Abstract
Chronic inflammation is a key contributor to obesity-related insulin resistance and type 2 diabetes (T2D). NLRP3 inflammasome activation plays an important role in impairing insulin signaling and insulin sensitivity. Adiponectin is an adipocyte-derived cytokine that has been shown to promote insulin sensitivity and exert anti-inflammatory properties, yet the detailed mechanism is still unclear. In this study, we aimed to investigate the anti-inflammatory effect of adiponectin on lipopolysaccharide (LPS) plus palmitic acid (PA)-induced THP-1 cells and to identify the underlying mechanism. We report here that adiponectin was able to inhibit interleukin (IL)-1β and IL-18 by suppressing NLRP3 inflammasome activation. Furthermore, we, for the first time, describe that adiponectin attenuates NLRP3 inflammasome activation by modulating the AMPK-ROS signaling pathway. These findings provide insight suggesting that adiponectin and NLRP3 inflammasome be considered molecular targets for the development of new treatment for T2D and the related metabolic diseases.
Keywords: Adiponectin, NLRP3 inflammasome, IL-1β, IL-18, AMPK
Introduction
The incidence of obesity, which gives rise to a state of chronic, low-grade inflammation that contributes to insulin resistance and type 2 diabetes, has increased dramatically and is a serious threat to global health [1-5]. Recent investigations suggest that obesity, obesity-linked insulin resistance, type 2 diabetes, and metabolic syndrome are chronic inflammatory diseases characterized by decreased adiponectin in the plasma [6,7]. Adiponectin is an adipocyte-derived cytokine that exerts anti-diabetic and anti-atherogenic properties. Adiponectin also has been shown to enhance insulin sensitivity and promote lipid metabolism in obesity-linked diseases through its anti-inflammatory action [8]. However, the detailed mechanism of the anti-inflammatory effect of adiponectin remains to be clarified.
A number of reports have established that hypoadiponectinemia is related to higher levels of inflammatory markers including IL-1β and IL-18. Ahonen et al. have suggested that a low adiponectin level and high IL-1β level in plasma predict the development of metabolic syndrome [9]. Kamio et al. demonstrated that pretreatment with 2 µg/ml adiponectin significantly reduced LPS-induced IL-1β mRNA expression [10]. In addition, Chandrasekar et al. have demonstrated that adiponectin can block IL-18-mediated vascular injury and inflammation through AMP-activated protein kinase (AMPK) activation [11]. The opposing relations of adiponectin with IL-1β and IL-18 led us to try to identify the underlying molecular mechanisms.
Numerous studies have shown that the synthesis and processing of IL-1β as well as IL-18 are tightly controlled by a molecular platform called NLRP3 inflammasome, which is composed of the Nod-like receptor protein NLRP3, the adaptor protein ASC, and pro-caspase-1 [12]. Upon stimulating by stimulators, NLRP3 protein is activated and recruits ASC and pro-caspase-1 to assemble the NLRP3 inflammasome, then triggers proteolytic cleavage of pro-caspase-1 into active caspase-1, which subsequently converts pro-IL-1β and pro-IL-18 into mature and biologically active forms IL-1β and IL-18 [13]. Interestingly, Wen et al. demonstrated that the AMPK-ROS signaling axis is involved in NLRP3 inflammasome activation in macrophages stimulated by LPS plus PA [14].
In the present study, we found that in patients with T2D, the circulating levels of adiponectin correlate inversely with IL-18. On this basis, we determined whether adiponectin attenuates IL-1β and IL-18 secretion from LPS+PA-induced THP-1 cells in vitro. In addition, we hypothesize that adiponectin inhibits IL-1β and IL-18 by suppressing NLRP3 inflammasome activation. Further, we investigate whether adiponectin exerts this biological effect via the AMPK-ROS pathway.
Materials and methods
Subjects and blood sampling
Thirty T2D patients (15 men and 15 women) within 5 years diagnosis were recruited from the Diabetes Clinic in the Third Xiangya Hospital of the Central South University. Twenty healthy subjects (10 men and 10 women) were matched for age and sex with the diabetic group. None of the subjects had evidence of infectious, allergic or other autoimmune diseases recently, and they did not use immunomodulatory drugs in the 6 months before sampling. The study was approved by the Ethics Committee of the Third Xiangya Hospital of the Central South University. Written informed consent was obtained from all study participants.
Venous blood from fasting subjects was collected between 6.00 and 8.00 a.m. to minimize possible circadian variations. Blood samples were allowed to clot at room temperature and the serum were immediately separated by centrifugation and stored in aliquots at -80 until analysis.
IL-18 and adiponectin assays
IL-18 and adiponectin levels were measured by enzyme-linked immunosorbent assay (ELISA) method according to manufacturer suggestions. IL-18 was determined by ELISA kit from MBL (No.7260) and adiponectin concentration was measured by ELISA kit from R&D Systems (No.DRP 300).
Cell culture and treatments
Monocytic THP-1 cells obtained from the American Type Culture Collection (ATCC) were grown at 37°C, under 5% CO2 in RPMI 1640 medium (Hyclone) supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/ml), streptomycin (100 mg/ml) and L-Glutamine (2.05 mM). The cells were cultured as a single-cell suspension and seeded onto 12-well plates at a density of 4 × 105 cells well-1. LPS and PA were supplemented to activate NLRP3 inflammasome with or without adiponectin.
Enzyme-linked immunosorbent assay for IL-1β and IL-18
THP-1 cells were pretreated with 100 ng/ml LPS for 3 h and then stimulated with 0.5 mM PA for another 8 h in the absence or presence of 2 µg/mL adiponectin. Supernatants were collected and stored at -80°C until used for cytokine determination. IL-1β and IL-18 were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D Systems).
Western blotting assay
Cells were harvested at the indicated time. Cell extracts (30 μg) were separated on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinyldene fluoride membranes (Millipore). Primary antibodies against NLRP3 (CST), AMPK (CST), phospho-AMPKα (CST), pro-caspase-1 (Abcam) and ASC (Abcam) were applied overnight at 4°C with a dilution of 1:1000. β-actin (1:5000 Sigma-Aldrich) was used as an internal control for equal protein loading. After primary antibody incubation, the blots were incubated with specific HRP-conjugated second antibody for 1 h at room temperature. The presence of bound antibody was detected using an ECL kit as the manufacturer’s manual.
Caspase-1 activity detection assay
Activity of caspase-1 was detected by using Caspase-1 activity assay kit (Beyotime, Shanghai China) according to the manufacturer’s protocol. The samples were read at 405 nm in a microtiter plate reader.
Small interfering RNA (siRNA) transfection
We designed several short-hairpin RNA fragments that might bind to the mRNA coding sequence of AMPKα1 and chose the one that most effectively inhibited AMPKα1 expression. The sense siRNA sequence targeting AMPKα1 was 5’-CGGGAUCAGUUAGCAACUATTUAGUUGCUAACUGAUCCCGTT-3’ (GenePharma). The inhibitory efficiency of siRNA probes was assessed by western blot analysis of AMPKα1 protein levels. In brief, cells were cultured for 24 h and grown to about 70% confluence. After this, cells were transfected with scramble siRNA or AMPKα1 siRNA by using of oligofectamine transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. All assays were performed 72 h after transfection of siRNA.
Measurement of reactive oxygen species
We measured mitochondrial ROS using MitoSOX (Molecular Probes). LPS-primed cells were treated with palmitate in the presence or absence of adiponectin. Cells were loaded with 5 µM MitoSOX for 15 min and washed twice with sterile PBS. Mean fluorescence intensity was determined using a CyAn ADP flow cytometer (DAKO). The ROS inhibitor NAC (N-acetyl-cysteine) was added 1 h before the addition of LPS. The ROS generation was assessed by the dichlorofluorescein fluorescence intensity (FL-1,530 nm) from 20,000 cells.
Statistical analysis
All data are presented as mean ± standard deviation values. The comparisons among different groups were made by one-way analysis of variance (ANOVA) followed by least significant difference (LSD) test as post hoc comparisons. Significant differences between groups were evaluated using SPSS 18.0 software (SPSS Inc., Chicago, IL). Differences with P < 0.05 were considered significant. Each experiment was repeated at least 3 times with similar results.
Results
Adiponectin concentration was negatively correlated with the level of IL-18
As shown in Figure 1, IL-18 level was significantly higher in T2D patients compared to the healthy control group (P < 0.01). Level of adiponectin was significantly lower in the diabetic patients when compared with healthy controls (P < 0.01). Notably, we found that the adiponectin concentrations were negatively correlated with IL-18 concentrations in both nondiabetic and diabetic subjects (r = -0.6386, P < 0.01). These results prompted us to ask whether adiponectin inhibits IL-18 in vitro. To address this question, we investigate the effect of adiponectin on LPS+PA-induced IL-18 and IL-1β production in THP-1 cells.
Figure 1.
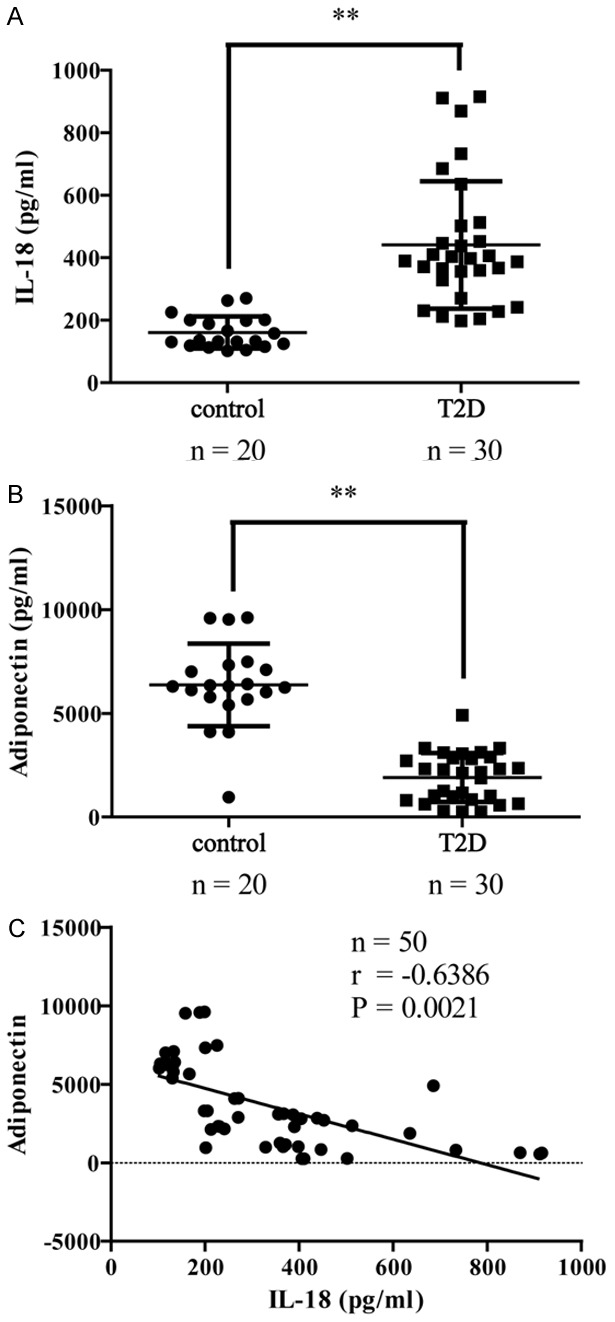
Adiponectin concentration was negatively correlated with the level of IL-18. (A) IL-18 and (B) adiponectin concentrations in serum of 30 diabetic patients and 20 non-diabetic subjects. Plots show mean ± SD by two-sided Student’s t-test. **P < 0.01 vs. the control group. (C) Scatter plots showing the correlation between adiponectin concentrations and IL-18 concentrations. Correlation coefficient r and P value were calculated by the Spearman’s rank correlation coefficient test.
Adiponectin significantly inhibited LPS+PA-induced IL-18 and IL-1β production from THP-1 cells
The THP-1 cells were stimulated with LPS+PA in the absence or presence of 2 µg/ml adiponectin. IL-18 and IL-1β levels in the suspensions were examined using ELISA in different groups. Compared to an untreated control group, THP-1 cells showed a significant increase in expression of IL-18 and IL-1β after LPS+PA stimulation (both P < 0.01; Figure 2), indicating that LPS and PA were effective stimulators of IL-18 and IL-1β secretion. However, in the adiponectin group, we saw that the secretion of IL-18 and IL-1β were significantly inhibited by adiponectin (P < 0.01; Figure 2). This result revealed that adiponectin has an effect of anti-IL-18 and anti-IL-1β.
Figure 2.
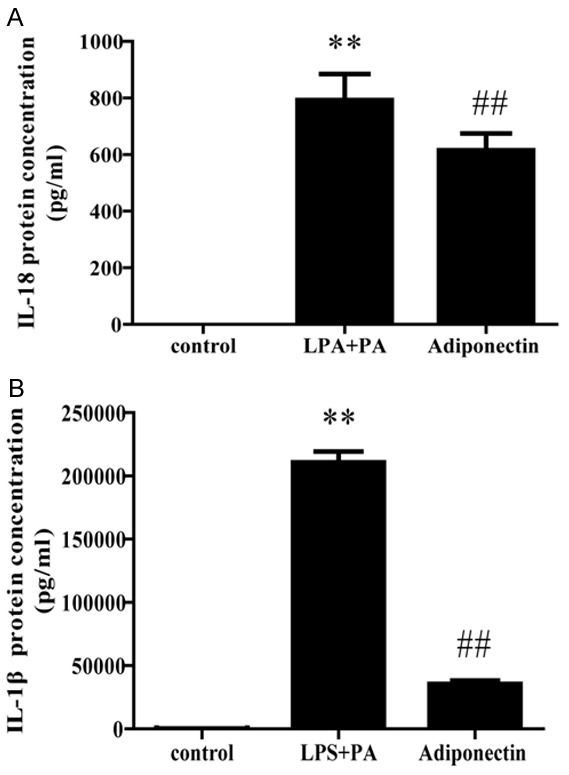
Effect of adiponectin on the LPS+PA induced secretion of IL-18 and IL-1β from THP-1 cells. Adiponectin group was pretreated with 2 μg/ml adiponectin for 12 h, and then stimulated with 100 ng/ml LPS for 3 hours and 0.5 mM PA for another 8 hours. Release of IL-18 and IL-1β was determined by ELISA. All data represent the mean ± SD of three independent experiments. **P < 0.01 vs. the control group; ##P < 0.01 vs. the LPS+PA group.
Adiponectin attenuates LPS+PA-induced NLRP3 inflammasome activation in THP-1 cells
Since the NLRP3 inflammasome is required to induce the maturation and secretion of IL-18 and IL-1β, we further asked whether adiponectin attenuates IL-18 and IL-1β secretion by impairment of the NLRP3 inflammasome, for which western blot analysis and caspase-1 activity detection assay were performed. As shown in Figure 3A-C, LPS+PA treatment significantly up-regulated NLRP3 and pro-caspase-1 expression, while did not modulate the expression level of ASC in comparison to untreated cells (P < 0.01 for both A and B; P > 0.05 for C). Interestingly, THP-1 cells co-incubated with LPS+PA and adiponectin exhibited significantly lower levels of NLRP3 and pro-caspase-1 compared to those exposed to LPS+PA only, although with no significant effect on ASC expression (Figure 3A-C). Similarly, LPS+PA stimulation showed a marked enhancement of caspase-1 activity in THP-1 cells and this rise in caspase-1 activity was significantly decreased by adiponectin treatment (Figure 3D). Taken together, these observations provide a mechanistic basis whereby adiponectin can attenuate LPS+PA-induced NLRP3 inflammasome activation in THP-1 cells.
Figure 3.
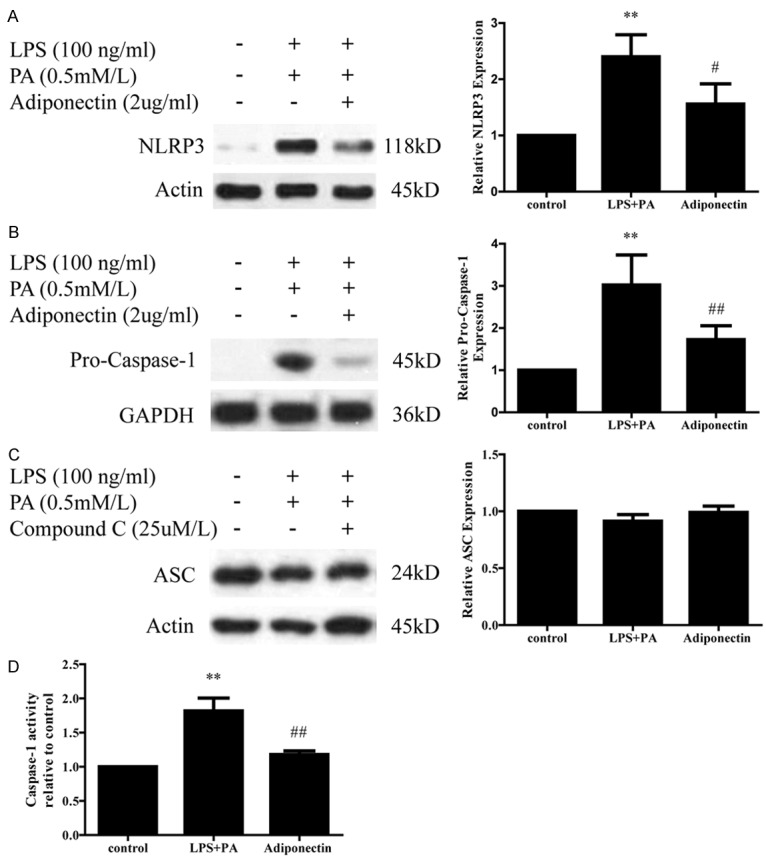
Effect of adiponectin on LPS+PA induced NLRP3 inflammasome activation in THP-1 cells. A. Expression of NLRP3 protein was detected by western blotting in different groups. Adiponectin treated group expressed lower level of NLRP3 protein than LPS+PA treated group. B and D. Level of pro-caspase-1 protein and caspase-1 activity were also lower in adiponectin treated group than LPS+PA treated group. C. Adiponectin has no effect on LPS+PA-induced ASC protein expression in THP-1 cells. All data represent the mean ± SD of three independent experiments. **P < 0.01 vs. the control group; #P < 0.05 vs. the LPS+PA group.
Adiponectin attenuates NLRP3 inflammasome activation through an AMPK-dependent mechanism
It has been demonstrated that adiponectin could ameliorate inflammation and insulin resistance through the activation of AMPK, and phosphorylation of AMPK is used as an indication of AMPK activation [15,16]. From Figure 4A we found that co-incubation with LPS+PA and adiponectin significantly increased the phosphorylation of AMPK in THP-1 cells compared with LPS+PA treatment only. In order to investigate if adiponectin inhibits NLRP3 inflammasome through an AMPK-dependent mechanism, we transfected THP-1 cells with siRNA directed against AMPKα1, which is a dominant form of AMPKα. The efficiency of siRNA-mediated AMPK downregulation was confirmed by western blotting showing reduced level of phosphorylated AMPK in siRNA-transfected cells (Figure 4B). As expected, NLRP3 was markedly increased in both scramble and AMPKα1 siRNA THP-1 cells stimulated with LPS+PA. Interestingly, we found adiponectin treatment inhibited NLRP3 expression in siControl THP-1 cells but not in siAMPKα1-transfected THP-1 cells (Figure 4C). Similarly, LPS+PA treatment significantly up-regulated caspase-1 activity in both scramble and AMPKα1 siRNA THP-1 cells in comparison to mock-treated cells. However, adiponectin reduced LPS+PA-mediated caspase-1 activation in scramble siRNA THP-1 cells but not in AMPKα1 siRNA THP-1 cells (Figure 4E). These findings indicate that adiponectin impaired NLRP3 inflammasome activation in an AMPK-dependent manner.
Figure 4.
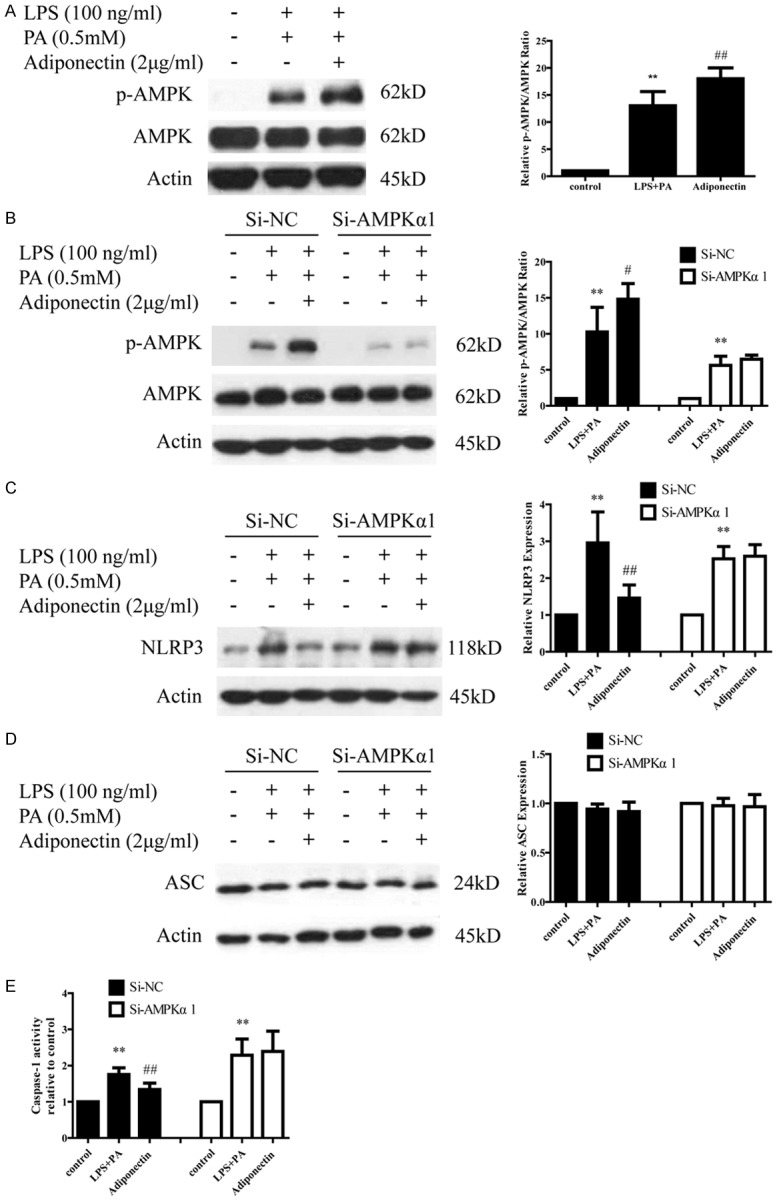
siRNA knockdown of AMPKα1 affects adiponectin by modulating LPS+PA-induced NLRP3 inflammasome activation. A. p-AMPK level was elevated in adiponectin treated group. B. p-AMPK and AMPK protein levels in Si-NC and Si-AMPKa THP-1 cells. C and E. The inhibition of NLRP3 protein and caspase-1 activity by adiponectin were abolished in Si-AMPKa THP-1 cells. D. Adiponectin has no effect on ASC protein expression in Si-NC and Si-AMPKa THP-1 cells. All data represent the mean ± SD of three independent experiments. **P < 0.01 vs. the control group; ##P < 0.01 vs. the LPS+PA group.
Adiponectin attenuates NLRP3 inflammasome activation by modulating AMPK-ROS signaling
Previous studies have demonstrated that NRLP3 inflammasome activation involves AMPK-ROS signaling, and mitochondrial generated reactive oxygen species (ROS) are essential for inflammasome activation [14,17]. To explore the effect of adiponectin on intracellular ROS generation and test whether adiponectin exerts a biological effect via the AMPK-ROS pathway, we determined the intracellular level of ROS using MitoSOX staining. As shown in Figure 5A, LPS+PA treatment substantially increased ROS production in THP-1 cells; however, adiponectin treatment significantly decreased the production of ROS. To examine further whether the decreased ROS production is AMPK-related, we assessed the LPS+PA-triggered generation of ROS in scramble and AMPKα1 siRNA THP-1 cells. As observed in Figure 5B, adiponectin significantly decreased ROS generation in scramble THP-1 cells but with no significant effect in AMPKα1 siRNA THP-1 cells, indicating that siRNA knockdown of AMPKα1 abolished the adiponectin-inhibited ROS activation in LPS+PA-induced THP-1 cells. Taken together, these observations provide insight into how adiponectin attenuates NLRP3 inflammasome activation by modulating AMPK-ROS signaling.
Figure 5.
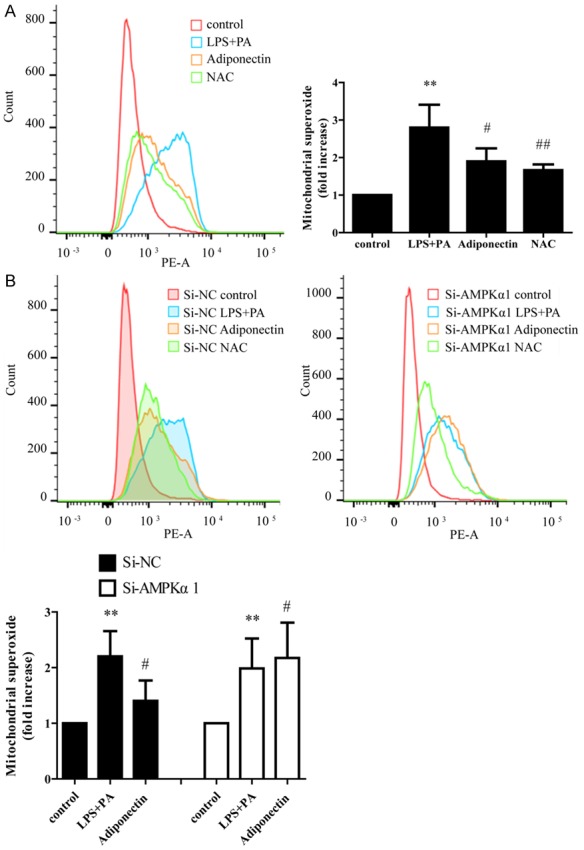
Effect of adiponectin on LPS+PA induced mitochondrial ROS production in Si-NC and Si-AMPKa THP-1 cells. A. Adiponectin inhibits mitochondrial ROS generation. The ROS inhibitor NAC (N-acetyl-cysteine) was added 1 hour before the addition of LPS as a positive control. B. THP-1 cells were transfected with scramble or AMPKα1 siRNA for 72 h, followed by stimulation with LPS (100 ng/mL) for 3 h and then treated with PA (0.5 mM) for 8 h with or without 2 μg/mL adiponectin. All data represent the mean ± SD of three independent experiments. **P < 0.01 vs. the control group; #P < 0.05 vs. the LPS+PA group; ##P < 0.01 vs. the LPS+PA group.
Discussion
Recent investigations suggest that obesity is a strong risk factor for insulin resistance and type 2 diabetes and is characterized by infiltration of adipose tissue by inflammatory cells and elevated levels of pro-inflammatory cytokines including Interleukin 1β (IL-1β), tumor necrosis factor α (TNFα), and Interleukin-6 (IL-6) [18]. It is demonstrated that the NLRP3 inflammasome senses obesity-associated ‘danger-signals’ and contributes to obesity-induced inflammation and insulin resistance. NLRP3 inflammasome can be activated by metabolic danger signals such as extracellular ATP, glucose, islet amyloid polypeptide (IAPP), free fatty acids, oxidized LDL, crystals of cholesterol, and uric acid [19]. Recently, progress has been made revealing a critical role for ROS in NLRP3 activation [20]. Some studies also have shown that LPS+PA causes ROS production and then induces the activation of the NLRP3 inflammasome [21]. Several previous studies have proposed that AMPK has emerged as an essential mediator of fatty acid metabolism, and it suppresses ROS production [22,23]. AMPK has also been shown to have an anti-inflammatory function in macrophages and it plays an important role during inflammasome activation by palmitate [24]. Thus, an AMPK-ROS signaling axis that regulates NLRP3 inflammasome activation was revealed [25].
Accumulating evidence has illustrated that adiponectin has positive actions on obesity-linked metabolic complications. Because of these beneficial effects, adiponectin has attracted tremendous scientific interest in recent years, and has been extensively studied both in human and animal models [26]. Both Freebies et al. and Berg et al. observed that injection of recombinant adiponectin in diabetic mice reduces plasma glucose to a near normal level [27], and adiponectin directly stimulates glucose utilization and improves insulin sensitivity via the activation of AMPK. Guo et al. reported that adiponectin knockout mice featuredhigh fat accumulation in the liver even with consumption of normal chow, which may result in dysfunction of mitochondria [28]. However, supplementation of adiponectin can rescue the mitochondria function with lowering the accumulation of ROS [29]. But the underlying mechanism remains unknown.
Here, we demonstrated that LPS+PA stimulation induced caspase-1 activation with IL-1β and IL-18 secretion. Moreover, we confirmed that adiponectin treatment significantly reduced the expression of NLRP3 and pro-caspase-1 and significantly inhibited caspase-1 activation in LPS+PA induced THP-1 cells. Considering the observed effect of adiponectin, we hypothesize that adiponectin most likely inhibits the production of IL-1β and IL-18 through the up-regulation of AMPK phosphorylation. Therefore, we confirmed the effect of AMPK phosphorylation on the anti-inflammatory influence of adiponectin in AMPKα1 knockdown THP-1 cells. Interestingly, we observed that adiponectin-triggered NLRP3 inflammasome activation was efficiently abolished by AMPKα1 siRNA, as compared with that of scramble THP-1 cells.
It has been reported that PA-enhanced ROS generation is negatively regulated by AMPK activation, which results in an increased βoxidation of PA in mitochondria and decreased overall lipid load inside cells. Moreover, AMPK upstream can inhibit ROS generation. Consistent with these previous studies, our study found that LPS+PA treatment induces ROS generation in THP-1 cells, which is prevented by adiponectin or an antioxidant NAC. Enhanced ROS generation is attenuated by adiponectin, suggesting that adiponectin can inhibit ROS generation. We further found that siRNA knockdown of AMPKα1 efficiently abolished adiponectin-inhibited ROS activation in comparison with that of scramble THP-1 cells, indicating that adiponectin attenuates NLRP3 inflammasome activation by modulating the AMPK-ROS pathway.
In conclusion, we observed a negative correlation between adiponectin and IL-18. Moreover, for the first time to our knowledge, we demonstrated that adiponectin inhibits LPS+PA-induced IL-1β and IL-18 secretion in THP-1 cells, at least in part, by acting at the NLRP3 inflammasome. In addition, we provided evidence that adiponectin suppresses NLRP3 inflammasome activation by modulating AMPK-ROS signaling. This is an important observation in that it suggests that adiponectin may control inflammation by up-regulating AMPK phosphorylation, and then reducing the ROS-initiated inflammatory response. Since adiponectin appears to affect multiple cytokines and inflammatory factors, our research suggests that targeting adiponectin may be an efficient therapy to modulate inflammatory responses in obesity-related disorders.
Acknowledgements
The study was funded by the National Natural Science Foundation of China (No. 81400831) and The New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (No. JY201718).
Disclosure of conflict of interest
None.
References
- 1.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nuñez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Müller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg BJ, van der Meer JW, Kanneganti T, Tack CJ, Netea MG. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polyakova E, Belyaeva O, Bazhenova E, Berkovich O, Berezina A, Ionin V, Bakulina A, Baranova E, Shlyakhto E. Insulin resistance and low serum adiponectin level as a risk factors of atherosclerosis and metabolic syndrome. Atherosclerosis. 2017;263:e252–e253. [Google Scholar]
- 5.Adabimohazab R, Garfinkel A, Milam EC, Frosch O, Mangone A, Convit A. Does inflammation mediate the association between obesity and insulin resistance? Inflammation. 2016;39:994–1003. doi: 10.1007/s10753-016-0329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks NL, Moore KS, Clark RD, Perfetti MT, Trent CM, Combs TP. Do low levels of circulating adiponectin represent a biomarker or just another risk factor for the metabolic syndrome? Diabetes Obes Metab. 2007;9:246–258. doi: 10.1111/j.1463-1326.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirose H, Yamamoto Y, Seino-Yoshihara Y, Kawabe H, Saito I. Serum high-molecular-weight adiponectin as a marker for the evaluation and care of subjects with metabolic syndrome and related disorders. J Atheroscler Thromb. 2010;17:1201–1211. doi: 10.5551/jat.6106. [DOI] [PubMed] [Google Scholar]
- 8.Nicolas S, Cazareth J, Zarif H, Guyon A, Heurteaux C, Chabry J, Petit-Paitel A. Globular adiponectin limits microglia pro-inflammatory phenotype through an adipoR1/NF-κB signaling pathway. Front Cell Neurosci. 2017;11:352. doi: 10.3389/fncel.2017.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahonen TM, Saltevo JT, Kautiainen HJ, Kumpusalo EA, Vanhala MJ. The association of adiponectin and low-grade inflammation with the course of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2012;22:285–291. doi: 10.1016/j.numecd.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Kamio N, Akifusa S, Yamaguchi N, Nonaka K, Yamashita Y. Anti-inflammatory activity of a globular adiponectin function on RAW264 cells stimulated by lipopolysaccharide from aggregatibacter actinomycetemcomitans. FEMS Immunol Med Microbiol. 2009;56:241–247. doi: 10.1111/j.1574-695X.2009.00573.x. [DOI] [PubMed] [Google Scholar]
- 11.Chandrasekar B, Boylston WH, Venkatachalam K, Webster NJ, Prabhu SD, Valente AJ. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-kB/PTEN suppression. J Biol Chem. 2008;36:24889–24898. doi: 10.1074/jbc.M804236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 13.He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-PYCARD inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg GR, Schertzer JD. AMPK promotes macrophage fatty acid oxidative metabolism to mitigate inflammation: implications for diabetes and cardiovascular disease. Immunol Cell Biol. 2014;92:340–345. doi: 10.1038/icb.2014.11. [DOI] [PubMed] [Google Scholar]
- 16.He C, Li H, Viollet B, Zou MH, Xie Z. AMPK suppresses vascular inflammation in vivo by inhibiting signal transducer and activator of transcription-1. Diabetes. 2015;64:4285–4297. doi: 10.2337/db15-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 18.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu JW, Lee MS. Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance. Arch Pharm Res. 2016;39:1503–1518. doi: 10.1007/s12272-016-0827-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinberg GR, Kemp BE. AMPK in health and diseas. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Kahn BB, Shi H. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaverkova H, Karasek D, Novotny D, Jackuliakova D, Halenka M, Lukes J, Frohlich J. Positive association of adiponectin with soluble vascular cell adhesion molecule sVCAM-1 levels in patients with vascular disease or dyslipidemia. Atherosclerosis. 2008;197:725–731. doi: 10.1016/j.atherosclerosis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 28.Guo R, Zhang Y, Turdi S, Ren J. Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: role of autophagy. Biochimica Et Biophysica Acta. 2013;1832:1136–1148. doi: 10.1016/j.bbadis.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]


