Abstract
Glucocorticoids are anti-inflammatory agents that are widely used in clinical practice. Increasing evidence has identified exosomes as important mediators in inflammation, but it is unknown whether glucocorticoids regulate exosome secretion and function. In the present study, we observed a reduction of exosome secretion in lipopolysaccharide (LPS)-induced RAW264.7 macrophages following treatment with dexamethasone. Importantly, exosomes isolated from LPS-induced RAW264.7 macrophages increased TNF-α and IL-6 production in RAW264.7 cells. However, this increase was less pronounced following treatment with exosomes isolated from dexamethasone-treated cells. Moreover, dexamethasone decreased expression of pro-inflammatory microRNA-155 in exosomes from LPS-induced RAW264.7 macrophages. We postulate that exosomes are novel targets in the anti-inflammatory effect of glucocorticoids in LPS-induced macrophage inflammatory responses. These findings will benefit the development of new approaches for anti-inflammatory therapeutics.
Keywords: Exosome, glucocorticoid, inflammation, microRNA-155
Introduction
Over the past few decades, glucocorticoids have been widely applied in the clinic to treat a variety of inflammatory and immune diseases such as asthma, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, sepsis, eczema, and psoriasis [1,2]. Today, the most common clinical use of glucocorticoids is for therapy of inflammatory disorders, in which they have become established as first-line treatment for both adults and children [1,3-6].
Given the importance of glucocorticoids in therapeutics, intense efforts by the pharmaceutical industry and academic research have been made to understand their mechanisms of action. At the cellular level, glucocorticoids reduce numbers of inflammatory cells, including T lymphocytes and mast cells, at sites of injury or activation [7-11]. The predominant effect of glucocorticoids is to switch off the expression of multiple inflammatory genes that are activated during acute and chronic inflammatory processes. Such genes encode inflammatory cytokines, adhesion molecules, chemokines, receptors, and enzymes, which are involved in the initiation or amplification of the immune system [9,12]. At higher concentrations, glucocorticoids have additional effects on the synthesis of anti-inflammatory proteins. A classic working model is the “glucocorticoid receptors (GR)” hypothesis, in which glucocorticoids diffuse readily across cell membranes and bind to GRs in the cytoplasm. GRs are normally bound by molecular chaperones or transcription factors such as heat shock protein-90, FK-binding protein, nuclear factor (NF)-κB, and Ap-1, and their binding regulates target gene expression [4,7]. Although the exact mechanisms of glucocorticoids are far from clear, the emergence of new inflammatory mediators is likely to offer further insights.
Exosomes are small particles with a diameter of 30 to 100 nm, which are released from both normal and diseased cells such as macrophages, T cells, and tissue cells [13-16]. They are composed of a lipid bilayer containing transmembrane proteins and components derived from donor cells. They have recently attracted interest as mediators of intercellular communication, and function as regulators in normal cell physiological activity, tumor pathogenesis, and most importantly in inflammation [13,14,17,18]. However, it remains unknown whether glucocorticoids regulate exosome secretion and function. To address this, we investigated the effect of glucocorticoids on exosomes using the lipopolysaccharide (LPS)-induced macrophage cell model. This is the first report to postulate that exosomes are a novel target involved in the anti-inflammatory effect of glucocorticoids.
Materials and methods
Cell lines and cell culture
RAW264.7 cells were originally obtained from ATCC and maintained in our institution. They were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, 2 μM glutamine, and 100 μg/ml streptomycin sulfate.
RNA extraction
RNA was extracted from cells using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s protocol, and then stored at -80°C.
mRNA expression analysis
A total of 1 μg RNA per sample was used to synthesize cDNA with the primerScript RT reagent Kit (TaKaRa, China) following the manufacturer’s protocol. Quantitative reverse transcription PCR (qRT-PCR) was performed using the SYBR Green Master kit (ABI, USA) according to the manufacturer’s instructions. Experiments were performed in triplicate. GAPDH was amplified for normalization as a housekeeping gene. Primers were designed using PrimerExpress software, and primer sequences were as follows: GAPDH: 5’-TGCACCACCAACT-GCTTAGC-3’ (forward) and 5’-GCATGGACTGTGGTCATGAG-3’ (reverse); TNF-α: 5’-GGCAGGTCTACTTTGGAGTCATTG-3’ (forward) and 5’-ACATTCG-AGGCTCCAGTGAATTCGG-3’ (reverse); IL-6: 5’-ACAACCACGGCCTTCC-CTACTT-3’ (forward) and 5’-CACGATTTCCCAGAGAACATGTG-3’ (reverse).
Enzyme-linked immunosorbent (ELISA) assay
Protein levels of TNF-α and IL-6 were measured by the ELISA kit (eBioscience, USA) according to the manufacturer’s instructions.
Exosome isolation
RAW264.7 macrophage cells were cultured in FBS-free DMEM medium, then treated with Dexamethasone (DEX, 10-7 M) or DMSO for 1 h prior to incubation with 1 μg/ml LPS. After 24 h, exosomes were isolated from culture supernatants and purified using ExoQuick (System BioSciences, USA). Exosome production was quantified by the BCA Protein Assay Kit (Pierce, USA).
Transmission electron microscopy (TEM)
Exosomes were re-suspended in 150 mM ammonium biocarbonate buffer. Experimental samples were placed on electron microscopy grids for 5 min, and the excess was wiped off using clean fibuff paper. The grids were dried at room temperature, then stained with 5 ml 2% filtered uranyl acetate solution. The grids were loaded onto a TITAN 80-300 Scanning Transmission Electron Microscope, and images were taken with Tecnai software (FEI).
MicroRNA (miRNA) expression analysis
Approximately 20 ng of RNA was converted into cDNA using the High-Capacity cDNA archive kit (ABI, USA) with a miR-155-specific primer (ABI, USA). The U6 gene (ABI, USA) was used as a normalization control.
Statistical analysis
Data are expressed as mean ± SEM of three independent repeats. Statistical tests were performed using PRISM 5.0 software (GraphPad, USA). P values less than 0.05 were identified as statistically significant.
Results
Glucocorticoids inhibit the LPS-induced macrophage inflammatory response
DEX is a representative member of the glucocorticoid family [19-21], and was used as an experimental drug in the present study. RAW264.7 cells were pretreated with DEX or DMSO for 1 h, then stimulated with LPS for 24 h. Real-time PCR analysis showed that DEX significantly decreased expression of TNF-α and IL-6 mRNA compared with DMSO controls (Figure 1A). Accordingly, TNF-α and IL-6 protein expression werealso significantly decreased following treatment with DEX (Figure 1B).
Figure 1.
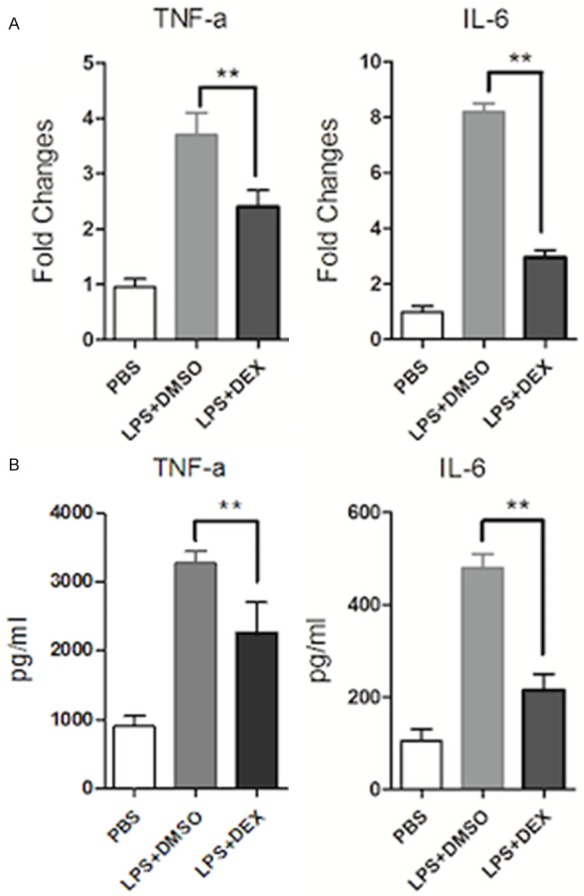
Glucocorticoids inhibit TNF-α and IL-6 production in the LPS-induced macrophage inflammatory response. RAW264.7 macrophages were pre-treated with DEX (10-7 M) or DMSO for 1 h, then treated with LPS (1 μg/ml) stimulation for 24 h. A: mRNA levels of TNF-α and IL-6 were measured by real-time PCR. Expression was normalized to that of endogenous GAPDH. B: Protein levels of TNF-αand IL-6 in the supernatant of culture cells were examined by ELISA. Data are representative of three independent experiments (**P<0.01).
Glucocorticoids reduce exosome secretion in LPS-induced RAW264.7 macrophage cells
To further investigate the effect of glucocorticoids on exosome secretion, we isolated exosomes from DEX- or DMSO control-treated RAW264.7 cells with LPS stimulation. Purified exosomes were confirmed and evaluated using TEM. As shown in Figure 2A, the purified vesicles were of the expected size of exosomes (30-100 nm). Quantification using the BCA assay showed that LPS increased the level of exosome secretion to almost twice that of the control. However, after DEX treatment, exosome secretion was significantly decreased (Figure 2B). These results suggest that glucocorticoids reduce exosome secretion in the LPS-induced macrophage inflammatory response.
Figure 2.
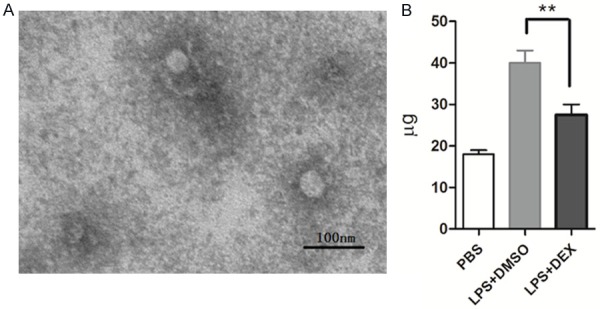
Glucocorticoids reduce exosome secretion in LPS-induced RAW264.7 macrophage cells. RAW264.7 cells cultured in serum-free medium were pre-treated with DEX (10-7 M) or DMSO for 1 h, then treated with LPS (1 μg/ml) stimulation for 24 h. A: Exosomes were evaluated using electron microscopy and images were taken with Tecnai software. B: Exosome production was quantified by a BCA Protein Assay Kit. Data are representative of three independent experiments (**P<0.01).
Glucocorticoids impair the inflammatory effect of exosome in LPS-induced RAW264.7 macrophage cells
To further investigate the effect of glucocorticoids on exosome function, RAW264.7 cells were treated with exosomes isolated from LPS-induced RAW264.7 cells with or without DEX. These exosomes were found to augment TNF-α and IL-6 production in RAW264.7 cells, but the increase was lower following treatment with exosomes isolated from DEX-treated cells (Figure 3). These results suggest that glucocorticoids regulate exosome function and impair the inflammatory effect of exosomes in LPS-induced RAW264.7 cells.
Figure 3.
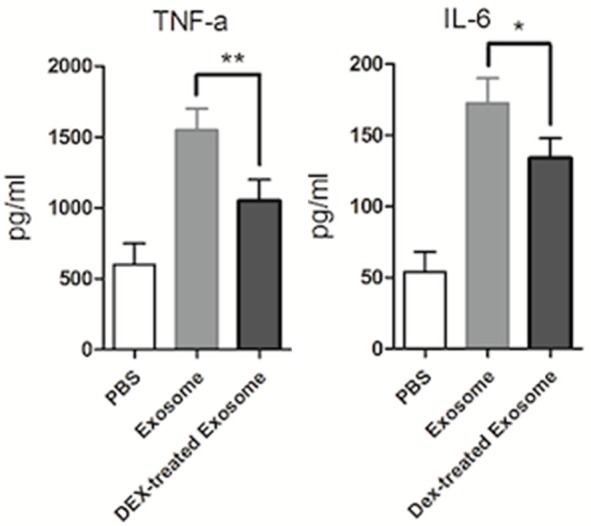
Glucocorticoids impaired the inflammatory effect of exosomes in LPS-induced RAW264.7 macrophage cells. RAW264.7 cells cultured in serum-free medium were pre-treated with DEX (10-7 M) or DMSO for 1 h, then treated with LPS (1 μg/ml) stimulation for 24 h. RAW264.7 macrophage cells were then treated with exosomes at 40 μg/ml for 24 h. Protein levels of TNF-α and IL-6 in the supernatant were examined by ELISA. Data are representative of three independent experiments (**P<0.01).
Glucocorticoids inhibit miR-155 expression in exosomes
miR-155 is a key pro-inflammatory miRNA while miR-146a is inhibitory [22-27]. To further investigate the effect of glucocorticoids on miR-155 and miR-146a expression in exosomes, we isolated RNA from exosomes and detected the expression of miR-155 and miR-146a by qPCR. As shown in Figure 4, miR-146a and miR-155 were both expressed in exosomes, and their expression was significantly increased following LPS stimulation. After DEX treatment, miR-155 expression was significantly decreased while that of miR-146a remained unchanged. These results suggested that glucocorticoids regulate exosome components and inhibit miR-155 expression in exosomes.
Figure 4.
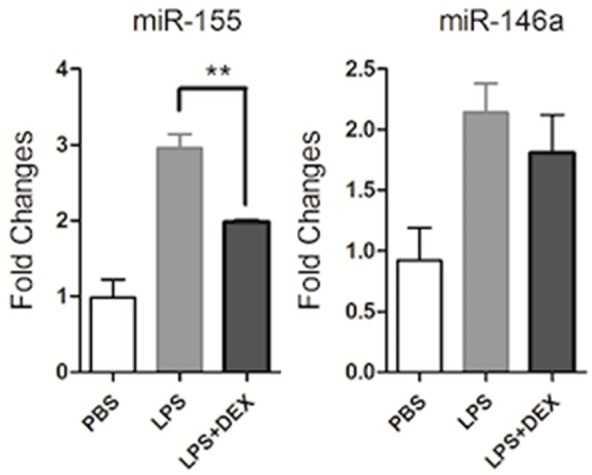
Glucocorticoids inhibit miR-155 expression in exosomes. RAW264.7 macrophage cells cultured in serum-free medium were pre-treated with DEX (10-7 M) or DMSO for 1 h, then treated with LPS (1 μg/ml) stimulation for 24 h. RNA was isolated from exosomes, and relative miR-155 and miR-146a expression levels were determined by real-time PCR and normalized to the endogenous control U6. Data are representative of three independent experiments (**P<0.01).
Discussion
Great progress has been made in the study of exosomes during recent years and they have been shown not only to be potential biomarkers but also mechanisms for the development of many physiological and pathological conditions [13,14]. Exosomes have immunostimulatory or immunosuppressive activities, and are therefore involved in regulation of the immune system [17,28].
Exosomes derived from dendritic cells carry MHC family proteins and co-stimulatory molecules such as CD80 and CD86, which are effective at stimulating T cell responses both in vitro and in vivo [29,30]. When purified exosomes from the body fluids of carcinoma patients were exposed to the human monocytic leukemia cell line THP-1, they induced expression of pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, and IL-23 at both the mRNA and protein level [31]. Moreover, exosomes derived from red blood cells also showed a pro-inflammatory effect by binding to monocytes and inducing the release of pro-inflammatory cytokines, which boosted in vitro T cell-mediated responses [28].
BALF exosomes from asthmatic patients have been reported totrigger secretion of pro-inflammatory leukotrienes and IL-8 in a human bronchial epithelial cell line [32]. Moreover, exosomes isolated from Mycobacterium avium-infected macrophages stimulated a pro-inflammatory response in resting macrophages [33,34]. Similarly, our study found that exosomes isolated from LPS-activated macrophages triggered the production of pro-inflammatory cytokines TNF-α and IL-6, indicating a pro-inflammatory role for exosomes in the macrophage inflammatory response. We also show that pre-treatment with glucocorticoids significantly reduced exosome secretion in the cell supernatant, which would contribute to the anti-inflammatory effect of glucocorticoids.
Exosomes are involved in cell-cell communication at the level of synapses or by delivering proteins and RNA, as well as modifying recipient cells within the microenvironment and at a distance through the systemic circulation. Recent reports also have shown that exosomes harbor genetic information in the form of miRNAs [13,14]. These are a group of non-coding small RNAs around 20-25 nucleotides in length. They regulate target genes through partial sequence complementarity to the 3’ untranslated region, causing mRNA degradation and/or translational repression [35,36]. They are also emerging as key regulators in the pathogenesis of inflammation. Indeed, several miRNAs areinduced in response to inflammatory signals, and functionally promote or inhibit inflammation [18,22,26,37,38].
miR-146a and miR-155 are two functional members of the TLR4 pathway. miR-146a was reported to target TRAF6, IRAK1, and IRAK2, and to inhibit NF-κB activation, thus limiting the inflammatory response [22,23]. Conversely, miR-155 transgenic mice exhibited increased pro-inflammatory cytokine production in response to LPS, suggesting a pro-inflammatory role for miR-155 [37,38]. Despite advances in our understanding of miRNA biology, little is known about regulation of miRNAs by glucocorticoids in the inflammatory process. In the present study, we found that miR-146a and miR-155 were both present within exosomes. The expression of miR-146a and miR-155 in exosomes was significantly upregulated following LPS stimulation, which is in line with previousobservations [23,26,37,39]. It is possible that increased miR-146a and miR-155 synthesis in macrophages would result in their increased expression within exosomes. Interestingly, treatment with DEX as a representative glucocorticoid decreased miR-155 expression in exosomes. Given the powerful pro-inflammatory effect of miR-155 in the inflammatory process, we propose that its downregulation by glucocorticoids helps limit inflammation, which may explain the loss of the inflammatory effect by exosomes from glucocorticoid-treated cells.
In summary, this study further extends the biological role of exosomes in inflammation and for the first time identifies exosomes as a possible novel target in the anti-inflammatory effect of glucocorticoids. These findings provide a basic rationale for the use of exosomes in drug development and the treatment of inflammation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81560668) and Xi Zang Min Zu University (xzmyzp02). We thank Sarah Williams, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.van den Berge M, Hiemstra PS, Postma DS. Genetics of glucocorticoids in asthma. N Engl J Med. 2011;365:2434–5. doi: 10.1056/NEJMc1112547. author reply 2435-6. [DOI] [PubMed] [Google Scholar]
- 2.Mapp CE. Inhaled glucocorticoids in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1960–1. doi: 10.1056/NEJM200012283432609. [DOI] [PubMed] [Google Scholar]
- 3.Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, Anderson P, Morgan NA. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of veterans affairs cooperative study group. N Engl J Med. 1999;340:1941–7. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 4.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 5.Schafer-Korting M, Kleuser B, Ahmed M, Holtje HD, Korting HC. Glucocorticoids for human skin: new aspects of the mechanism of action. Skin Pharmacol Physiol. 2005;18:103–14. doi: 10.1159/000084907. [DOI] [PubMed] [Google Scholar]
- 6.Ahluwalia A. Topical glucocorticoids and the skin--mechanisms of action: an update. Mediators Inflamm. 1998;7:183–93. doi: 10.1080/09629359891126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adcock IM. Glucocorticoids: new mechanisms and future agents. Curr Allergy Asthma Rep. 2003;3:249–57. doi: 10.1007/s11882-003-0047-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Chen S, Li C, Zhu Y, Peng B. Skewed T-cell subsets and enhanced macrophages phagocytosis in the spleen of patients with immune thrombocytopenia failing glucocorticoids. Int J Hematol. 2011;94:248–54. doi: 10.1007/s12185-011-0908-6. [DOI] [PubMed] [Google Scholar]
- 9.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 10.Schewitz LP, Lee RW, Dayan CM, Dick AD. Glucocorticoids and the emerging importance of T cell subsets in steroid refractory diseases. Immunopharmacol Immunotoxicol. 2009;31:1–22. doi: 10.1080/08923970802334848. [DOI] [PubMed] [Google Scholar]
- 11.Shuto T, Imasato A, Jono H, Sakai A, Xu H, Watanabe T, Rixter DD, Kai H, Andalibi A, Linthicum F, Guan YL, Han J, Cato AC, Lim DJ, Akira S, Li JD. Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via a negative cross-talk with p38 MAP kinase. J Biol Chem. 2002;277:17263–70. doi: 10.1074/jbc.M112190200. [DOI] [PubMed] [Google Scholar]
- 12.Barnes PJ. Glucocorticoids. Chem Immunol Allergy. 2014;100:311–6. doi: 10.1159/000359984. [DOI] [PubMed] [Google Scholar]
- 13.Andre F, Escudier B, Angevin E, Tursz T, Zitvogel L. Exosomes for cancer immunotherapy. Ann Oncol. 2004;15(Suppl 4):iv141–4. doi: 10.1093/annonc/mdh918. [DOI] [PubMed] [Google Scholar]
- 14.Chivet M, Javalet C, Hemming F, Pernet-Gallay K, Laulagnier K, Fraboulet S, Sadoul R. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41:241–4. doi: 10.1042/BST20120266. [DOI] [PubMed] [Google Scholar]
- 15.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribeiro MF, Zhu H, Millard RW, Fan GC. Exosomes function in pro- and anti-angiogenesis. Curr Angiogenes. 2013;2:54–9. doi: 10.2174/22115528113020020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. J Neuroinflammation. 2014;11:68. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.London NJ, Chiang A, Haller JA. The dexamethasone drug delivery system: indications and evidence. Adv Ther. 2011;28:351–66. doi: 10.1007/s12325-011-0019-z. [DOI] [PubMed] [Google Scholar]
- 20.Greenough A. Gains and losses from dexamethasone for neonatal chronic lung disease. Lancet. 1998;352:835–6. doi: 10.1016/S0140-6736(05)60002-5. [DOI] [PubMed] [Google Scholar]
- 21.van de Beek D. Brain teasing effect of dexamethasone. Lancet Neurol. 2007;6:203–4. doi: 10.1016/S1474-4422(07)70041-8. [DOI] [PubMed] [Google Scholar]
- 22.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, Lithgow GJ, Campisi J. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging. 2009;1:402–11. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahid MA, Pauley KM, Satoh M, Chan EK. miR-146a is critical for endotoxin-induced tolerance: implication in innate immunity. J Biol Chem. 2009;284:34590–9. doi: 10.1074/jbc.M109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Boldin MP, Yu Y, Liu CS, Ea CK, Ramakrishnan P, Taganov KD, Zhao JL, Baltimore D. miR-146a controls the resolution of T cell responses in mice. J Exp Med. 2012;209:1655–70. doi: 10.1084/jem.20112218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leah E. Rheumatoid arthritis: miR-155 mediates inflammation. Nat Rev Rheumatol. 2011;7:437. doi: 10.1038/nrrheum.2011.101. [DOI] [PubMed] [Google Scholar]
- 26.Okoye IS, Czieso S, Ktistaki E, Roderick K, Coomes SM, Pelly VS, Kannan Y, Perez-Lloret J, Zhao JL, Baltimore D, Langhorne J, Wilson MS. Transcriptomics identified a critical role for Th2 cell-intrinsic miR-155 in mediating allergy and antihelminth immunity. Proc Natl Acad Sci U S A. 2014;111:E3081–90. doi: 10.1073/pnas.1406322111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vigorito E, Kohlhaas S, Lu D, Leyland R. miR-155: an ancient regulator of the immune system. Immunol Rev. 2013;253:146–57. doi: 10.1111/imr.12057. [DOI] [PubMed] [Google Scholar]
- 28.Danesh A, Inglis HC, Jackman RP, Wu S, Deng X, Muench MO, Heitman JW, Norris PJ. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood. 2014;123:687–96. doi: 10.1182/blood-2013-10-530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobo-Vujanovic A, Munich S, Vujanovic NL. Dendritic-cell exosomes cross-present Toll-like receptor-ligands and activate bystander dendritic cells. Cell Immunol. 2014;289:119–27. doi: 10.1016/j.cellimm.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Wang L, Lin Z, Tao L, Chen M. More efficient induction of antitumor T cell immunity by exosomes from CD40L gene-modified lung tumor cells. Mol Med Rep. 2014;9:125–31. doi: 10.3892/mmr.2013.1759. [DOI] [PubMed] [Google Scholar]
- 31.Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, Marmé F, Umansky L, Umansky V, Eigenbrod T, Sammar M, Altevogt P. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288:36691–702. doi: 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qazi KR, Torregrosa Paredes P, Dahlberg B, Grunewald J, Eklund A, Gabrielsson S. Proinflammatory exosomes in bronchoalveolar lavage fluid of patients with sarcoidosis. Thorax. 2010;65:1016–24. doi: 10.1136/thx.2009.132027. [DOI] [PubMed] [Google Scholar]
- 33.Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007;282:25779–89. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang JJ, Chen C, Xie PF, Pan Y, Tan YH, Tang LJ. Proteomic analysis and immune properties of exosomes released by macrophages infected with Mycobacterium avium. Microbes Infect. 2014;16:283–91. doi: 10.1016/j.micinf.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Clark EA, Kalomoiris S, Nolta JA, Fierro FA. Concise review: MicroRNA function in multipotent mesenchymal stromal cells. Stem Cells. 2014;32:1074–82. doi: 10.1002/stem.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yendamuri S, Calin GA. The role of microRNA in human leukemia: a review. Leukemia. 2009;23:1257–63. doi: 10.1038/leu.2008.382. [DOI] [PubMed] [Google Scholar]
- 37.O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci U S A. 2009;106:7113–8. doi: 10.1073/pnas.0902636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–9. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 39.Cheng Y, Kuang W, Hao Y, Zhang D, Lei M, Du L, Jiao H, Zhang X, Wang F. Downregulation of miR-27a* and miR-532-5p and upregulation of miR-146a and miR-155 in LPS-induced RAW264.7 macrophage cells. Inflammation. 2012;35:1308–13. doi: 10.1007/s10753-012-9443-8. [DOI] [PubMed] [Google Scholar]


