Abstract
Background: Bladder cancer is the most common malignancy in the urothelial tract. Invasive cancer has a poor prognosis compared to non-invasive cancer, and identifying the cancer type is useful in determining the most appropriate treatment. In this study, the expression ratios of E-cadherin in non-invasive urothelial carcinoma of the bladder were investigated. The goal of the study was to predict possible invasion in the recurrence of these cases. Material and methods: Seventy-two recurrent non-invasive cases of urothelial carcinoma of the bladder were retrospectively analyzed. An immunohistochemical study of E-cadherin was performed of the baseline tissue sections. An evaluation was carried out of the intensity of membranous staining of E-cadherin and the percentage of cells that stained positive. Results: Invasive cancer was identified in the recurrence material of 14 of the 72 urothelial carcinomas of the bladder that were originally non-invasive based on the baseline samples. The rate of invasion in the recurrence material was significantly higher in cases in which the loss of E-cadherin expression was observed. Conclusion: If E-cadherin expression is negative or weak, close clinical follow-up of patients is necessary, even if the initial diagnosis is non-invasive urothelial carcinoma of the bladder. This is because the rate of invasion in these cases is higher in recurrent cases.
Keywords: Urothelial carcinoma of the bladder, invasion, E-cadherin expression
Introduction
Bladder cancer is the most common malignancy in the urothelial tract. It is the ninth most frequently diagnosed cancer worldwide. Incidence rates are consistently lower in women than in men, although sex differences vary greatly among countries. It is most common in older people but can occur at any age. The observed patterns and trends of bladder cancer incidence worldwide appear to reflect the prevalence of tobacco smoking, although infection with Schistosoma haematobium and other risk factors are also major causes in selected populations [1].
Approximately 70-80% of cases of urothelial carcinoma of the bladder are limited to the mucosa or submucosa. Patients are diagnosed with muscle-invasive bladder cancer when the cancer cells invade the bladder detrusor muscle [1,2]. Moreover, 20% of initially diagnosed non-invasive bladder tumors develop into muscle-invasive cancer within five years following organ-preserving treatment [3]. Bladder cancer has become common globally, with an incidence ≥ 400000 annually. Approximately 165000 people die from this cancer annually [1,4]. It is important to try to predict recurrence and progression in patients with non-invasive bladder cancer as this informs the selection of treatment strategies after transurethral resection of bladder tumor. Therefore, the European Organization for Research and Treatment of Cancer (EORTC) developed a risk table that provides a scoring system based on the risk of recurrence and progression. According to the EORTC risk table, the six most significant clinical and pathologic factors in bladder cancer that are predictive of poor prognosis are multiple tumor focus, large tumor size (> 3 cm), prior recurrence, T category, concomitant carcinoma in situ (CIS), and a high-grade histopathologic diagnosis [5]. As per the institutional standardized postoperative protocol, the patients are followed-up every 3-4 months for the first two years after transurethral resection, and thereafter every six months for the next three years, and then annually [6].
E-cadherin is an important molecule in cell-cell adhesion in epithelial tissue. It is localized on the surface of the epithelial cells in regions of cell-cell contact known as adherence junctions [7,8]. E-cadherin is essential for the formation and maintenance of epithelia [9]. The loss of epithelial cell polarity and decreased E-cadherin expression are the main features of epithelial-mesenchymal transition [10]. The latter plays a role in tumor invasion and metastasis [11]. The disappearance of tight junctions between the cells can cause morphological change and increase the extent of invasion and metastatic capacity of the epithelial cells [12].
In addition to the role of E-cadherin in normal cells, this highly conserved gene in also highly influential in malignant cell transformation. Most tumors have abnormal cellular architecture and loss of tissue integrity can lead to local invasion. The loss of E-cadherin is an most important risk factor for the metastasis of several carcinomas and correlates with increased tumor invasiveness [13,14].
Material and methods
Data from the archives at the Pathology Department, Erzincan University Mengücek Gazi Education and Research Hospital, Erzincan, Turkey, were analyzed in this research. The study subjects were aged 31-88 years (a mean age of 64.7 years). The majority were aged 60-70 years. Fifty-six of them were men and 16 were women.
An immunohistochemical study of E-cadherin, using primary antibodies of E-cadherin (MU390-UC®, Biogenex, 1/20 ER1 20), was performed on the paraffin-embedded tissue sections of 72 recurrent cases of non-invasive bladder urothelial carcinoma. The sections were stained using a fully automated immunohistochemistry device (Leica BOND-MAX®; Leica Biosystems, Melbourne, Australia). After immunohistochemistry tissue processing, the sections were dehydrated using a graded series of ethanol and xylene and then covered with a mounting medium (Entellan®; Merck Millipore, Darmstadt, Germany). The samples stained for E-cadherin were evaluated under an Olympus® pX53 microscope (200 ×) (Olympus Optical Co., Tokyo, Japan). Photographs were taken with an Olympus®Camera adaptor U-TVO.5XG-3 (Olympus Optical Co., Tokyo, Japan).
The membranous staining intensity of E-cadherin was classified using four groups: no staining (a score of 0), weak staining (a score of 1), moderate staining (a score of 2), and strong staining (a score of 3). The percentage of positively stained cells with E-cadherin was classified using five groups: 0-9% (a score of 0), 10-24% (a score of 1), 25-49% (a score of 2), 50-74% (a score of 3), and ≥ 75% (a score of 4). The staining positivity of E-cadherin was calculated using the formula of total score = intensity score × percentage score [15]. Dependent on the degree of staining with E-cadherin, the cases were classified using three groups:
●Group 1: Negative staining (a total score of 1-2) (Figure 1).
Figure 1.

Group 1 (negative staining with E-cadherin): E-cadherin staining score 2 (× 200).
●Group 2: Weakly positive staining (a total score of 3-6) (Figure 2).
Figure 2.
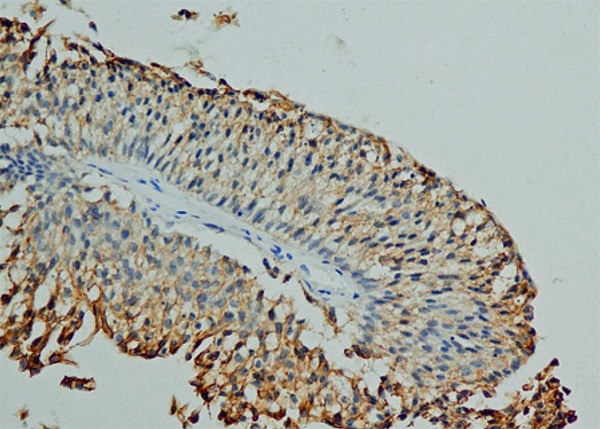
Group 2 (weakly positive E-cadherin staining): E-cadherin staining score 6 (× 200).
●Group 3: Strongly positive (a total score of ≥ 7) (Figure 3).
Figure 3.

Group 3 (strongly positive E-cadherin staining): E-cadherin staining score 12 (× 200).
The rate of invasion in recurrent cases with and without the loss of E-cadherin expression was also investigated. A pathological evaluation was performed according to the 2004 World Health Organization/International Society of Urologic Pathologists classification. Non-invasive urothelial neoplasm was defined as malignancy limited to the mucosa (pTa) and invasive urothelial neoplasm was defined as lamina propria (pT1) or muscularis propria (detrusor muscle) invasion (pT2).
Statistical analysis
The data were encoded, then transmitted to a computer and analyzed using SPSS® version 20.0. The descriptive data characteristics are expressed as mean ± standard deviation. The Kruskal-Wallis H test was used to compare the groups. Statistical significance was determined to be a P-value ≤ 0.050.
Results
Initially, 344 bladder transurethral resections and bladder biopsy materials were reviewed between 2007 and 2017. Of these, non-invasive urothelial carcinoma of the bladder was identified in 192 patients and invasion of the lamina propria or muscularis propria was found in 152.
Recurrence was detected in 72 of the 192 non-invasive cases. In these patients, the earliest recurrence occurred in the fourth month post treatment and average time to recurrence was 16 months. Invasion was present in the follow-up samples of 14 (19%) of the 72 originally non-invasive cases (based on baseline tissue sampling). Invasion of the lamina propria and muscularis propria was demonstrated in the follow-up sample of a patient identified with non-invasive urothelial carcinoma based on the initial assessment (Figure 4, 5 and 6).
Figure 4.

Non-invasive urothelial carcinoma (arrow) (HE × 200).
Figure 5.
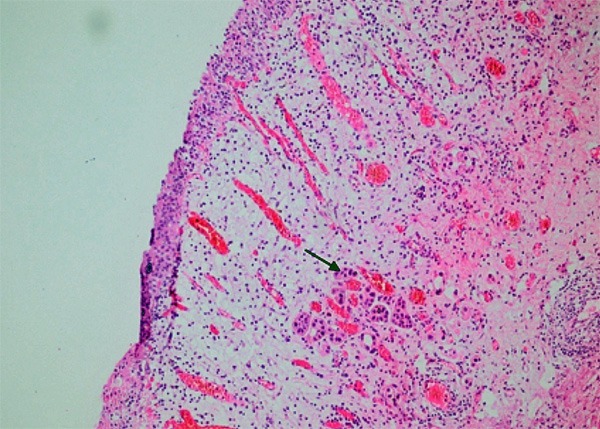
The invasion of lamina propria in the recurrent material (arrow) (HE × 200).
Figure 6.
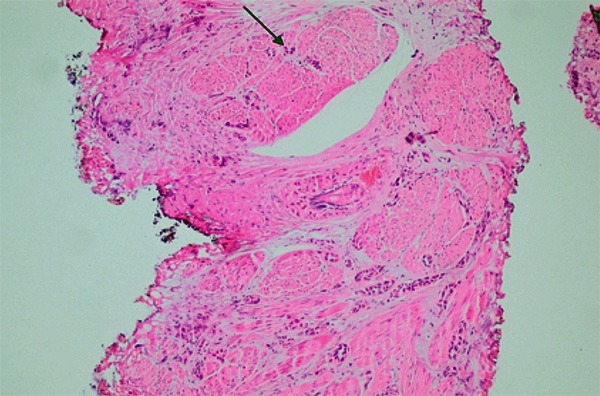
The invasion of muscularis propria in the recurrent material (arrow) (HE × 200).
Invasion was identified in 11 (20%) of the 56 men (78%) and in 3 (19%) of the 16 women (22%). A statistically significant difference was not found between sex and the rate of invasion in the follow-up samples (P ≥ 0.050).
The tumors were high and low grade in 47 (65%) and 25 cases (35%), respectively. Invasion was present in 10 (21%) of the high-grade and in 4 (16%) of the low-grade cases. A statistically significant difference was not observed between the follow-up samples showing recurrence in terms of differentiation in severity of tumor and rate of invasion (P ≥ 0.050).
The E-cadherin staining results (intensity score × percentage score) were as follows:
●Group 1 (negative staining with E-cadherin): Invasive cancer was observed in the follow-up samples of 3 (38%) of the 8 patients initially diagnosed with non-invasive urothelial carcinoma of the bladder.
●Group 2 (weakly positive E-cadherin staining): Invasive cancer was observed in the follow-up samples of 6 (29%) of the 21 patients initially diagnosed with non-invasive urothelial carcinoma of the bladder.
●Group 3 (strongly positive E-cadherin staining): Invasive cancer was observed in the follow-up samples of 5 (12%) of the 43 patients initially diagnosed with non-invasive urothelial carcinoma of the bladder.
A statistically significant difference was found between groups 1 and 3 (P ≤ 0.050) and between groups 2 and 3 (P ≤ 0.050) for the rate of invasion in the follow-up samples showing recurrence, but was not found between groups 1 and 2 (P ≥ 0.050).
Discussion
Bladder cancer is the most common urinary system malignancy. It is the fifth most common cancer in Western countries and the ninth most frequently diagnosed cancer worldwide. Its incidence is over 400000 annually, with an estimated prevalence of 2.7 million [16-18]. Over 90% of bladder cancer in western countries comprises urothelial carcinoma and 70-80% of these tumors are non-invasive [19,20]. High rates of recurrence and invasion are reported in patients with non-invasive bladder cancer owing to the aggressive nature of these tumors [21-23].
Tumor invasion is often associated with the down regulation of E-cadherin expression concomitant with a suppression of the cell junctions, while decreased levels of E-cadherin expression have been reported in higher-grade urothelial cancer [24].
Cadherin in the epithelial tissue is a calcium-dependent adhesion protein that promotes tight cell-cell junction. E-cadherin adjusts the connections of nearby epithelial cells, thereby maintaining cell polarity [25,26]. These cell adhesion molecules are important in preventing the progression of cancer, including that of the bladder [27].
The loss of E-cadherin expression affects the invasion and metastasis of various neoplasms, including bladder cancer, hepatocellular carcinoma, and breast cancer [28].
He et al. demonstrated that the loss of E-cadherin expression played an important role in the development of various tumors, such as pancreatic cancer, non-small cell lung cancer, and colorectal cancer [29]. Gao et al. also showed that decreased E-cadherin expression was critical in ovarian tumorigenesis [30].
Conclusion
If E-cadherin expression is found to be negative or weak, close clinical follow-up of patients is necessary, even if the initial diagnosis non-invasive urothelial carcinoma of the bladder. This is because the rate of invasion in these cases is higher in recurrent cases.
A routine pathologic assessment of E-cadherin expression in urothelial carcinoma of the bladder may be useful in expediting an early diagnosis of invasive cancer and thus would be beneficial to the follow-up and treatment of such patients.
Disclosure of conflict of interest
None.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M, van Rhijn BW, Shariat SF, Soukup V, Sylvester RJ, Zigeuner R. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71:447–61. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Black PC. Fine-tuning risk stratification for non-muscle-invasive bladder cancer. Eur Urol. 2016;69:70–1. doi: 10.1016/j.eururo.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage TaT1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–75. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Kim JK, Moon KC, Jeong CW, Kwak C, Kim HH, Ku JH. Papillary urothelial neoplasm of low malignant potential (PUNLMP) after initial TUR-BT: comparative analyses with non-invasive low-grade papillary urothelial carcinoma (LGPUC) J Cancer. 2017;8:2885–91. doi: 10.7150/jca.20003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gumbiner BM. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 8.Serrels A, Canel M, Brunton VG, Frame MC. Src/FAK-mediated regulation of E-cadherin as a mechanism for controlling collective cell movement: insights from in vivo imaging. Cell Adh Migr. 2011;5:360–5. doi: 10.4161/cam.5.4.17290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallin WJ, Sorkin BC, Edelman GM, Cunningham BA. Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1987;84:2808–12. doi: 10.1073/pnas.84.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu GL, Yang HJ, Liu T, Lin YZ. Expression and significance of E-cadherin, N-cadherin, transforming growth factor-β1 and Twist in prostate cancer. Asian Pac J Trop Med. 2014;7:76–82. doi: 10.1016/S1995-7645(13)60196-0. [DOI] [PubMed] [Google Scholar]
- 11.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 13.Vleminckx K, Vakaet L Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion supressor role. Cell. 1991;66:107–19. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 14.Kong DB, Chen F, Sima N. Focal adhesion kinases crucially regulate TGFβ-induced migration and invasion of bladder cancer cells via Src kinase and E-cadherin. Onco Targets Ther. 2017;10:1783–92. doi: 10.2147/OTT.S122463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Zhang K, Zhou L, Wu W, Jiang T, Cao J, Huang K, Qiu Z, Huang C. Expression and potential correlation among forkhead box protein M1, Caveolin-1 and E-cadherin in colorectal cancer. Oncol Lett. 2016;12:2381–8. doi: 10.3892/ol.2016.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 17.Van Rhijn BW, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, Witjes JA, Zlotta AR. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. 2009;56:430–42. doi: 10.1016/j.eururo.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–93. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace DM, Bryan RT, Dunn JA, Begum G, Bathers S. Delay and survival in bladder cancer. BJU Int. 2002;89:868–78. doi: 10.1046/j.1464-410x.2002.02776.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 21.Pasin E, Josephson DY, Mitra AP, Cote RJ, Stein JP. Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Rev Urol. 2008;10:31–43. [PMC free article] [PubMed] [Google Scholar]
- 22.Montie JE, Abrahams NA, Bahnson RR, Eisenberger MA, El-Galley R, Herr HW, Hudes GR, Kuzel TM, Lange PH, Patterson A, Pollack A, Richie JP, Sexton WJ, Shipley WU, Small EJ, Trump DL, Walther PJ, Wilson TG. Bladder cancer. Clinical guidelines in oncology. J Natl Compr Canc Netw. 2006;4:984–1014. doi: 10.6004/jnccn.2006.0083. [DOI] [PubMed] [Google Scholar]
- 23.Yun SJ, Kim WJ. Role of the epithelial-mesenchymal transition in bladder cancer: from prognosis to therapeutic target. Korean J Urol. 2013;54:645–50. doi: 10.4111/kju.2013.54.10.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ismail AF, Oskay Halacli S, Babteen N, De Piano M, Martin TA, Jiang WG, Khan MS, Dasgupta P, wells CM. PAK5 mediates cell: cell adhesion integrity via interaction with E-cadherin in bladder cancer cells. Biochem J. 2017;474:1333–46. doi: 10.1042/BCJ20160875. [DOI] [PubMed] [Google Scholar]
- 25.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–35. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 26.Rangel MC, Karasawa H, Castro NP, Nagaoka T, Salomon DS, Bianco C. Role of Cripto-1 during epithelial-to-mesenchymal transition in development and cancer. Am J Pathol. 2012;180:2188–200. doi: 10.1016/j.ajpath.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryan RT. Cell adhesion and urothelial bladder cancer: the role of cadherin switching and related phenomena. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140042. doi: 10.1098/rstb.2014.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang W, Hao Z, Han JL, Zhu DJ, Jin ZF, Xie WL. CAV-1 contributes to bladder cancer progression by inducing epithelial-to-mesenchymal transition. Urol Oncol. 2014;32:855–63. doi: 10.1016/j.urolonc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 29.He X, Chen Z, Jia M, Zhao X. Downregulated E-cadherin expression indicates worse prognosis in asian patients with colorectal cancer: evidence from meta-analysis. PLoS One. 2013;8:e70858. doi: 10.1371/journal.pone.0070858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao J, Zhu Y, Nilsson M, Sundfeldt K. TGF-b isoforms induce EMT independent migration of ovarian cancer cells. Cancer Cell Int. 2014;14:72. doi: 10.1186/s12935-014-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


