Abstract
2,067 women who underwent cervical cancer screening were included in this study. p16/Ki-67 and p16/mcm2 were performed on the remaining liquid-based cytology (LBC) samples of 125 HPV-positive women and 114 randomly selected HPV-negative women. Women with HR-HPV infection or cytological abnormalities (≥ASC-US) were referred for colposcopy and biopsy. A third-year follow up visit was performed on all women except for CIN2+. The expression of p16/Ki-67 and p16/mcm2 in the HPV16/18 group and in the other 12 HR-HPV group was significantly higher than that in HPV negative group (P<0.05), with odds ratios (ORs) of 16.27 (95% CI: 4.38-60.47) and 4.52 (95% CI: 2.16-9.45) for p16/Ki-67, and 31.28 (95% CI: 6.33-154.56) and 9.10 (95% CI: 4.52-18.33) for p16/mcm2, respectively. The sensitivities to detect CIN2+ and CIN3 + were 94.1% (95% CI: 73.0-99.0) and 92.9% (95% CI: 68.5-98.7) for p16/Ki-67, and 88.2% (95% CI: 65.7-96.7) and 85.7% (95% CI: 60.1-96.0) for p16/mcm2, respectively. Both the sensitivities of the two biomarkers were significantly higher than that of LBC and HPV16/18 genotyping (P<0.05). The three-year cumulative risks of CIN2+ were 69.0%, 48.4%, 34.8% and 50.0% for p16/Ki-67, p16/mcm, LBC and HPV16/18 genotyping. Women who tested positive on both p16/Ki-67 and p16/mcm2 at baseline had the highest RR value (39.64 [95% CI: 9.78-160.72]) of progressing to CIN2+ when compared to those who were negative for both. To conclude, p16/Ki-67 and p16/mcm2 dual staining can enhance the sensitivity of cytology in a single round of screening, and they can be predictors of high grade cervical lesions in the following years.
Keywords: p16/Ki-67, p16/mcm2, immunocytochemical dual staining, HPV, cervical cancer
Introduction
Cervical cancer is the fourth most common malignancy of women in the world. In 2012, there were about 530,000 new cases and 270,000 deaths worldwide. Of these, more than 85% occurred in poor areas, accounting for about 12% of all female malignancies [1]. Although HPV prophylactic vaccines have been marketed, screening is still the primary means of preventing cervical cancer today [2]. In 2013, WHO recommended HPV testing alone to be used as the first-line screening method to detect cervical cancer [3]. However, most HPV infections are transient and are spontaneously cleared by the body without causing any disease. It is not feasible to refer all HPV-positive women to further invasive procedures. Over the years, a serious lack of cytopathic doctors exacerbated the cervical cancer screening issue in China. Therefore, we urgently need to find new biomarkers to manage HPV-positive women [4].
p16 overexpression is one of the potential biomarkers that has the potential to distinguish HPV-positive women. As is known, HR-HPV E7 protein competitively inhibits the binding of CDK4 to the pRb protein which results in the inactivation of the pRb protein E2F family transcription factor, E2F release, and increased gene expression levels, so that proliferation potential of cervical cells leads to p16 overexpression [5-8]. A large number of reports in the cervical intraepithelial neoplasia (CIN) and cervical cancer using immunohistochemistry (IHC) can detect p16 expression, and the positive rate increases with gradual increases in the lesions [9-13]. At present, pathologists have used p16 IHC as an adjunctive diagnostic method to improve the accuracy of CIN diagnosis [14-16]. p16 immunocytochemical staining can triage for HPV-positive women [17,18]. However, p16 expression can also occur in metaplasia or atrophic cells as well as in normal cervical columnar cells. In addition, the impact of cytological specimens leads to poor specificity [19]. Thus, p16 alone is not enough to be used as a triage test for HPV-positive women, which leads us to the use of the nuclear antigen Ki-67 and the antibody mcm2.
The nuclear antigen Ki-67, a biomarker of cell proliferation, is expressed in all cell cycles beyond the G0 period [20]. The microchromosome maintenance protein-2 (mcm2) is involved in DNA replication in all eukaryotic cells by loading the complex onto DNA prior to replication, and in helicase activity by solubilizing DNA to allow DNA synthesis to participate in DNA replication in all eukaryotic cells [21,22]. Previous studies have been reported that two antibodies, mcm2 and topoisomerase IIα (TOP2A), in conjunction with immunocytochemistry techniques known as ProExTMC, have been used as an adjunctive diagnostic method for squamous intraepithelial lesions [23,24]. So far, there is no report on the combined detection of p16 and mcm2 antibodies. As a function of cell cycle regulators, Ki-67 and mcm2 may have potential overlap with the efficacy and clinical efficacy. Based on the above hypothesis, we conducted this study to evaluate the clinical performance of p16/Ki-67 and p16/mcm2 dual staining in a screening population in China.
Materials and methods
Study participants and procedures
From April 2015 to October 2015, we recruited 2,067 eligible women in Shuangliu County, Sichuan Province, China. This was a population-based prospective study conducted under the National Cervical Cancer Screening Program. The inclusion criteria were as follows: 1) between 35 and 64 years of age; 2) no history of cervical disease; 3) no pregnancy; 4) knowledgeable of the study procedures and able to sign the informed consents. After a well-trained health worker completed the questionnaire, the gynecologist collected a cervical exfoliative cytology sample from the cervix of each participant and transferred it to the ThinPrep preservation solution (Hologic Inc., Bedford, MA). The specimens were then transported to a central laboratory for HPV DNA testing. For HPV-positive women, liquid-based cytology (LBC) was performed. HPV16/18 or the other 12 high-risk HPV-positive (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) strains combined with cytological diagnosis of unidentified atypical squamous cells or worse (≥ASC-US) were referred to colposcopy, followed by directed cervical biopsies. We performed immunocytochemical dual staining of p16/Ki-67 (MXB, Fuzhou, China) and p16/mcm2 (MXB, Fuzhou, China) for the remaining cytological samples of all HPV-positive women and a random sample of negatives (the negatives were also tested with LBC). It is important to note that the clinical management of HPV-positive women is not based on dual staining results. For each participant, a third year follow-up visit was performed with co-testing by HPV DNA and LBC. This study was approved by the Sichuan University Medical Ethics Committee. All participants agreed to participate in cervical cancer screening and signed the informed consent forms.
HPV DNA test
A 1 ml aliquot was removed from ThinPrep cytology specimens to detect for high-risk HPV using a HPV Genotyping Real time PCR Kit (Liferiver, Shanghai, China), which detects viral DNA by nucleic acid hybridization with a probe set for 14 HR-HPV types, including specific HPV16 and HPV18 types as well as 12 other pooled high-risk types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68). All procedures were performed in strict accordance with the kit’s instructions.
Immunocytochemistry dual-staining
A second and third monolayer slide was prepared from the residual PreservCyt material using a ThinPrep 2000 system to perform p16/Ki-67 and p16/mcm2 immunocytochemical dual staining. Immunostaining for p16/Ki-67 was performed using the p16/Ki-67 Detection KIT (Immunocytochemistry, MXB, Fuzhou, China), which contains an antibody cocktail comprising a mouse monoclonal antibody (clone MX007) directed at the p16 protein and a rabbit monoclonal antibody (clone MIB-1) against the Ki-67 protein. Secondary antibodies included goat antimouse fragment antigen-binding antibody fragments for the detection of p16 with horseradish peroxidase (HRP) and goat antirabbit antibody fragments for the detection of Ki-67 with a polymer reagent conjugated to alkaline phosphatase (AP). HRP-mediated conversion of 3, 3’-diaminobenzidine (DAB) chromogen, and AP-mediated conversion of Fast Red chromogen emerged the red and yellow-brown staining of p16 and Ki-67, respectively. Finally, we used hematoxylin to counterstain cytology slide with a 3-step mounting procedure including 85%, 95% and pure ethanol medium, each for 1 minute. p16/mcm2 dual staining was performed using an antibody cocktail composed of the p16 antibody (clone MX007) and the rabbit anti-human mcm2 monoclonal antibody (clone SP85) according to the manufacturer’s instructions. Other procedures were similar, with the p16/Ki-67 dual staining as described above. Slides with at least one cervical epithelial cell that simultaneously showed red cytoplasmic immunostaining (p16) and brownish nuclear immunostaining (Ki-67) or moderate to intense yellow-brown nuclear staining (mcm2) were defined as positive results regardless of the morphological features, while those without any double-stained cells were recorded as negative results. All slides were cover slipped for viewing by a trained cytotechnologist under a lighted microscope, and the cytotechnologist was blinded to other testing results. The staining patterns of cervical cells for p16/Ki-67 positive and p16/mcm2 positive are shown in Figure 1.
Figure 1.
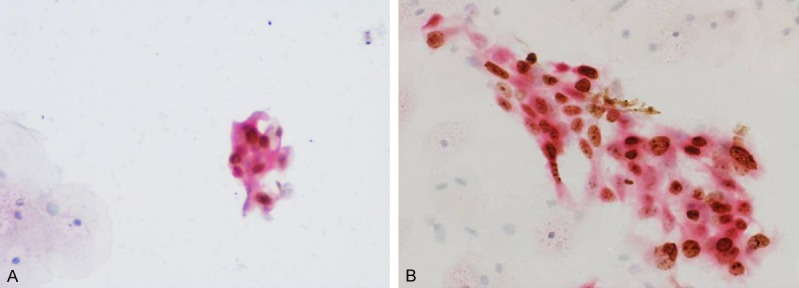
Staining patterns of cervical cell (A) p16 and Ki-67 positive staining, (B) p16 and mcm2 positive staining.
Liquid-based cytology
The cytological diagnosis was strictly described in terms of TBS system terminology. First, two experienced cytopathologists at the West China Second University Hospital of Sichuan University conducted a blind reading, independently. If the diagnoses were inconsistent, then a blind reading was conducted by a third cytopathologist, and the final results were based on the majority. Positive cytology is defined as ≥ASC-US.
Colposcopy and histopathology
Colposcopy was performed within one month after the screening positive results were determined. Direct biopsies were taken at suspicious or abnormal sites, and the endoscopic curettages (ECC) were performed when the scales were at the junction of the neck or there were lesions into the neck. The biopsy specimens were immediately fixed with 10% neutral buffered formalin for 24 hours. At the West China Second University Hospital of Sichuan University, the biopsy specimens were routinely paraffin embedded and sliced. First, two histopathologists read the slides, independently; if the results were inconsistent, a third histopathologist read the slides, and the final results were based on the majority. Pathological diagnosis was based on WHO classification and diagnostic criteria, including normal cervical tissue, CIN1, CIN2, CIN3, squamous cell carcinoma (SCC), and adenocarcinoma (ADC). The final pathological diagnosis was based on the worst lesion if multiple biopsies were taken. Those samples that were HPV negative, cytology normal, colposcopy negative, or histology CIN1 were recorded as negative results. Those histology results of CIN2, CIN3, SCC and ADC (i.e., CIN2+) were recorded as positive results.
Statistical analysis
The study analysis targeted women with complete screening, triage, and diagnostic confirmation results. Diagnostic accuracy of p16/Ki-67, p16/mcm2, LBC and HPV16/18 genotyping for the detection of CIN2+ and CIN3+ was evaluated by calculating the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), with 95% confidence intervals (CIs). We compared the efficacy of different triaging strategies by calculating risk ratios (RR) with 95% CIs: (1) a HPV DNA test in combination with cytology triage; (2) screening for specific HPV16 and HPV18, which conferred an extremely high risk for the progression to CIN3 [25]; (3) biomarkers with p16/Ki-67 or p16/mcm2 dual-staining after primary HPV screening. A Chi squared test of proportions was used to test linear associations between triag-ing methods and increasing severity of histological diagnoses. Associations between p16/ki-67 or p16/mcm2 and HR-HPV were examined using logistic regression models by reporting the odds ratios (ORs) with 95% CIs. McNe-mar tests were used to compare paired matching data such as sensitivities and specificities. All analysis was performed using SPSS version 19.0 (IBM, Armonk, NY, USA) using a statistically significant level of 0.05 (two-sided).
Results
Finally, 198 women were included in this analysis (Figure 2). The mean age of the participants was 49.3±8.1 years. Of these participants, 91 (46.0%) were HPV positive. 175 (88.4%) were cytology normal, 10 (5.1%) were ASC-US, 2 (1.0%) were ASC-H, 9 (4.5%) had low-grade squamous intraepithelial lesions (LSILs), and 2 (1.0%) had high-grade squamous intraepithelial lesions (HSILs). The baseline pathological diagnoses were 174 (87.9%) negative, 7 (3.5%) CIN1, 3 (1.5%) CIN2, 12 (6.1%) CIN3, 1 (0.5%) squamous carcinoma, and 1 (0.5%) adenocarcinoma.
Figure 2.
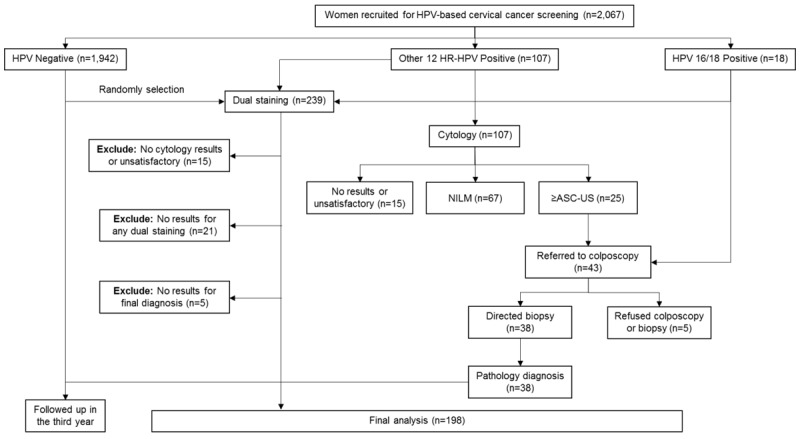
Flow chart and procedures involved in every step of the study. Abbreviations: ASC-US, atypical squamous cells-undetermined significance; NILM, no cervical intraepithelial neoplasia or malignancy.
As shown in Table 1, the overall positivity of p16/Ki-67 (26.3%) and p16/mcm2 (37.9%) dual-staining were higher than LBC (11.6%) and HPV16/18 genotyping (6.6%) (P<0.05). The positivty of both p16/Ki-67 and p16/mcm2 increased significantly with histology severity (both Ptrend<0.01). It increased from 19.5% in women with a normal diagnosis to 100% in those with cancer for p16/Ki67 and from 31.6% to 100% for p16/mcm2.
Table 1.
p16/Ki-67, p16/mcm2, LBC and HPV16/18 positivity with baseline histology categories, N (%)
| Category | p16/Ki-67 | p16/mcm2 | LBC (≥ASC-US) | HPV16/18 genotyping |
|---|---|---|---|---|
| Normal | 34 (19.5) | 55 (31.6) | 13 (7.5) | 5 (2.9) |
| CIN1 | 2 (28.6) | 5 (71.4) | 2 (28.6) | 3 (42.9) |
| CIN2 | 3 (100.0) | 3 (100.0) | 0 (0.0) | 0 (0.0) |
| CIN3 | 11 (91.7) | 10 (83.3) | 7 (58.3) | 4 (33.3) |
| Cancer | 2 (100.0) | 2 (100.0) | 1 (50.0) | 1 (50.0) |
| Total | 52 (26.3) | 75 (37.9) | 23 (11.6) | 13 (6.6) |
Abbreviations: CIN, cervical intraepithelial neoplasia; CIN2+, cervical intraepithelial neoplasia grade 2 or worse; CIN3+, cervical intraepithelial neoplasia grade 3 or worse; LBC, liquid-based cytology.
All participants were divided into three groups based on HPV status to analyze the association between HR-HPV infection and p16/Ki-67 or p16/mcm2 dual-staining results. Table 2 showed a strong association between p16/Ki-67 and p16/mcm2 dual-staining and HPV status. Compared with HPV negatives, p16/Ki-67 expression in the HPV16/18 group and in the 12 HR-HPV group was significantly higher, with odds ratios (ORs) of 16.27 (95% CI: 4.38-60.47) and 4.52 (95% CI: 2.16-9.45), respectively. It was more obvious in p16/mcm2 dual-staining, with ORs of 31.28 (95% CI: 6.33-154.56) and 9.10 (95% CI: 4.52-18.33), respectively.
Table 2.
The associations of p16/Ki-67, p16/mcm2 expression and HR-HPV infection, N (%)
| Biomarkers | HR-HPV negative | Other 12 HR-HPV positive | HPV16/18 positive |
|---|---|---|---|
| p16/Ki67 | |||
| + | 13 (12.1) | 30 (38.5) | 9 (69.2) |
| - | 94 (87.9) | 48 (61.5) | 4 (30.8) |
| P value | - | <0.001 | <0.001 |
| OR (95% CI) | Ref. | 4.52 (2.16-9.45) | 16.27 (4.38-60.47) |
| p16/mcm2 | |||
| + | 16 (15.0) | 48 (61.5) | 11 (84.6) |
| - | 91 (85.0) | 30 (38.5) | 2 (15.4) |
| P value | - | <0.001 | <0.001 |
| OR (95% CI) | Ref. | 9.10 (4.52-18.33) | 31.28 (6.33-154.56) |
Abbreviations: OR, odds ratio; CI, confidence interval; Other 12 HR-HPV positive: positive for any of the 12 HPV types (HPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68), and negative for HPV16/18; Ref, reference.
Sensitivities, specificities, PPVs, NPVs, and colposcopy referral rates for the detection of CIN2+ and CIN3+ at baseline are shown in Table 3. The sensitivity of p16/Ki-67 dual-staining to detect CIN2+ and CIN3+ were 94.1% (95% CI: 73.0-99.0) and 92.9% (95% CI: 68.5-98.7), which were not statistically different from p16/mcm2 (88.2% [95% CI: 65.7-96.7] for CIN2+, 85.7% [95% CI: 60.1-96.0] for CIN3+), but higher than LBC and HPV16/18 genotyping (both P<0.05). The specificity of p16/Ki-67 for the detection of CIN2+ and CIN3+ were 80.1% (95% CI: 73.7-85.3) and 78.8% (95% CI: 72.3-84.1), both significantly higher than p16/mcm but lower than LBC and HPV16/18 genotyping.
Table 3.
Clinical performance characteristics of p16/Ki-67, p16/mcm2, LBC and HPV16/18 genotyping for detection of CIN2+ and CIN3+ at baseline, % (95% CI)
| Methods | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| CIN2+ | ||||
| p16/Ki-67 | 94.1 (73.0-99.0) | 80.1 (73.7-85.3) | 30.8 (19.9-44.3) | 99.3 (96.2-99.9) |
| p16/mcm2 | 88.2 (65.7-96.7) | 66.9 (59.7-73.3) | 20.0 (12.5-30.4) | 98.4 (94.3-99.6) |
| LBC | 47.1 (26.2-69.0) | 91.7 (86.8-94.9) | 34.8 (18.8-55.1) | 94.9 (90.5-97.3) |
| HPV16/18 | 29.4 (13.3-53.1) | 95.6 (91.5-97.7) | 38.5 (17.7-64.5) | 93.5 (89.0-96.3) |
| CIN3+ | ||||
| p16/Ki-67 | 92.9 (68.5-98.7) | 78.8 (72.3-84.1) | 25.0 (15.2-38.2) | 99.3 (96.2-99.9) |
| p16/mcm2 | 85.7 (60.1-96.0) | 65.8 (58.7-72.2) | 16.0 (9.4-25.9) | 98.4 (94.3-99.6) |
| LBC | 57.1 (32.6-78.6) | 91.9 (87.0-95.0) | 35.8 (18.8-55.1) | 96.6 (92.7-98.4) |
| HPV16/18 | 35.7 (16.3-61.2) | 95.7 (91.7-97.8) | 38.5 (17.7-64.5) | 95.1 (91.0-97.4) |
Abbreviations: CIN2+, cervical intraepithelial neoplasia grade 2 or worse; CIN3+, cervical intraepithelial neoplasia grade 3 or worse; LBC, liquid-based cytology; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
150 women were followed up with a final diagnosis in the third year. The three-year cumulative risks for p16/Ki-67, p16/mcm, LBC, HPV16/18 to detect CIN2+ were 69.0%, 48.4%, 34.8% and 50.0%. Women who tested positive on both p16/Ki-67 and p16/mcm2 at baseline had the highest risk (RR: 39.64 [95% CI: 9.78-160.72]) to detect CIN2+ in the three years, followed by p16/Ki-67-positive and p16/mcm2-negative, HPV16/18-positive, LBC-positive. p16/Ki-67-negative women had the lowest risk, no matter what the p16/mcm2 result was (Table 4).
Table 4.
Third-year cumulative rates and relative risks (RR) of p16/Ki-67, p16/mcm2, LBC and HPV16/18 genotyping for CIN2+
| Test results | N | Cumulative rate % (95% CI) | RR (95% CI) |
|---|---|---|---|
| p16/Ki-67 and p16/mcm2 | |||
| -/- | 111 | 1.80 (0.50-6.33) | Ref. |
| -/+ | 10 | 0.00 (0.00-27.75) | 1.02 (0.99-1.04) |
| +/- | 8 | 62.50 (30.58-86.31) | 34.69 (7.94-151.55) |
| +/+ | 21 | 71.43 (50.04-86.19) | 39.64 (9.78-160.72) |
| LBC | |||
| - | 127 | 11.02 (6.68-17.65) | 6.12 (1.42-26.33) |
| + | 23 | 34.78 (18.81-55.11) | 19.30 (4.38-85.06) |
| HPV16/18 | |||
| - | 140 | 12.14 (7.72-18.59) | 6.74 (1.59-28.55) |
| + | 10 | 50.00 (23.66-76.34) | 27.75 (6.15-125.21) |
Abbreviations: RR, relative risk; CI, confidence interval; LBC, liquid-based cytology; Ref, reference.
Discussion
These results show that the positivity of p16/Ki-67 and p16/mcm2 increased with the severity of histology diagnoses. Under normal circumstances, p16 and Ki-67 do not co-express in the same cell, but p16 and Ki-67 may co-express after cell cycle regulation [25,26]. Previous studies have shown that the overexpression of mcm2 is detected in HR-HPV-induced cervical lesions [27]. Our study found that there was a strong association between p16/Ki-67 expression and HPV infections, especially in the HPV16/18-positive group, in which p16/Ki-67 positivity was higher than that in the other 12 HR-HPV group. A similar result was observed in p16/mcm2 dual staining. One possible explanation may be that HPV16/18 is more closely related to cervical cancer and therefore may secrete more HPV16/18 proteins [28,29].
Our results suggested that p16/Ki-67 was more sensitive, and similar specific than LBC and HPV16/18 genotyping, which was consistent with previous studies in different populations [30,31]. As a new biomarker, p16/mcm2 was as sensitive as p16/Ki-67, but a little less specific. We found that 8 and 6 women with CIN3+ diagnoses who were missed by LBC were identified by p16/Ki-67 and p16/mcm, respectively. This means that using p16/Ki-67 and p16/mcm2 can greatly improve the sensitivity of cytology-based screening.
This study also performed a prospective analysis on the three-year cumulative risks and relative risks for different tests. We found that women with both p16/Ki-67-negative and p16/mcm2-negative had the lowest risk, while women with the two biomarkers both positive had the highest risk. Therefore, it is an effective way to predict high grade cervical lesions by using p16/Ki-67 and p16/mcm2 dual staining, especially p16/Ki-67.
We acknowledge our study had some limitations. Because our study was based on the National Cancer Screening Program in China, we only uses two referral standards (i.e., women who were HPV16/18 positive or 12 other HPV positive types and ≥ASC-US), which could misclassify some cases and overestimate the specificities of HPV testing and LBC. This is why our results were higher than those reported in other studies, and we found 5 CIN2+ at the follow-up round. However, this study focused on comparisons among different tests, and three-year cumulative risks were also evaluated.
In summary, p16/Ki-67 and p16/mcm2 immunocytochemical dual staining can effectively improve the sensitivity of cytology in detecting high grade cervical lesions in initial screenings. Moreover, they can effectively predict high grade cervical lesions in the following years. Since p16/Ki-67 and p16/mcm2 dual staining are simple and easy to interpret, general technical staff can complete the procedure after a short-term training, and the tests have good reproducibility. Therefore, we believe that p16/Ki-67 and p16/mcm2 can be used as indicators of referral for HPV-positive women in China, and the women who test negative don’t need to be screened again for an extended period of time.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 81602504). We wish to thank all members of the project from CICAMS and the cooperative hospitals in China and the women who participated in this study. The proofreading of this manuscript by Johnathan Zeng from the University of Pennsylvania is also highly appreciated.
Disclosure of conflict of interest
None.
References
- 1.Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available at: http://globocan.iarc.fr/Default.aspx. Accessed March 14, 2018.
- 2.Herrero R, Gonzalez P, Markowitz LE. Present status of human papillomavirus vaccine development and implementation. Lancet Oncol. 2015;16:e206–e216. doi: 10.1016/S1470-2045(14)70481-4. [DOI] [PubMed] [Google Scholar]
- 3.WHO Guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. World Health Organization. 2013 [PubMed] [Google Scholar]
- 4.Arbyn M, Ronco G, Cuzick J, Wentzensen N, Castle PE. How to evaluate emerging technologies in cervical cancer screening? Int J Cancer. 2009;125:2489–2496. doi: 10.1002/ijc.24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D, von Knebel Doeberitz M. Overex-pression of p16(ink4a) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 6.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, Duensing S, Sheets EE, Munger K, Crum CP. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884–891. doi: 10.1097/00000478-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 8.von Knebel Doeberitz M. New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur J Cancer. 2002;38:2229–2242. doi: 10.1016/s0959-8049(02)00462-8. [DOI] [PubMed] [Google Scholar]
- 9.Agoff SN, Lin P, Morihara J, Mao C, Kiviat NB, Koutsky LA. p16(INK4a) expression correlates with degree of cervical neoplasia: a comparison with Ki-67 expression and detection of high-risk HPV types. Mod Pathol. 2003;16:665–673. doi: 10.1097/01.MP.0000077518.78046.0C. [DOI] [PubMed] [Google Scholar]
- 10.Saqi A, Pasha TL, McGrath CM, Yu GH, Zhang P, Gupta P. Overexpression of p16(INK4A) in liquid-based specimens (SurePath) as marker of cervical dysplasia and neoplasia. Diagn Cytopathol. 2002;27:365–370. doi: 10.1002/dc.10205. [DOI] [PubMed] [Google Scholar]
- 11.Murphy N, Ring M, Killalea AG, Uhlmann V, O’Donovan M, Mulcahy F, Turner M, McGuinness E, Griffin M, Martin C, Sheils O, O’Leary JJ. p16(INK4A) as a marker for cervical dyskaryosis: CIN and cGIN in cervical biopsies and ThinPrep smears. J Clin Pathol. 2003;56:56–63. doi: 10.1136/jcp.56.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahebali S, Depuydt CE, Segers K, Moeneclaey LM, Vereecken AJ, Van Marck E, Bogers JJ. p16INK4a as an adjunct marker in liquid-based cervical cytology. Int J Cancer. 2004;108:871–876. doi: 10.1002/ijc.11589. [DOI] [PubMed] [Google Scholar]
- 13.Tringler B, Gup CJ, Singh M, Groshong S, Shroyer AL, Heinz DE, Shroyer KR. Evaluation of p16(INK4a) and pRb expression in cervical squamous and glandular neoplasia. Hum Pathol. 2004;35:689–696. doi: 10.1016/j.humpath.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Horn LC, Reichert A, Oster A, Arndal SF, Trunk MJ, Ridder R, Rassmussen OF, Bjelkenkrantz K, Christiansen P, Eck M, Lorey T, Skovlund VR, Ruediger T, Schneider V, Schmidt D. Immunostaining for p16INK4a used as a conjunctive tool improves interobserver agreement of the histologic diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol. 2008;32:502–512. doi: 10.1097/PAS.0b013e31815ac420. [DOI] [PubMed] [Google Scholar]
- 15.Ordi J, Garcia S, del Pino M, Landolfi S, Alonso I, Quintó L, Torné A. p16 INK4a immunostaining identifies occult CIN lesions in HPV-positive women. Int J Gynecol Pathol. 2009;28:90–97. doi: 10.1097/PGP.0b013e31817e9ac5. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Kuhn L, Denny LA, De Souza M, Taylor S, Wright TC Jr. Impact of utilizing p16INK4A immunohistochemistry on estimated performance of three cervical cancer screening tests. Int J Cancer. 2007;120:351–356. doi: 10.1002/ijc.22172. [DOI] [PubMed] [Google Scholar]
- 17.Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Gillio-Tos A, De Marco L, Giorgi-Rossi P, Pontenani G, Rosso S, Sani C, Sintoni C, Segnan N, Zorzi M, Cuzick J, Rizzolo R, Ronco G New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2008;9:937–945. doi: 10.1016/S1470-2045(08)70208-0. [DOI] [PubMed] [Google Scholar]
- 18.Carozzi F, Gillio-Tos A, Confortini M, Del Mistro A, Sani C, De Marco L, Girlando S, Rosso S, Naldoni C, Dalla Palma P, Zorzi M, Giorgi-Rossi P, Segnan N, Cuzick J, Ronco G NTCC working group. Risk of high-grade cervical intraepithelial neoplasia during follow-up in HPV-positive women according to baseline p16-INK4A results: a prospective analysis of a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2013;14:168–176. doi: 10.1016/S1470-2045(12)70529-6. [DOI] [PubMed] [Google Scholar]
- 19.Wentzensen N, Bergeron C, Cas F, Eschenbach D, Vinokurova S, von Knebel Doeberitz M. Evaluation of a nuclear score for p16INK4a-stained cervical squamous cells in liquid-based cytology samples. Cancer. 2005;105:461–467. doi: 10.1002/cncr.21378. [DOI] [PubMed] [Google Scholar]
- 20.Heatley MK. Ki67 protein: the immaculate deception? Histopathology. 2002;40:483. doi: 10.1046/j.1365-2559.2002.01390.x. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez MA, Tachibana KE, Laskey RA, Coleman N. Control of DNA replication and its potential clinical exploitation. Nat Rev Cancer. 2005;5:135–141. doi: 10.1038/nrc1548. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzato M, Caudroy S, Bronner C, Evrard G, Simon M, Durlach A, Birembaut P, Clavel C. Cell cycle and/or proliferation markers: what is the best method to discriminate cervical high-grade lesions? Hum Pathol. 2005;36:1101–1107. doi: 10.1016/j.humpath.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Depuydt CE, Makar AP, Ruymbeke MJ, Benoy IH, Vereecken AJ, Bogers JJ. BD-ProExC as adjunct molecular marker for improved detection of CIN2+ after HPV primary screening. Cancer Epidemiol Biomarkers Prev. 2011;20:628–637. doi: 10.1158/1055-9965.EPI-10-0818. [DOI] [PubMed] [Google Scholar]
- 24.Walts AE, Bose S. p16, Ki-67, and BD ProExC immunostaining: a practical approach for diagnosis of cervical intraepithelial neoplasia. Hum Pathol. 2009;40:957–964. doi: 10.1016/j.humpath.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Ravarino A, Nemolato S, Macciocu E, Fraschini M, Senes G, Faa G, Negri G. CINtec PLUS immunocytochemistry as a tool for the cytologic diagnosis of glandular lesions of the cervix uteri. Am J Clin Pathol. 2012;138:652–656. doi: 10.1309/AJCP00INMGIFYFNQ. [DOI] [PubMed] [Google Scholar]
- 26.Singh M, Mockler D, Akalin A, Burke S, Shroyer A, Shroyer KR. Immunocytochemical colocalization of P16(INK4a) and Ki-67 predicts CIN2/3 and AIS/adenocarcinoma. Cancer Cytopathol. 2012;120:26–34. doi: 10.1002/cncy.20188. [DOI] [PubMed] [Google Scholar]
- 27.Santin AD, Zhan F, Bignotti E, Siegel ER, Cané S, Bellone S, Palmieri M, Anfossi S, Thomas M, Burnett A, Kay HH, Roman JJ, O’Brien TJ, Tian E, Cannon MJ, Shaughnessy J Jr, Pecorelli S. Gene expression profiles of primary HPV16- and HPV18-infected early stage cervical cancers and normal cervical epithelium: identification of novel candidate molecular markers for cervical cancer diagnosis and therapy. Virology. 2005;331:269–291. doi: 10.1016/j.virol.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 28.Castle PE, Stoler MH, Wright TJ, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011;12:880–890. doi: 10.1016/S1470-2045(11)70188-7. [DOI] [PubMed] [Google Scholar]
- 29.Ghittoni R, Accardi R, Hasan U, Gheit T, Sylla B, Tommasino M. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes. 2010;40:1–13. doi: 10.1007/s11262-009-0412-8. [DOI] [PubMed] [Google Scholar]
- 30.Wentzensen N, Fetterman B, Castle PE, Schiffman M, Wood SN, Stiemerling E, Tokugawa D, Bodelon C, Poitras N, Lorey T, Kinney W. p16/Ki-67 dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:v257. doi: 10.1093/jnci/djv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petry KU, Schmidt D, Scherbring S, Luyten A, Reinecke-Lüthge A, Bergeron C, Kommoss F, Löning T, Ordi J, Regauer S, Ridder R. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol. 2011;121:505–509. doi: 10.1016/j.ygyno.2011.02.033. [DOI] [PubMed] [Google Scholar]


