009;66:1700–17. doi: 10.1007/s00018-009-8631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis DA, Schmid SI, Howley PM. Repression of the integrated papillomavirus E6/E7 promoter is required for growth suppression of cervical cancer cells. J Virol. 2000;74:2679–86. doi: 10.1128/jvi.74.6.2679-2686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilfrich R, Hariri J. Prognostic relevance of human papillomavirus L1 capsid protein detection within mild and moderate dysplastic lesions of the cervix uteri in combination with p16 biomarker. Anal Quant Cytol Histol. 2008;30:78–82. [PubMed] [Google Scholar]
- 28.McMurray HR, Nguyen D, Westbrook TF, McAnce DJ. Biology of human papillomaviruses. Int J Exp Pathol. 2001;82:15–33. doi: 10.1046/j.1365-2613.2001.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan YB, Ye L, Wang TY, Wu GP. Correlation between morphology and human telomerase gene amplification in bronchial brushing cells for the diagnosis of lung cancer. Diagn Cytopathol. 2010;38:402–6. doi: 10.1002/dc.21235. [DOI] [PubMed] [Google Scholar]
- 30.Liu YZ, Wang Z, Fang LL, Li L, Cao J, Xu X, Han YL, Cai Y, Wang LX, Wang MR. A potential probe set of fluorescence in situ hybridization for detection of lung cancer in bronchial brushing specimens. J Cancer Res Clin Oncol. 2012;138:1541–9. doi: 10.1007/s00432-012-1232-0. [DOI] [PubMed] [Google Scholar]


