Abstract
Bladder cancer is one of the most common cancers in the world. Despite advanced development made to improve the diagnosis and therapy techniques for bladder cancer, patients always have a poor outcome based on its high potential for metastasis. MiR-195 was reported to have close relevance with the process of bladder cancer. However, the molecular mechanism of miR-195 underlying bladder cancer metastasis and epithelial-mesenchymal transition (EMT) remains unclear. The present study was done to explore the function of miR-195 on EMT and cell migration in bladder cancer. In the present study, we detected the level of miR-195 in 25 matched human bladder cancer tissues and normal adjacent tissues, as well as bladder cancer cell lines or normal cells. Additionally, we determined the effects of miR-195 on expression of CDK4, and the miR-195/CKD4 signaling cascade on cell cycle, invasion, migration, and viability. Results showed that miR-195 was down expressed in bladder cancer tissues and cell lines, which inhibited EMT, cell migration, and invasion. We identify CDK4, an early G1 cell cycle regulator, as a downstream target of miR-195. Also, we found that miR-195 could induce G1-phase arrest, inhibit cell invasion, migration, and viability through down-regulation of CDK4 expression in 5637 and BIU-87 cells. Our experimental data suggest an important role for miR-195/CDK4 in bladder tumorigenesis and provide a potential therapeutic strategy for bladder cancer.
Keywords: miR-195, CDK4, epithelial-mesenchymal transition, bladder cancer
Introduction
Bladder cancer is one of the most common cancers in the developed world, and urothelial carcinoma is the most common type of bladder cancer, with an incidence that ranks ninth in the globe among all kinds of cancers [1]. The lifetime cost for patients with bladder cancer is the highest among all cancers on a per-patient basis [2]. Until now, bladder carcinoma patients always have bad outcomes due to high metastatic potential in spite of advanced improvements made in surgical techniques and the molecular mechanisms underlying bladder cancer occurrence and progression remain unclear [3,4].
It is reported that the occurrence and progression of bladder cancer involves various genetic changes, including chromosomal anomalies, genetic polymorphisms, and genetic and epigenetic alterations [5]. MicroRNAs (miRNAs) are small (18-25 nucleotide), endogenously expressed, noncoding, and single-stranded RNA molecules [6]. They modulate genes expression at the post-transcriptional level primarily by binding to the 3’-untranslated region (UTR) of a target messenger RNA (mRNA). MiRNAs can regulate gene expression by inhibiting protein translation of a target mRNA, or through degrading their target mRNA by affecting its stability, thereby inhibiting protein synthesis indirectly [6]. More than half of miRNA genes are located in cancer-related genomic regions or in fragile sites [7]. Aberrant expression of miRNAs in cancers are associated with poor prognosis, especially in bladder cancer [8-10]. miRNAs, as oncogenes or tumor suppressors exert pivotal influence on cancer Epithelial-mesenchymal transition (EMT) [11,12], which is a process in which epithelial cells transfer toward the mesenchymal state, modify the adhesion molecules expressed, and allow cells to adopt a migratory or more invasive behavior [13]. EMT has been shown to trigger dissociation of carcinoma cells from primary carcinomas, transfer to more aggressive cell phenotype, and subsequently result in cancer metastasis [14]. EMT is a common phenomenon in malignant tumors, such as gastric cancer [15], lung cancer [16], pancreatic cancer [17], and bladder cancer [18,19].
The previous study showed that miR-195 exhibits diverse expression patterns and functions differently according to different cancer types [20-22]. In bladder cancer, Chen Zhao et al. [20] reported that miR-195 was significantly downregulated in bladder cancer tissues and inhibited cell proliferation in bladder cancer via suppression of cell division control protein 42 homolog/signal transducer and activator of transcription-3 signaling. Similarly, Yiwei Lin et al. [23] reported that miR-195 is down-regulated in human bladder cancer tissue and repressed T24 cell growth. However, the biological function of miR-195 on EMT process in bladder cancer was not well elucidated.
In the present study, we focused on the role of miR-195 on the EMT in bladder cancer. First, we detected the expression level of miR-195 in bladder cancer tissues and cell lines, followed by determination of the function of miR-195 on cell migration, invasion, and EMT. Finally, we investigated the impact of miR-195 on its downstream gene CDK4. Our current study, for the first time, show the function of miR-195 on cell EMT and migration in bladder cancer. Based on this work, we hope to find a promising biomarker for the early detection and therapeutic targeting of bladder cancer.
Materials and methods
Patient tissue samples collection
The present study was approved by the Ethics Committee of Shanghai Huashan Hospital. Informed consent was obtained from each participator of the study. 25 bladder cancer tissues, in addition to their paired adjacent normal tissues, were collected from patients without chemotherapy or radiation in the Department of Urology, Huashan Hospital, Fudan University (Shanghai, China). Tissue samples were identified by two experienced pathologists without consideration for the clinical data and immediately frozen in liquid nitrogen following surgical removal.
Cell culture
Human normal bladder cell line SV-HUC-1, bladder cancer cell lines 5637 and BIU-87 were purchased from American Type Culture Collection (ATCC, USA), bladder cancer cell line UMUC-3 were purchased from Cancer Institute & Hospital of the Chinese Academy of Medical Sciences (Shanghai, China). SV-HUC-1 cells were cultured in F-12K Medium, 5637 cells were cultured in RPMI-1640 medium, and UMUC-3 and BIU-87 cell lines were cultured in Eagles Minimum Essential Medium (DMEM), all medium was supplemented with 10% heat-inactivated fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 mg/ml) in a humidified atmosphere containing 5% CO2 maintained at 37°C.
miR-195/CDK4 overexpression or down expression and cell transfection
Synthesis of miR-195 mimic, miR-195 inhibitor, and their negative control duplex (named as NC in figures) lacking significant homology was previously reported [23]. siRNAs targeting of human CDK4 (No.SR300734), pCMV6-CDK4 (No.SC112998), or their controls were all purchased from OriGene (USA). All human sequences were applied for transient gain of function study.
Transfection was performed using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA) when cell confluency was 50-60% in medium without antibiotics, according to the manufacturer’s instructions. The knockdown or overexpressed efficiencies of miR-195/CDK4 were evaluated by qPCR after cells transfection with miR-195 mimic or miR-195 inhibitor 24 hours.
Mimic-miR-195 (sense): UAGCAGCACAGAAAUAUUGGC; Mimic-NC (sense): ACUACUGAGUGACAGUAGA. Inhibitor-miR-195: GCCAAUAUUUCUGUGCUGCUA; Inhibitor-NC: CAGUACUUUUGUGUAGUACAA.
Reverse-transcription and real-time quantitative polymerase chain reaction (qPCR) analysis
Small RNA was isolated from bladder tissue and adjacent tissue samples by using RNAiso for small RNA (TaKaRa, Japan) and reversely transcribed using the One Step PrimeScript miRNA cDNA Synthesis Kit (TaKaRa, Japan), while total RNA from transfected 5637, BIU-87 cells was extracted with RNAiso plus (TaKaRa, Japan) and transcribed into cDNA with the PrimeScript RT reagent Kit (TaKaRa, Japan). The resulting cDNA was quantified by real-time RT-PCR using SYBR Premix Ex Taq (TaKaRa, Japan). The relative expression level of miR-195 and CDK4 was calculated by the 2-ΔΔCt method after normalization with reference to expression of U6 small nuclear RNA or GAPDH, respectively. U6 was used as a reference gene. The qPCR conditions were set as follows: 95°C for 5 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 15 sec.
MiR-195 forward primer: 5’-TAGCAGCACAGAAATATTGGC-3’; Reverse primer: Uni-miR qPCR primer (Takara, Dalian, China). U6 forward primer: 5’-ACGCAAATTCGTGAAGCGTT-3’; Reverse primer: Uni-miR qPCR primer (Takara, Dalian, China).CDK4 forward primer: TGAAATTGGTGTCGGTGCCT; Reverse primer: CAGTCGCCTCAGTAAAGCCA. N-cadherin forward primer: TGC GGT ACAGTG TAA CTG GG; Reverse primer: GAA ACC GGG CTA TCT GCT CG. Vimentin forward primer: GGACCAGCTAACCAACGACA; Reverse primer: AAGGTCAAGACGTGCCAGAG. Snail forward primer: TCGGAAGCCTAACTACAGCGA; Reverse primer: AGATGAGCATTGGCAGCGAG. Slug forward primer: AGATGC ATATTCGGA CCC AC; Reverse primer: CCT CAT GTT TGT GCA GGA GA. Twist forward primer: CTCGGACAAGCTGAGCAAGA; Reverse primer: GCTCTGGAGGACCTGGTAGA. E-cadherin forward primer: AACAGGATGGCTGAAGGTGA; Reverse primer: CCTTCCATGACAGACCCCTT. GAPDH forward primer: GCACCGTCAAGGCTGAGAAC; Reverse primer: TGGTGAAGACGCCAGTGGA.
Western blotting analysis
Cells were lysed by RIPA buffer (Beyotime, Shanghai, China) supplemented with protease inhibitors. The proteins were separated with 10% SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF; Millipore, USA) membrane, subsequently incubated the PVDF membrane with 5% nonfat milk dissolved by Tris buffered saline with Tween 20 containing (TBST) at room temperature for 1 h. The PVDF membrane was then further incubated with rabbit polyclonal anti-CDK4 (No.ab64533), rabbit monoclonal anti-N-cadherin (No.ab18203), rabbit monoclonal anti-E-cadherin (No.ab152102), rabbit monoclonal anti-Vimentin (No.ab92547), rabbit monoclonal anti-Slug (No.ab27568), rabbit monoclonal anti-Snail (No.ab180714), rabbit monoclonal anti-Twist (No.ab50887), and rabbit monoclonal anti-GAPDH (No.ab181602) primary antibodies (Abcam, Cambridge, UK), respectively, at room temperature for 3 hours. Subsequent to being washed three times in phosphate-buffered saline (PBS) with Tween 20, the membrane was incubated with the goat anti-rabbit secondary antibody (Abcam) at room temperature for 40 min. Chemiluminescent detection was carried out using an enhanced chemiluminescence kit (Pierce Chemical, Rockford, IL, USA), and relative protein expression was analyzed using Image-Pro Plus software 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Relative protein expression was shown as the density ratio versus GAPDH.
Cell proliferation detection
The cells were seeded in 96-well plates at a density of 1 × 104 cells per well and a Cell Counting Kit-8 assay (CCK-8; Dojindo, Japan) was used to assess the proliferation potential. Duplicate sets of 5 wells each were assessed for each time point. At every 24 hours after seeding, the absorbance was measured at 450 nm using a plate reader (model 680; Bio-Rad, Hertfordshire, UK). Each assay was repeated at least 3 times.
Cell colony formation assay
5637, BIU-87, and UMUC-3 cells in the experimental or control group (200 cells/well) were plated in triplicate into 6-well plates and cultured for 2 weeks. Cells were then stained with 0.1% crystal violet, and the colony formation rate was calculated using the following equation: colony efficiency (%) = (number of colonies/number of seeded cells) × 100.
Cell cycle
A549 cells were digested by enzyme and centrifuged at 1000 rpm for 5 min. After washed with PBS twice, the cells were fixed with 1 ml precooled 70% ethanol at 4°C for 24 h. Next, the cells were treated with propidium iodide (PI) and RNase A at 37°C for 30 min. Then the cells were tested by flow cytometry at 488 nm to test DNA content. FACS data were analyzed using FlowJo software (Tree Star, Inc.).
Luciferase assays
CDK4 3’-UTR-luciferase reporter vector was created by ligating the CDK4 3’-UTR PCR products into the XhoI and NotI restriction sites of the psiCHECK-2TM Vector (Promega, Madison, Wisconsin, USA). Bladder cancer cells 5637 and BIU-87 were grown in a 24-well plate, subsequently with co-transfected with 50 nM of either miR-195 mimic or miR-195 inhibitor or their negative control oligo and CDK4 3’-UTR-luciferase reporter vector. The relative luciferase activity was measured by Dual-Luciferase Reporter Assay System (Promega, USA) 48 h after transfection.
Statistical analysis
Each assay was repeated at least 3 times. Data were expressed as mean ± standard deviation (SD) of three independent experiments. All analyses were performed using GraphPad Prism version 6.0 or SPSS 17.0. Two-tailed Student’s t-test was used to evaluate the differences in protein expression and mRNA levels. P<0.05 was considered to be statistically significant.
Results
Overexpression of miR-195 in bladder cancer is associated with patients’ poor prognosis
To assess the potential role of miR-195 in bladder cancer, we examined the mRNA expression of miR-195 in 25 human bladder cancer samples and paired normal adjacent bladder tissues using qPCR. As shown in Figure 1A, expression of miR-195 was lower in bladder cancer samples compared with their corresponding normal tissues. Statistical analyses suggested that the average expression levels of miR-195 in bladder cancer samples were lower than that in paired normal tissues (P<0.001). We also determined the expression of miR-195 in bladder cancer cell lines 5637, BIU-87, UMUC-2, and normal bladder cell SV-HUC-1. Similarly, the level of miR-195 was significantly downregulated in 5637, BIU-87, and UMUC-2 compared with that in SV-HUC-1 (Figure 1B). In addition, we analyzed overall survival in bladder cancer patients with high miR-195 level or low miR-195 level by Kaplan-Meier curves (KM), and the results showed that patients with high expression of miR-195 had shorter overall survival than patients with low expression of miR-195 (Figure 1C). Besides, as shown in Tables 1, 2, patients with low levels of miR-195 always had a higher stage of malignancy cancer, higher incidence rate of cancer progression, and lower 5-year survival compared with those patients with high levels of miR-195. These differences were statistically significant (P<0.05). All the data indicated that miR-195 exerted anti-tumor effects in bladder cancer.
Figure 1.

miR-195 overexpression in bladder cancer and cell lines and association with poor prognosis in patients. A: The expression level of miR-195 in bladder cancer tissues and its matched adjacent non-tumor tissues. B: The expression level of miR-195 in bladder cancer cell lines 5637, BIU-87, UMUC-3, and normal bladder cell line SV-HUC-1. C: Kaplane-Meier curves (KM) of overall survival in bladder cancer patients with high miR-195 level or low miR-195 level. (n=3, **P<0.01, ***P<0.001).
Table 1.
Correlation of miR-195 expression level and clinicopathologic features of patients with bladder cancer
| Clinical features | N | mir-195 expression level | P values | |
|---|---|---|---|---|
|
| ||||
| Low expression | High expression | |||
| Sex | ||||
| Male | 97 | 55 | 42 | 0.515 |
| Female | 49 | 25 | 24 | |
| Age (years) | ||||
| ≤50 | 65 | 35 | 30 | 0.837 |
| >50 | 81 | 45 | 36 | |
| Stage | ||||
| Ta | 48 | 15 | 33 | 0.001 |
| T1 | 56 | 35 | 21 | |
| ≥T2 | 42 | 35 | 7 | |
| Grade | ||||
| Low malignant potential | 42 | 25 | 17 | 0.914 |
| Low grade | 43 | 23 | 16 | |
| High grade | 61 | 34 | 27 | |
| Carcinoma in situ | ||||
| Yes | 36 | 21 | 16 | 0.815 |
| No | 110 | 60 | 50 | |
| Recurrence | ||||
| Yes | 53 | 42 | 11 | 0.001 |
| No | 93 | 39 | 54 | |
| Progression | ||||
| Yes | 36 | 32 | 4 | 0.001 |
| No | 110 | 53 | 57 | |
Table 2.
The correlation of 5-year survival and miR-195 expression in bladder cancer patients
| Clinical features | N | Overall survival | |
|---|---|---|---|
|
| |||
| 5-year survival | P values | ||
| Sex | |||
| Male | 97 | 44 | 0.21 |
| Female | 49 | 32 | |
| Age (years) | |||
| ≤50 | 65 | 30 | 0.899 |
| >50 | 81 | 36 | |
| Stage | |||
| Ta | 48 | 35 | 0.002 |
| T1 | 56 | 25 | |
| ≥T2 | 42 | 6 | |
| Grade | |||
| Low malignant potential | 42 | 25 | 0.241 |
| Low grade | 43 | 21 | |
| High grade | 61 | 20 | |
| Carcinoma in situ | |||
| Yes | 36 | 16 | 0.948 |
| No | 110 | 50 | |
| Mir-195 | |||
| High | 66 | 46 | 0.001 |
| Low | 80 | 20 | |
miR-195 inhibits cell migration, invasion, and cloning efficiency in bladder cancer cells
To confirm the anti-tumor effects of miR-195 in bladder cancer, we recruited miR-195 mimics and inhibitor to upregulate and downregulate the expression level of miR-195 in 5637 and BIU-87 cells. As shown in Figure 2A, the knockdown efficiency of inhibitor-miR-195 was about 80.0%, and the expression level of miR-195 increased about 12-fold when cells were transfected with miR-195 mimics in both 5637 and BIU-87 cells, compared with control group. The migration and invasion abilities of 5637 and BIU-87 cells were repressed when upregulated the level of miR-195, the migration and invasion abilities of 5637 and BIU-87 cells were facilitated when downregulated the level of miR-195 (Figure 2B-D). We also examined the cloning efficiency influenced by miR-195 in 5637 and BIU-87 cells. Similarly, overexpression of miR-195 could inhibit cell cloning efficiency, whereas knocking down miR-195 could promote cell cloning efficiency in both 5637 and BIU-87 cells. These results suggest that miR-195 serves as a cancer suppressor in bladder cancer, inhibiting cell migration, invasion, and cloning efficiency in bladder cancer cells.
Figure 2.
miR-195 inhibits cell migration, invasion, and cloning efficiency in bladder cancer cells. (A) The expression level of miR-195 in 5637 and BIU-87 cells with different treatments: inhibitor-miR-195, inhibitor-NC, mimic-NC and mimic-miR-195. Cell migration was determined by wound healing assay, (B) in 5637 cells, and (C) in BIU-87 cells. (D) Transwell assay was performed to detect the invasion ability of 5637 and BIU-87 cells with different treatments. (E) Cloning formation assay was carried out to test the cloning efficiency of 5637and BIU-87 cells in different group. (n=3, ##P<0.01, ###,***P<0.001).
miR-195 regulates EMT in bladder cancer cells
Since EMT is closely related to cancer metastasis, and miR-195 could inhibit cell migration, we investigated the function of miR-195 on EMT in bladder cancer cell lines 5637 and BIU-87. The mRNA and protein expression levels of N-cadherin, Vimentin, Slug, Snail, and Twist in both 5637 and BIU-87 cells decreased significantly after miR-195 overexpression, and the mRNA and protein expression of E-cadherin increased significantly after cells were treated with miR-195 mimics (Figure 3). All these data indicate that miR-195 modulates EMT process, making cells acquire more characteristics of epithelial cells.
Figure 3.
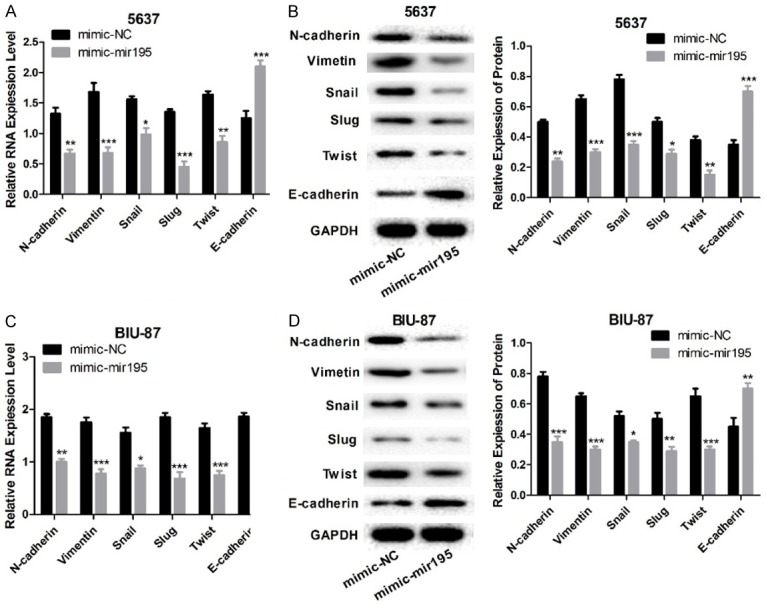
miR-195 regulates EMT in bladder cancer cells. A, B: The mRNA and protein expression levels of N-cadherin, Vimentin, Slug, Snail, Twist, and E-cadherin in 5637 cells after cells overexpressed miR-195. C, D: The mRNA and protein expression levels of N-cadherin, Vimentin, Slug, Snail, Twist, and E-cadherin in BIU-87 cells after cell overexpressed miR-195. (n=3, *P<0.05, **P<0.01, ***P<0.001).
miR-195 regulates cell cycle and suppresses cell invasion via downregulation of CDK4
As shown in previous studies [23], CDK4, an early G1 cell cycle regulator, is a downstream gene which is regulated by miR-195. In the current study, we examined the effects of miR-195 on bladder cancer cell cycle. First, we transfected 5637 and BIU-87 cells with 50 nM miR-195 mimics or miR-195 inhibitor and assessed the impact of miR-195 on the expression of CKD4 by both qPCR and Western blot analysis. As shown in Figure 4A and 4B, expression of CDK4 increased or decreased when cells had downregulated or upregulated expression of miR-195. We performed luciferase assays to determine the relationship between miR-195 and CDK4, and the fluorescence intensity was enhanced when miR-195 was knocked down and the fluorescence intensity was impaired when miR-195 was overexpressed (Figure 5B), suggesting miR-195 negative regulation of CDK4 expression in bladder cancer cells. Also, we evaluated the cell cycle distribution 48 h after transfection of miR-195 mimics or miR-195 inhibitor, and the results indicated that miR-195 could induce G1-phase arrest (Figure 4C, 4D).
Figure 4.
miR-195 regulates cell cycle via downregulating CDK4 expression. A: The mRNA level of CDK4 in 5637 and BIU-87 cells with different treatments: si-miR-195, si-NC, vector-NC and vector-miR-195. B: Protein expression of CDK4 in 5637 and BIU-87 cells. C, D: Flow cytometry were used to assess the effect of miR-195 on cell cycle both in 5637 and BIU-87 cells. (n=3, #P<0.05, **P<0.01, ###,***P<0.001).
Figure 5.

miR-195 suppresses cell invasion via downregulation of CDK4 expression in bladder cancer cells. A: QPCR was used to detect the degree of knockdown or overexpression of CDK4. B: Luciferase activity of CDK4 was determined through luciferase assay. C, D: Cell variety influenced by miR-195/CDK4 signal molecular was tested by CCK-8. E: Cell invasion influenced by miR-195/CDK4 signal molecular was tested by Transwell assay. (n=3, *P<0.05, ##P<0.01, ###,***P<0.001).
Finally, to explore the role of miR-195/CDK4 signaling on cell invasion, we applied siRNAs or vector plasmid targeting to the human CDK4 gene to downregulate or upregulate expression of CDK4 in 5637 and BIU-87 cells, and siRNA-3 showed the best knockdown efficiency in both 5637 and BIU-87 cells (Figure 5A), hence we chose siRNA-CDK4-3 for further study. Cell viability and invasion were inhibited significantly when cells were transfected with siRNA-CDK4 and miR-195 inhibitor together compared with cells treated with miR-195 inhibitor, either in 5637 or BIU-87 cells (Figure 5C-E), demonstrating that CDK4 serves as a oncogene, exerting cancer promoting activity in bladder tumors, and miR-195 regulates cell cycle and suppresses cell invasion and growth via downregulating CDK4.
Discussion
Bladder cancer remains a major clinical challenge due to its poor prognosis and limited treatment options to prevent its recurrence. The oncogenesis of bladder cancer involves multiple oncogenes and suppressor genes changes. Recently, an increasing number of studies have demonstrated that aberrant expression of miRNAs is a common event in bladder cancer patients [24,25]. Tölle A et al. summarized that miRNAs could be considered as ideal tumor biomarkers or therapeutic targets for malignant bladder tumor from 79 papers [26]. Our study showed the role of a specific tumor-suppressor gene, miR-195, in bladder cancer.
In the present study, it was demonstrated that miR-195 was markedly downregulated in bladder cancer tissues and cell lines when compared with that in the matched normal adjacent tissues and cells. Our results are consistent with previous studies [20,23,27]. The present findings suggest that miR-195 might act as a cancer suppressor gene in bladder cancer cell proliferation. At the time of the first diagnosis, approximately 70% to 80% of bladder cancers are non-muscle-invasive bladder cancers (NMIBCs) and the remaining 20% to 30% are muscle-invasive bladder cancers (MIBCs). But, almost 50% of patients with MIBC already have occurred distant metastases at the time of diagnosis [28]. Patients with NMIBC are at risk of recurrence or progression into MIBC, and thus the prognosis of bladder cancer is exceedingly poor considering the high rate of metastasis [28]. Our study demonstrates that miR-195 is able to inhibit bladder cancer cell cloning efficiency, invasion, and migration, as well as regulate EMT. EMT is a multistep process in which epithelial cells lose their epithelial characteristics and gain mesenchymal characteristics, such as motility and invasive properties [29]. EMT is controlled by a group of transcriptional repressors, namely, Zeb-1, Zeb-2, Twist, Snail, and Slug. Snail1, Snail2, Zeb-1, and Zeb-2 are zinc-finger transcription factors that bind directly to the E-boxes of the promoter of the E-cadherin-encoding gene, thereby repressing E-cadherin expression [30]. In this study, we detected mRNA and protein expression of N-cadherin, Vimentin, Slug, Snail, Twist, and E-cadherin in 5637 cells after cells overexpressed miR-195, and the mRNA and protein expression levels of N-cadherin, Vimentin, Slug, Snail, and Twist in both 5637 and BIU-87 cells were decreased significantly after cells overexpressed miR-195. The mRNA and protein expression of E-cadherin also increased significantly after cells were treated with miR-195 mimics. All these data indicate that miR-195 modulates EMT, making cells acquire more characteristics of epithelial cells with enhanced invasive capacity.
Serine-threonine kinase cyclin-dependent kinase 4 (CDK4), as a pivotal cell cycle regulator, triggers an important cascade of events in G1-phase via efficiently catalyzing Rb phosphorylation. A previous study reported that CKD4 is one of the downstream genes controlled by miR-195 [23,31], and has been considered as a desirable target for cancer therapies [32-34]. We also determined the relationship of miR-195 and CDK4 in bladder cancer, qPCR and Western blotting and luciferase activity analyses showed that miR-195 could negatively regulate expression of CDK4. Therefore, we conclude that miR-195 might disturb the cell cycle by inducing G1-phase arrest through downregulation of CDK4 expression in bladder cancer. Additionally, we detected a role for CDK4 in cell viability and invasion in bladder cancer. We applied siRNAs or vector plasmid targeting to human CDK4 gene to downregulate or upregulate the expression of CDK4 in 5637 and BIU-87 cells. Results suggested that both cell viability and invasion were inhibited significantly when cells were knocked down for CDK4 and miR-195 together compared with cells treated with miR-195 inhibitor, either in 5637 or BIU-87 cells. These data demonstrate that CDK4 serves as an oncogene, exerting cancer promoting activity in bladder tumors, and miR-195 regulates cell cycle and suppresses cell invasion and growth via downregulating CDK4. These findings are inconsistent with a previous study by Lamb R et al. [35], which confirmed that cyclin D1 and CDK4/6 exerted alternate roles in regulation of migration and stem-like cell activity in breast cancer. Furthermore, Zhang, T et al. [36] reported that miR-124 retarded bladder cancer growth by directly targeting CDK4, suggesting that CDK4 could be implicated as an oncogene and play vital effects in oncogenesis.
In summary, our study confirms that miR-195 is frequently downregulated in bladder cancer. MiR-195 serves as a tumor suppressor in bladder cancer cells, and exerts crucial roles in inducing G1-phase arrest, promoting cell growth, migration, and invasion by targeting CDK4. Our experimental data suggest an important role of miR-195 in bladder tumorigenesis and provide a potential therapeutic strategy for bladder cancer.
Acknowledgements
This research was supported by Zhejiang Provincial Natural Science Foundation of China (Grant No.LY16H160016).
Disclosure of conflict of interest
None.
References
- 1.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–93. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–30. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 3.Scarpato KR, Tyson MD, Clark PE. Natural biology and management of nonmuscle invasive bladder cancer. Curr Opin Oncol. 2016;28:210–5. doi: 10.1097/CCO.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simms MS, Mann G, Kockelbergh RC, Mellon JK. The management of lymph node metastasis from bladder cancer. Eur J Surg Oncol. 2005;31:348–56. doi: 10.1016/j.ejso.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Kim WJ, Bae SC. Molecular biomarkers in urothelial bladder cancer. Cancer Sci. 2008;99:646–52. doi: 10.1111/j.1349-7006.2008.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X, Du L, Duan W, Wang R, Yan K, Wang L, Li J, Zheng G, Zhang X, Yang Y, Wang C. Serum microRNA expression signatures as novel noninvasive biomarkers for prediction and prognosis of muscle-invasive bladder cancer. Oncotarget. 2016;7:36733–36742. doi: 10.18632/oncotarget.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki H, Yoshiike M, Nozawa S, Usuba W, Katsuoka Y, Aida K, Kitajima K, Kudo H, Hoshikawa M, Yoshioka Y, Kosaka N, Ochiya T, Chikaraishi T. Expression level of urinary Micro-RNA-146a-5p is increased in patients with bladder cancer and decreased in those after transurethral resection. Clin Genitourin Cancer. 2016;14:e493–e499. doi: 10.1016/j.clgc.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Xu T, Qin L, Zhu Z, Wang X, Liu Y, Fan Y, Zhong S, Wang X, Zhang X, Xia L, Zhang X, Xu C, Shen Z. MicroRNA-31 functions as a tumor suppressor and increases sensitivity to mitomycin-C in urothelial bladder cancer by targeting integrin alpha5. Oncotarget. 2016;7:27445–57. doi: 10.18632/oncotarget.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaravinos A. The regulatory role of MicroRNAs in EMT and cancer. J Oncol. 2015;2015:865816. doi: 10.1155/2015/865816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y, Tang H. MicroRNAs regulate the epithelial to mesenchymal transition (EMT) in cancer progression. Microrna. 2014;3:108–17. doi: 10.2174/2211536603666141010115102. [DOI] [PubMed] [Google Scholar]
- 13.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Du Y, Jiang B, Song S, Pei G, Ni X, Wu J, Wang S, Wang Z, Yu J. Metadherin regulates actin cytoskeletal remodeling and enhances human gastric cancer metastasis via epithelial-mesenchymal transition. Int J Oncol. 2017;51:63–74. doi: 10.3892/ijo.2017.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirza S, Jain N, Rawal R. Evidence for circulating cancer stem-like cells and epithelialmesenchymal transition phenotype in the pleurospheres derived from lung adenocarcinoma using liquid biopsy. Tumour Biol. 2017;39:1010428317695915. doi: 10.1177/1010428317695915. [DOI] [PubMed] [Google Scholar]
- 17.Karnevi E, Rosendahl AH, Hilmersson KS, Saleem MA, Andersson R. Impact by pancreatic stellate cells on epithelial-mesenchymal transition and pancreatic cancer cell invasion: Adding a third dimension in vitro. Exp Cell Res. 2016;346:206–15. doi: 10.1016/j.yexcr.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Iskender B, Izgi K, Hizar E, Jauch J, Arslanhan A, Yuksek EH, Canatan H. Inhibition of epithelial-mesenchymal transition in bladder cancer cells via modulation of mTOR signalling. Tumour Biol. 2016;37:8281–91. doi: 10.1007/s13277-015-4695-1. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Kidiyoor A, Hu Y, Guo C, Liu M, Yao X, Zhang Y, Peng B, Zheng J. Evaluation of transforming growth factor-beta1 suppress Pokemon/epithelial-mesenchymal transition expression in human bladder cancer cells. Tumour Biol. 2015;36:1155–62. doi: 10.1007/s13277-014-2625-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C, Qi L, Chen M, Liu L, Yan W, Tong S, Zu X. microRNA-195 inhibits cell proliferation in bladder cancer via inhibition of cell division control protein 42 homolog/signal transducer and activator of transcription-3 signaling. Exp Ther Med. 2015;10:1103–1108. doi: 10.3892/etm.2015.2633. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Wang N, Wei H, Yin D, Lu Y, Zhang Y, Zhang Q, Ma X, Zhang S. MicroRNA-195 inhibits proliferation of cervical cancer cells by targeting cyclin D1a. Tumour Biol. 2016;37:4711–20. doi: 10.1007/s13277-015-4292-3. [DOI] [PubMed] [Google Scholar]
- 22.Su K, Zhang T, Wang Y, Hao G. Diagnostic and prognostic value of plasma microRNA-195 in patients with non-small cell lung cancer. World J Surg Oncol. 2016;14:224. doi: 10.1186/s12957-016-0980-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Lin Y, Wu J, Chen H, Mao Y, Liu Y, Mao Q, Yang K, Zheng X, Xie L. Cyclin-dependent kinase 4 is a novel target in micoRNA-195-mediated cell cycle arrest in bladder cancer cells. FEBS Lett. 2012;586:442–7. doi: 10.1016/j.febslet.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Schubert M, Junker K, Heinzelmann J. Prognostic and predictive miRNA biomarkers in bladder, kidney and prostate cancer: Where do we stand in biomarker development? J Cancer Res Clin Oncol. 2016;142:1673–95. doi: 10.1007/s00432-015-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin N, Jin X, Gu X, Na W, Zhang M, Zhao R. Screening biomarkers of bladder cancer using combined miRNA and mRNA microarray analysis. Mol Med Rep. 2015;12:3170–6. doi: 10.3892/mmr.2015.3739. [DOI] [PubMed] [Google Scholar]
- 26.Tolle A, Ratert N, Jung K. miRNA panels as biomarkers for bladder cancer. Biomark Med. 2014;8:733–46. doi: 10.2217/bmm.14.26. [DOI] [PubMed] [Google Scholar]
- 27.Ichimi T, Enokida H, Okuno Y, Kunimoto R, Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama K, Tsujimoto G, Nakagawa M, Seki N. Identification of novel microRNA targets based on microRNA signatures in bladder cancer. Int J Cancer. 2009;125:345–52. doi: 10.1002/ijc.24390. [DOI] [PubMed] [Google Scholar]
- 28.Yun SJ, Kim WJ. Role of the epithelial-mesenchymal transition in bladder cancer: from prognosis to therapeutic target. Korean J Urol. 2013;54:645–50. doi: 10.4111/kju.2013.54.10.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-toepithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–8. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 31.Sekiya Y, Ogawa T, Iizuka M, Yoshizato K, Ikeda K, Kawada N. Down-regulation of cyclin E1 expression by microRNA-195 accounts for interferon-beta-induced inhibition of hepatic stellate cell proliferation. J Cell Physiol. 2011;226:2535–42. doi: 10.1002/jcp.22598. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Yu B, Wu Y, Lee RJ, Lee LJ. Efficient down-regulation of CDK4 by novel lipid nanoparticle-mediated siRNA delivery. Anticancer Res. 2011;31:1619–26. [PubMed] [Google Scholar]
- 33.McCain J. First-in-class CDK4/6 inhibitor palbociclib could usher in a new wave of combination therapies for HR+, HER2- breast cancer. P T. 2015;40:511–20. [PMC free article] [PubMed] [Google Scholar]
- 34.Witkiewicz AK, Cox D, Knudsen ES. CDK4/6 inhibition provides a potent adjunct to Her2-targeted therapies in preclinical breast cancer models. Genes Cancer. 2014;5:261–72. doi: 10.18632/genesandcancer.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamb R, Lehn S, Rogerson L, Clarke RB, Landberg G. Cell cycle regulators cyclin D1 and CDK4/6 have estrogen receptor-dependent divergent functions in breast cancer migration and stem cell-like activity. Cell Cycle. 2013;12:2384–94. doi: 10.4161/cc.25403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang T, Wang J, Zhai X, Li H, Li C, Chang J. MiR-124 retards bladder cancer growth by directly targeting CDK4. Acta Biochim Biophys Sin (Shanghai) 2014;46:1072–9. doi: 10.1093/abbs/gmu105. [DOI] [PubMed] [Google Scholar]




