Abstract
The morphological, immunohistochemical, and immunopathological analyses of muscle biopsy are essential for the diagnosis of idiopathic inflammatory myopathies (IIMs). However, they are also one of the most common causes of misdiagnosis. Although several diagnostic criteria have been proposed for the diagnosis of IIMs, misdiagnosis still remains common in clinical practice. The present study aims to characterize the inflammatory profile of IIMs, including the expression of MHC-I, MHC-II, MAC and infiltrating cells. We also investigated the sensitivity and specificity of MHC-I and MHC-II immunostaining for the diagnosis of IIMs. We found that the expression of MHC-I and MHC-II was both higher in IIMs than in non-inflammatory myopathies (NIMs). The distribution of MHC-I in IIMs is different from that of MHC-II. MHC-I is mainly located in the sarcoplasms, while MHC-II is located mostly on the sarcolemmas. Moreover, our findings suggest that MAC may be a potential marker to diagnose DM, and the combination of MHC-I and MHC-II immunostaining results in a higher sensitivity and specificity for IIM diagnosis, especially for DM. In addition, infiltrating cells in PM were mainly CD8+ cells, but we found in DM and NIMs they were primarily CD4+ cells, which is consistent with previous studies. Lastly, glucocorticoid treatment and disease duration have little effect on the MHC-I and MHC-II expression pattern. Our findings indicate that the immunostaining of inflammatory markers such as MHC-I, MHC-II, CD4, CD8, CD303 and MAC are of diagnostic value for IIMs regardless of the immunosuppression regime and disease duration.
Keywords: Polymyositis, dermatomyositis, major histocompatibility complex, inflammatory cells, membrane attack
Introduction
The idiopathic inflammatory myopathies (IIMs) are a heterogeneous group of disorders comprised of dermatomyositis (DM), polymyositis (PM), inclusion body myositis (IBM), and necrotizing autoimmune myopathy [1-3]. The most remarkable myopathological feature of IIMs is the infiltration of inflammatory cells surrounding muscle fascicles or invading muscle fibers [4]. However, inflammatory infiltrates are not specific to IIMs and can be present in other genetic and acquired myopathies such as muscular dystrophy and myasthenia gravis, which, if omitting the clinical pictures, may lead to the misdiagnosis of IIMs [5-7].
The 1975 Bohan and Peter criteria are still some of the most widely-used criteria in clinical practice [1,2]. However, the criteria proposed in earlier times do not emphasize immunological characterization as part of the muscle pathology investigation, except for the Dalakas criteria [5], the first criteria introducing the profiling of inflammatory cells, in which CD8+ cells invading major histocompatibility complex (MHC) class I upregulated muscle fibers are used to define PM and thus significantly improve diagnostic specificity. The ENMC criteria [8] also use CD8+ cells invading non-necrotic fibers or ubiquitous MHC-I expression for PM diagnosis, and MAC deposition or perifascicular MHC-I expression for DM. However, one recent study shows that the CD8-MHC-I complex can also be present in PM, IBM and other unclassifiable myositis patients, which challenges the specificity of the CD8-MHC-I complex for PM [9]. Meanwhile, the distribution of MHC-I and MHC-II molecules has seldom been evaluated in previous studies.
Immunological profiling of muscle biopsy holds significant diagnostic value and provides clues to pathogenesis. Firstly, both MHC-I and MHC-II are cell surface molecules whose major biological function is to present exogenous and endogenous antigens to immune cells. They are histochemically undetectable in normal human muscle fibers but upregulated in IIMs [10,11]. Despite the generally accepted diagnostic value of ubiquitous or perifascicular expressions of MHC-I and MHC-II in PM and DM, they were also found to be upregulated in other primary muscle disorders like muscular dystrophy, which compromises the specificity of MHC-I immunostaining for IIM diagnosis [12]. Secondly, the deposition of membrane attack complex (MAC) can be found in the blood vessels, muscle fibers, and skin lesions of DM. MAC is believed to be involved in perimysial and perifascicular damage, which is the characteristic histological change of perifascicular atrophy [13,14]. Lastly, in DM, where the humoral mediated process is considered as the main pathogenic event, the inflammatory cells are predominantly CD4+ cells and B lymphocytes, while in PM, CD8+ cells invading MHC-I upregulated muscle indicates cellular immunity is the core etiology [15,16].
Although the expressions of MHC-I and MHC-II are emphasized in the diagnosis of IIMs, in our opinion, the diagnostic value of MHC-I and MHC-II may be underestimated. In the present study, we aim to determine the expression patterns of MHC-I, MHC-II, MAC and inflammatory cells in PM, DM and non-inflammatory myopathy (NIM) patients from our neuromuscular center. Particular attention was also paid to the utility of distribution of MHC-I and MHC-II in differentiating IIMs from NIMs. We also investigated the effect of disease duration and glucocorticoid on immunohistochemical staining in PM and DM.
Materials and methods
Patients
With the approval of the Ethics Committee of Xiangya Hospital, Central South University, a total of 89 Chinese patients, including 71 cases of IIMs and 18 cases of NIMs, in the neuromuscular center of Xiangya Hospital of Central South University from 2012 to 2016 were included. The diagnosis of IIM was made according to the Bohan and Peter criteria [1,2]. The IIM patients included 44 cases of PM and 27 cases of DM, but no IBM cases were included as the low number of patients might skew the statistical analysis. NIMs consisted of 12 cases of dystrophinopathy and 6 of dysferlinopathy with secondary muscle inflammation, diagnosed based on pathological findings of muscle biopsy and/or genetic testing. In addition, 6 cases that exhibited no muscle pathology changes and were devoid of any neuromuscular diseases were recruited as the normal control group. All patients provided written consent forms.
Muscle biopsies
Open muscle biopsies were taken from biceps brachii or gastrocnemius. The muscle samples were immediately frozen in isopentane cooled with liquid nitrogen and stored at -80°C. Subsequent routine histological and immunohistochemical analysis was performed on the 8 µm cryostat sections.
Routine histological staining
Routine histological staining techniques included hematoxylin and eosin (H&E), modified Gömöri trichrome, acid phosphatase, periodic acid-Schiff (PAS), Oil red O, NADH-TR, ATPase (pH = 4.3, 4.6, 11.0), succinic dehydrogenase (SDH), and cytochrome C oxidase (COX).
Immunohistochemical staining
Sections were fixed for 10 minutes in acetone at -20°C, incubated in 0.3% H2O2 solution (Sigma) in phosphate-buffered saline (PBS 0.01 M, pH 7.4, Sigma) for 10 minutes and subsequently blocked in 10% fetal bovine serum (Sigma) for 45 minutes. After the PBS wash, the samples were incubated with a primary antibody (Table 1) at 37°C for 3 hours. Then, they were rinsed in PBS and treated with a secondary antibody for 30 min at 37°C. An ABC kit was used (VECTASTAIN Elite ABC Kit PK-6100) according to the manufacturer’s recommendations, and the samples were treated with DAB (Sigma) for several minutes. Finally, the sections were sealed in neutral balsam.
Table 1.
Antibodies employed in the present study
| Antibody | Species | Dilution | Company |
|---|---|---|---|
| MHC-I | Mouse anti-human monoclonal | 1:100 | BioLegend |
| MHC-II | Rabbit anti-human monoclonal | 1:100 | Abcam |
| CD4 | Mouse anti-human monoclonal | 1:100 | BD Biosciences |
| CD8 | Mouse anti-human monoclonal | 1:200 | BD Biosciences |
| CD303 | Mouse anti-human monoclonal | 1:100 | BD Biosciences |
| MAC | Mouse anti-human monoclonal | 1:100 | Abcam |
Light microscopy
All muscle samples were evaluated blindly by two experienced myopathologists. The patterns of MHC-I and MHC-II staining were categorized as sarcolemmal and sarcoplasmic. Moreover, sarcolemmal staining was classified as being completed sarcolemmal or partial sarcolemmal, depending on whether it involved the entire surface of the myofibers or was patchy in its distribution. The expression of MHC-I and MHC-II was assessed under at least six random different views of 200 magnification. The ratio of positively stained fibers to total non-necrotic myofibers was calculated.
The quantification of CD4+ and CD8+ cells was assessed in at least six random microscopic fields at 200 magnification. The ratios of immunoreactive inflammatory cells to total inflammatory cells in all the fields were calculated. The quantification of CD303+ plasmacytoid dendritic cells (pDCs) was performed with the hot spot method [17,18] and used for myositis because of the relatively low frequency of inflammatory cell infiltration. This method allows the quantification of cells in hot spots, defined as muscle areas containing the highest density of positive cells. Accordingly, six hot spots per muscle section were selected for CD303+ pDC quantification.
The patterns of MAC were classified as endomysial, perimysial, capillary perivascular, or endomysial arteriolar and were assessed by observing the whole section. The ratio of positively stained cases to the total cases was calculated in each specific region.
Data analysis
The Pearson chi-square (χ2) test was used to compare the frequency distribution of categorical variables. Student’s t-test or one-way ANOVA followed by Bonferroni’s post-hoc test were used to compare the mean values among various groups of cases and controls. The statistical analysis was performed using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) Version 20. A p-value <0.05 was considered statistically significant.
Results
Demographic and clinical features
There was a female predominance in IIM patients (male:female = 27:44), compared with the male predominance in NIM (male:female = 5:1, P = 0.001), which was partly due to the male dystrophinopathy patients. The CK levels of NIM patients were generally higher than those of IIMs (P = 0.023), while they were even higher in DM than in PM. Further details of the demographic statistics of the subgroups are listed in Table 2.
Table 2.
Clinical characteristics of each subgroup
| Group | Subgroup | No. | Male:female | Age (year, Mean ± SEM) | Disease duration (month, Mean ± SEM) | CK (U/L, Mean ± SEM) |
|---|---|---|---|---|---|---|
| IIMs | PM | 44 | 9:13 | 44.6±2.2 | 15.2±3.7 | 2307.7±381.2 |
| DM | 27 | 1:2 | 36.5±3.9 | 8.5±2.1 | 4222.8±734.1 | |
| NIMs | Dystrophinopathy | 12 | 5:1 | 16.4±3.9 | 98.0±20.4 | 5205.8±912.6 |
| Dysferlinoapthy | 6 | 5:1 | 35.0±2.0 | 90.0±36.1 | 5528.8±901.5 |
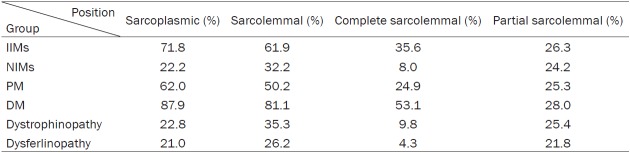
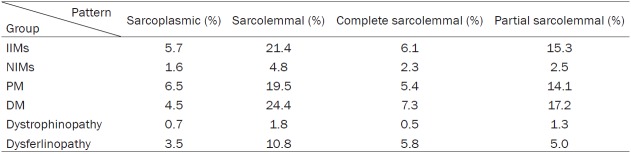
Patterns of MHC-I and MHC-II staining
All IIM and NIM cases showed MHC-I positive staining except for one PM patient, while on MHC-II staining, 70.4% of the IIMs and 22.2% of the NIMs showed positivity (P = 0.001). The average proportions of positive staining for MHC-I and MHC-II in IIMs, NIMs and each subgroup are shown in Tables 3, 4. There was no MHC-I or MHC-II immunoreactivity in any of the normal control samples (Figure 1), and no case with MHC-II immunopositivity in the absence of MHC-I staining.
Table 3.
Average proportions of positive staining for MHC-I in each group
Table 4.
Average proportions of positive staining for MHC-II in each group
Figure 1.
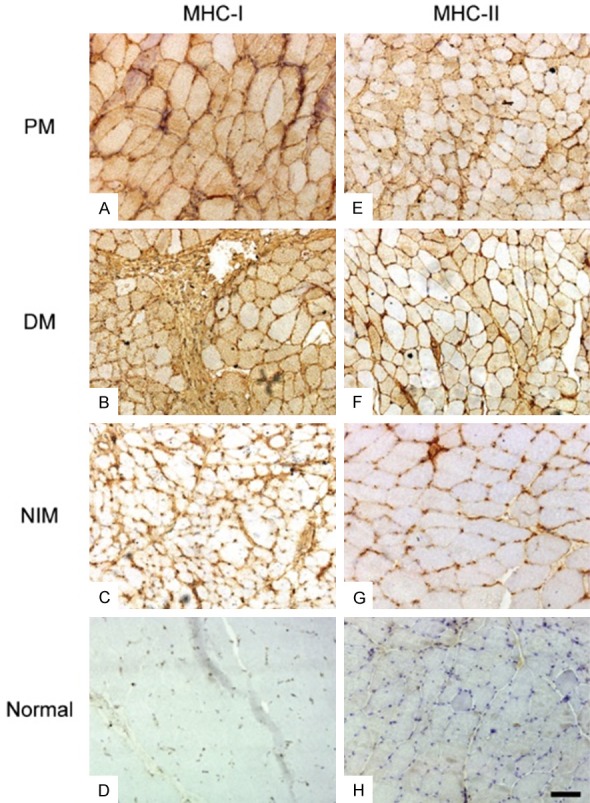
MHC-I and -II immunostainings. In PM (A, E), MHC-I staining was more sarcoplasmic while MHC-II immunoreactivity was mostly sarcolemmal. In DM (B, F), staining of sarcolemmal MHC-I was more complete than partial, and MHC-II immunostaining was mostly sarcolemmal. In NIMs (C, G), both MHC-I and MHC-II staining were mainly partial sarcolemmal. There was no immunoreactivity in normal control samples (D, H). (Original magnification ×200, Scale bar = 300 μm).
In terms of sarcolemmal staining, the pattern of MHC-I in the IIMs was more complete than partial (P = 0.012), but it was more partial than complete in the NIMs (P = 0.000). MHC-I immunoreactivity in the IIMs was mainly in the sarcoplasms instead of on the sarcolemmas (P = 0.001). More specifically, MHC-I staining was more sarcoplasmic than sarcolemmal in PM (P = 0.001), while in DM, the staining of sarcolemmal MHC-I was more complete than partial (P = 0.000).
The MHC-II immunoreactivity in the IIMs was mostly sarcolemmal rather than sarcoplasmic (P = 0.000), and more partial than complete (P = 0.000). In the NIMs, there was no difference between the MHC-II sarcolemmal and sarcoplasmic staining.
Diagnostic value of MHC-I and MHC-II
We used 35% and 20% as the cut-off points for the MHC-I sarcoplasmic and MHC-II partial sarcolemmal staining positivity respectively, which resulted in a sensitivity of 0.859 and a specificity of 0.833 for IIMs. To compare the DM with the NIMs, if MHC-I complete sarcolemmal staining was observed in more than 25% of the muscle fibers or MHC-II partial sarcolemmal staining in more than 22.5% of the muscle fibers, the sensitivity would be 0.963 and the specificity be 1.000 for DM. In the case of MHC-I sarcoplasmic staining being positive in more than 35% of the muscle fibers or MHC-II complete sarcolemmal staining in more than 15.5% of the muscle fibers, the sensitivity and specificity of diagnosis of PM was 0.841 and 0.833 respectively.
Given that one single index was used for IIMS diagnosis, MHC-I showed a better diagnostic value than MHC-II. For example, for the differentiation of the DM from the NIMs, MHC-I had a sensitivity and a specificity as 0.926 and 1.000 respectively, while MHC-II had only 0.778 and 0.778 respectively. However, by combining MHC-I and MHC-II, the sensitivity and specificity for DM diagnosis was improved to 0.963 and 1.000 respectively.
Profile of the infiltrating inflammatory cells
In PM, inflammatory cells were mainly located in clusters in the endomysium as well as the perimysium, whereas in DM, they were mainly perivascular and perifascicular. In NIMs, scattered inflammatory cells were observed mainly in the endomysium and perimysium (Figure 2). The frequencies of inflammatory cells in all groups are listed in Table 5. No inflammatory cells were found in the normal control group.
Figure 2.
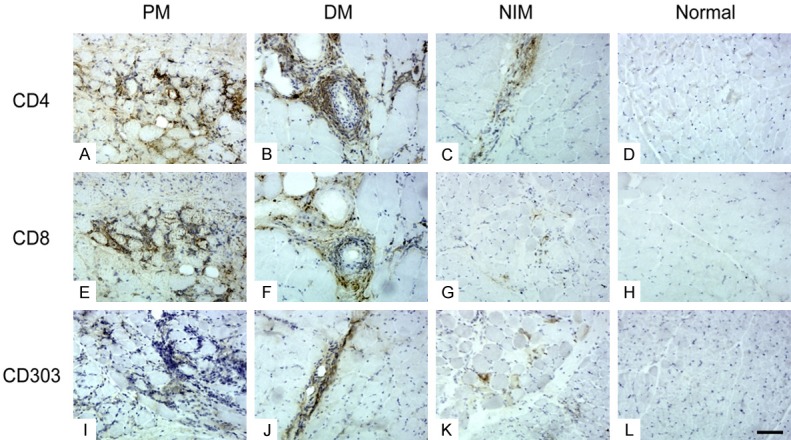
Inflammatory marker staining. In PM (A, E, I), inflammatory cells were mainly located in clusters in the endomysium, whereas in DM (B, F, J), they were mostly perivascular and perifascicular. In NIMs (C, G, K), scattered inflammatory cells were present mainly in the endomysium and perimysium. There were no inflammatory cells in the normal control group (D, H, L). (Original magnification ×200, Scale bar = 300 μm).
Table 5.
Frequencies of inflammatory cells in IIMs and NIMs
| Group | CD4+ cell (%) | CD8+ cell (%) | CD303+ cell (%) |
|---|---|---|---|
| IIMs | 17.25 | 14.54 | 7.48 |
| NIMs | 8.61 | 3.44 | 3.22 |
| PM | 14.5 | 18.4 | 9.5 |
| DM | 21.7 | 8.2 | 4.2 |
| Dystrophinopathy | 7.8 | 2.8 | 1.4 |
| Dysferlinopathy | 10.3 | 4.7 | 6.8 |
CD4+, CD8+ and CD303+ cells were more frequent in IIMs than in NIMs (Figure 3). Moreover, CD8+ and CD303+ cells were more frequent in PM than DM (P<0.001, 0.01). In accordance with previous studies, the majority of the inflammatory cells in PM were CD8+ cells (P = 0.043), but CD4+ cells were the majority in DM and NIMs (P = 0.000, 0.000).
Figure 3.
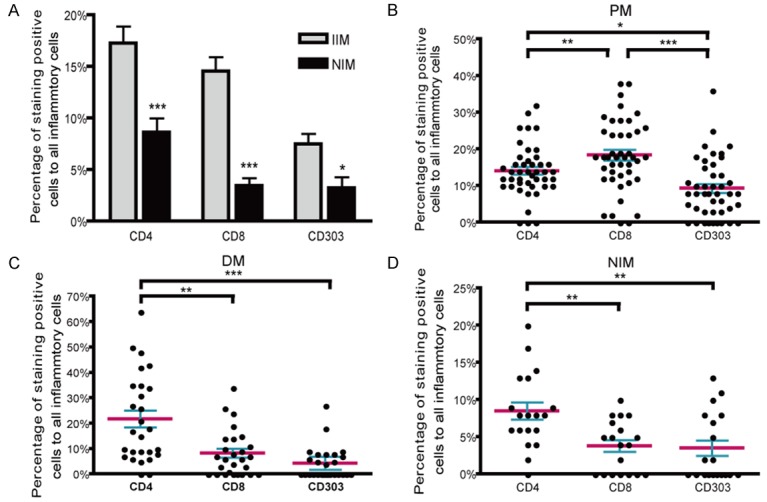
Proportions of inflammatory cells in IIMs and NIMs. CD4+, CD8+ and CD303+ cells were more frequent in IIMs than in NIMs (A). In PM (B), the majority of inflammatory cells were CD8+ cells. In DM (C) and NIMs (D), inflammatory cells were mainly CD4+ cells. The mean and SEM of the samples are indicated. *P<0.05, **P<0.01, ***P<0.001.
Immunohistostaining pattern of MAC
DM patients demonstrated more frequent capillary perivascular MAC than PM patients (P = 0.000). No NIMs showed MAC positivity on perivascular spaces. The frequencies of MAC positive staining in all groups are listed in Table 6. No other significant difference was observed in the endomysial, perimysial, or endomysial arteriolar MAC staining patterns. On the basis of perivascular MAC staining in DM and other myopathies including PM and NIMs, MAC showed a sensitivity of 0.889 and specificity of 0.903 to differentiate DM from other myopathies. The normal control cases showed no MAC immunoreactivity (Figure 4).
Table 6.
Positive rates of MAC at different location in each group
| Group | Endomysium (%) | Perimysium (%) | Capillary perivascular (%) | Endomysial arteriole (%) | Total positivity (%) |
|---|---|---|---|---|---|
| PM | 54.5 | 15.9 | 13.6 | 36.4 | 93.2 |
| DM | 33.3 | 29.6 | 88.9 | 37.0 | 92.6 |
| Dystrophinopathy | 8.3 | 8.3 | 0.0 | 8.3 | 41.7 |
| Dysferlinopathy | 83.3 | 0 | 0.0 | 66.7 | 83.3 |
Figure 4.
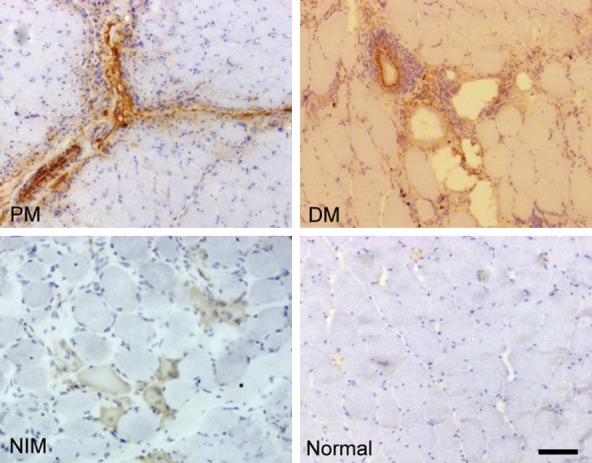
MAC immunostaining. PM showed perimysial immunoreactivity, DM showed arteriolar staining, NIMs showed non-specific necrotic fiber immunostaining, and the normal control cases showed no MAC immunoreactivity. (Original magnification ×200, Scale bar = 300 μm).
Effects of disease duration and glucocorticoid on inflammatory profiles of PM and DM
Based on the disease duration upon the muscle biopsy date, PM and DM patients were categorized into two groups respectively: the S group (duration shorter than three months) and the L group (duration longer than three months). PM-L consisted of 28 cases and PM-S 16, while DM-L included 19 cases and DM-S 8. The frequencies of CD4+ and CD303+ cells were higher in DM-L than in DM-S (P = 0.003, 0.011). No other significant difference was identified in MHC-I, MHC-II and CD8+ T cells between PM-L and PM-S, or between DM-L and DM-S.
PM and DM patients were also divided into two groups depending on the usage of glucocorticoid: GC (glucocorticoid used in the 4 weeks before biopsy) and NGC group (no glucocorticoid used in the 4 weeks before biopsy). PM-NGC consisted of 37 cases and PM-GC 7 cases, DM-NGC 19 cases and DM-GC 8 cases. There was no significant difference in MHC-I, MHC-II and inflammatory cell subtypes between PM-GC and PM-NGC, or between DM-GC and DM-NGC group.
Profile of MHC-I, MHC-II and infiltrating inflammatory cells between isolated IIMs and overlap IIMs
The IIM patients were classified into two groups based on whether or not they were associated with connective tissue diseases (CTDs) at the time of muscle biopsy: isolated IIMs and overlap IIMs. The overlap IIMs composed of five cases, including three DM patients associated with rheumatoid arthritis, one DM patient with systemic sclerosis, and one PM patient with systemic lupus erythematosus. The overlap IIMs accounted for 7.0% of all the IIMs, in which the overlap DMs were 14.8% of the DMs and the overlap PMs were 2.3% of the PMs in our study. No significant difference was detected in MHC-I, MHC-II and inflammatory cell subtypes either between isolated IIMs and overlap IIMs, or between isolated DM and overlap DM group.
Discussion
In the present study, the patterns of sarcolemmal and sarcoplasmic staining of MHC-I and MHC-II in non-necrotic myofibers were evaluated qualitatively. The sarcolemmal staining pattern was further categorized as complete or partial. The salient point of our study is that by quantifying MHC-I and MHC-II staining in the sarcolemmas and sarcoplasms, we find that MHC-I and MHC-II are good diagnostic markers in combination to diagnose IIMs with a sensitivity and specificity both higher than 0.833. For DM diagnosis, the sensitivity and specificity are both higher than 0.963, which is relatively high compared with previous studies [11,12]. The distribution of MHC-I and MHC-II immunoreactivity in IIMs and NIMs was also different in our research. In IIMs, MHC-I is mostly expressed in sarcoplasms, while MHC-II is basically expressed in sarcolemmas. No significant difference was identified on the MHC-I and MHC-II distribution of sarcoplasms and sarcolemmas in NIMs. Our study is the first to report and quantify the distribution of MHC-I and MHC-II in IIMs muscles for IIM diagnosis.
The percentages of CD4+, CD8+ and CD303+ cells in IIMs are higher than those in NIMs in our research, which suggests that the inflammation in dystrophinopathy and dysferlinopathy is more likely a secondary immune response to muscle damage. It has long been established that interstitial and perivascular inflammation in PM are predominantly CD8+ cells, and in DM CD4+ cells [15,16]. In comparison, CD4+ cell-predominant focal inflammatory infiltrates can sometimes be found on muscle biopsies of dysferlinopathy [19,20]. In our study, CD8+ cells are the major inflammatory cells in PM, and CD4+ cells are most abundant in DM and NIMs. These findings again support the conclusion that cytotoxicity mediates muscle fiber injuries in PM and the humoral immunity in DM. Dendritic cells (DCs) are professional antigen-presenting cells supporting adaptive and innate immune responses [21]. They can be divided into two types: myeloid DCs (mDCs) involved in specific adaptive immune response and plasmacytoid DCs (pDCs) which have a key role in innate immunity. pDCs are found mainly in DM while mDCs predominate in PM and IBM [22,23]. In the present study, CD303+ pDCs are more frequent in PM than DM, which is contrary to previous findings. This may be due to the following reasons. Firstly, the hot spot method of CD303+ pDCs calculation in our study was different from the quantification method used in previous studies. Secondly, CD303+ pDCs in DM showed a more scattered distribution in the perimysium than in PM in our study, combined with the hot spot method for CD303+ pDCs quantification, hence in our study the percentage of CD303+ pDCs in PM is higher than DM. Lastly, the immune response in Chinese IIMs may be different from that in other populations.
DM is considered a microangiopathy caused by MAC mediated complement cascade activation [24-27]. In our study, MAC positivity can be observed in the endomysium, perimysium, on the walls of intramuscular endomysial capillaries and arterioles in IIMs as well as NIMs. Taking distribution patterns into consideration, capillary and perivascular deposition of MAC in DM is much higher than in PM and NIMs. MAC deposition in the capillary walls of endomysial microvessels is deemed highly specific to DM, especially in childhood DM [13,24]. However, one recent study by Braczynski [28] reports that the capillary MAC deposits’ diagnostic value may be overestimated for DM. In our study, although the microvessel MAC immunopositivity is also detected in other myositis, endomysial capillary MAC immunopositivity for the diagnosis of DM gives a relatively high sensitivity of 0.889 and specificity of 0.903. Thus, we confirm that capillary perivascular deposition of MAC can be used as an important part of the diagnostic workup for DM.
It has been shown that immunosuppressive treatment in IIMs has little effect on MHC-I expression, hence making MHC-I a useful diagnostic marker for IIMs regardless of previous treatment [29]. Recent studies have also discovered that corticosteroid therapy has little influence on the presence or degree of inflammatory infiltrates in IIMs [30]. Our study shows that glucocorticoid treatment within 4 weeks before biopsy does not affect the immunoreactivity of MHC-I and MHC-II, nor does it change the subtype of inflammatory cells. Moreover, we have shown that the disease duration also has little effect on the expression of MHC-I and MHC-II, which has not been reported before. This phenomenon suggests that the overexpression of MHC-I and MHC-II is sustained throughout the whole disease duration and plays an essential role in disease pathogenesis. Besides, the CD303+ pDCs in our study, at the late stage of DM, were higher than in the early stage, which refutes an initiation role of pDCs in DM etiology.
In our study, IIMs are categorized into two groups: isolated and overlap. We find that there is no difference in the immunoreactivity of MHC-I, MHC-II and the subtype of inflammatory cells between the isolated and overlap groups in IIMs, which has been only rarely reported before. The underlying mechanism remains elusive, but we consider these phenomena strongly indicate a common immunologic pathogenesis between overlap and isolated IIMs [31].
In conclusion, our study shows that by the quantitative analysis of sarcolemmal and sarcoplasmic staining, MHC-I and MHC-II are sensitive and specific markers for IIM diagnosis, and especially for DM diagnosis. In addition, our findings indicate that regardless of the disease duration, the immunosuppression regime, or CTD overlapping, the immunohistochemical staining for inflammatory markers, such as MHC-I, MHC-II, CD4, CD8, CD303 and MAC, are of diagnostic value for IIMs.
Acknowledgements
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The present study was supported by the Young Scientists Fund of National Natural Science Foundation of China (grant No. 81601094).
Disclosure of conflict of interest
None.
References
- 1.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 3.Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;372:1734–1747. doi: 10.1056/NEJMra1402225. [DOI] [PubMed] [Google Scholar]
- 4.Dalakas MC. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991;325:1487–1498. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- 5.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971–982. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 6.Fanin M, Angelini C. Muscle pathology in dysferlin deficiency. Neuropathol Appl Neurobiol. 2002;28:461–470. doi: 10.1046/j.1365-2990.2002.00417.x. [DOI] [PubMed] [Google Scholar]
- 7.Mastaglia FL. When the treatment does not work: polymyositis. Pract Neurol. 2008;8:170–174. doi: 10.1136/jnnp.2007.142562. [DOI] [PubMed] [Google Scholar]
- 8.Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, Vencovsky J, de Visser M, Hughes RA. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, the Netherlands. Neuromuscul Disord. 2004;14:337–345. doi: 10.1016/j.nmd.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Ikenaga C, Kubota A, Kadoya M, Taira K, Uchio N, Hida A, Maeda MH, Nagashima Y, Ishiura H, Kaida K, Goto J, Tsuji S, Shimizu J. Clinicopathologic features of myositis patients with CD8-MHC-1 complex pathology. Neurology. 2017;89:1060–1068. doi: 10.1212/WNL.0000000000004333. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez Cruz PM, Luo YB, Miller J, Junckerstorff RC, Mastaglia FL, Fabian V. An analysis of the sensitivity and specificity of MHC-I and MHC-II immunohistochemical staining in muscle biopsies for the diagnosis of inflammatory myopathies. Neuromuscul Disord. 2014;24:1025–1035. doi: 10.1016/j.nmd.2014.06.436. [DOI] [PubMed] [Google Scholar]
- 11.Salaroli R, Baldin E, Papa V, Rinaldi R, Tarantino L, De Giorgi LB, Fusconi M, Malavolta N, Meliconi R, D’Alessandro R, Cenacchi G. Validity of internal expression of the major histocompatibility complex class I in the diagnosis of inflammatory myopathies. J Clin Pathol. 2012;65:14–19. doi: 10.1136/jclinpath-2011-200138. [DOI] [PubMed] [Google Scholar]
- 12.Nagappa M, Nalini A, Narayanappa G. Major histocompatibility complex and inflammatory cell subtype expression in inflammatory myopathies and muscular dystrophies. Neurol India. 2013;61:614–621. doi: 10.4103/0028-3886.125264. [DOI] [PubMed] [Google Scholar]
- 13.Jain A, Sharma MC, Sarkar C, Bhatia R, Singh S, Gulati S, Handa R. Detection of the membrane attack complex as a diagnostic tool in dermatomyositis. Acta Neurol Scand. 2011;123:122–129. doi: 10.1111/j.1600-0404.2010.01353.x. [DOI] [PubMed] [Google Scholar]
- 14.Pestronk A, Schmidt RE, Choksi R. Vascular pathology in dermatomyositis and anatomic relations to myopathology. Muscle Nerve. 2010;42:53–61. doi: 10.1002/mus.21651. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Z, Yang C, Wang J, Feng Q, Chen Q, Yang P. Altered chemokine receptor expression in the peripheral blood lymphocytes in polymyositis and dermatomyositis. Cytokine. 2017;99:316–321. doi: 10.1016/j.cyto.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Venalis P, Lundberg IE. Immune mechanisms in polymyositis and dermatomyositis and potential targets for therapy. Rheumatology (Oxford) 2014;53:397–405. doi: 10.1093/rheumatology/ket279. [DOI] [PubMed] [Google Scholar]
- 17.Page G, Chevrel G, Miossec P. Anatomic localization of immature and mature dendritic cell subsets in dermatomyositis and polymyositis: Interaction with chemokines and Th1 cytokine-producing cells. Arthritis Rheum. 2004;50:199–208. doi: 10.1002/art.11428. [DOI] [PubMed] [Google Scholar]
- 18.Tournadre A, Porcherot M, Cherin P, Marie I, Hachulla E, Miossec P. Th1 and Th17 balance in inflammatory myopathies: interaction with dendritic cells and possible link with response to high-dose immunoglobulins. Cytokine. 2009;46:297–301. doi: 10.1016/j.cyto.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Yin X, Wang Q, Chen T, Niu J, Ban R, Liu J, Mao Y, Pu C. CD4+ cells, macrophages, MHC-I and C5b-9 involve the pathogenesis of dysferlinopathy. Int J Clin Exp Pathol. 2015;8:3069–3075. [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JH, Park YE, Kim SI, Kim JI, Lee CH, Park KH, Kim DS. Differential immunohistological features of inflammatory myopathies and dysferlinopathy. J Korean Med Sci. 2009;24:1015–1023. doi: 10.3346/jkms.2009.24.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg SA, Pinkus GS, Amato AA, Pinkus JL. Myeloid dendritic cells in inclusionbody myositis and polymyositis. Muscle Nerve. 2007;35:17–23. doi: 10.1002/mus.20649. [DOI] [PubMed] [Google Scholar]
- 23.Lopez de Padilla CM, Vallejo AN, McNallan KT, Vehe R, Smith SA, Dietz AB, Vuk-Pavlovic S, Reed AM. Plasmacytoid dendritic cells in inflamed muscle of patients with juvenile dermatomyositis. Arthritis Rheum. 2007;56:1658–1668. doi: 10.1002/art.22558. [DOI] [PubMed] [Google Scholar]
- 24.Goncalves FG, Chimelli L, Sallum AM, Marie SK, Kiss MH, Ferriani VP. Immunohistological analysis of CD59 and membrane attack complex of complement in muscle in juvenile dermatomyositis. J Rheumatol. 2002;29:1301–1307. [PubMed] [Google Scholar]
- 25.Buckley AF, Bossen EH. Skeletal muscle microvasculature in the diagnosis of neuromuscular disease. J Neuropathol Exp Neurol. 2013;72:906–918. doi: 10.1097/NEN.0b013e3182a7f0b8. [DOI] [PubMed] [Google Scholar]
- 26.Gitiaux C, Kostallari E, Lafuste P, Authier FJ, Christov C, Gherardi RK. Whole microvascular unit deletions in dermatomyositis. Ann Rheum Dis. 2013;72:445–452. doi: 10.1136/annrheumdis-2012-201822. [DOI] [PubMed] [Google Scholar]
- 27.Kissel JT, Mendell JR, Rammohan KW. Microvascular deposition of complement membrane attack complex in dermatomyositis. N Engl J Med. 1986;314:329–334. doi: 10.1056/NEJM198602063140601. [DOI] [PubMed] [Google Scholar]
- 28.Braczynski AK, Harter PN, Zeiner PS, Drott U, Tews DS, Preusse C, Penski C, Dunst M, Weis J, Stenzel W, Mittelbronn M. C5b-9 deposits on endomysial capillaries in non-dermatomyositis cases. Neuromuscul Disord. 2016;26:283–291. doi: 10.1016/j.nmd.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 29.van der Pas J, Hengstman GJ, ter Laak HJ, Borm GF, van Engelen BG. Diagnostic value of MHC class I staining in idiopathic inflammatory myopathies. J Neurol Neurosurg Psychiatry. 2004;75:136–139. [PMC free article] [PubMed] [Google Scholar]
- 30.Pinhata MM, Nascimento JJ, Marie SK, Shinjo SK. Does previous corticosteroid treatment affect the inflammatory infiltrate found in polymyositis muscle biopsies? Clin Exp Rheumatol. 2015;33:310–314. [PubMed] [Google Scholar]
- 31.Colafrancesco S, Priori R, Valesini G. Inflammatory myopathies and overlap syndromes: update on histological and serological profile. Best Pract Res Clin Rheumatol. 2015;29:810–825. doi: 10.1016/j.berh.2016.02.005. [DOI] [PubMed] [Google Scholar]