Abstract
Interleukin-6 (IL-6) is an inflammatory cytokines that plays a role in the development of cancer. Several studies have examined the relationship between the IL-6 -174G>C polymorphism and bladder cancer, but these results are inconclusive. Therefore, we conducted a meta-analysis to explore the association between IL-6 -174G>C polymorphism and bladder cancer risk. A comprehensive literature search was performed to identify eligible studies regarding the IL-6 -174G>C polymorphism and bladder cancer. Effect sizes under fixed- and random-effects models were calculated using odds ratios (ORs) with 95% confidence intervals (CIs). Finally, five case-control studies were included in the subsequent analyses. In the fixed-effect analysis, significantly higher bladder cancer risks of 1.20 (95% CI = 1.07-1.36) and 1.30 (95% CI = 1.08-1.56) were found for the dominant model (C/C+G/C vs. G/G) and recessive model (C/C vs. G/C+G/G), respectively. Especially for the Asian population, significantly greater bladder cancer risks of 1.63 (95% CI = 1.32-2.00) and 1.54 (95% CI = 1.07-2.21) were observed for the dominant model (C/C+G/C vs. G/G) and the recessive model (C/C vs. G/C+G/G), respectively. Non-significantly increased risks of bladder cancer were observed for the dominant and recessive models under the random-effects analysis. The major findings of this meta-analysis suggest that IL-6 -174G>C polymorphism is significantly associated with bladder cancer risk in the Asian population. Further studies with a larger sample size are needed to validate the effects of IL-6 polymorphisms on bladder cancer risk.
Keywords: Bladder cancer, interleukin, meta-analysis, polymorphism
Introduction
Inflammation is a self-limiting host defense mechanism against mechanical chemical or biological injury. Etiological factors eliciting chronic inflammation related to cancer development include microbial-related (Helicobacter pylori gastritis for gastric cancer and MALT lymphoma) or virus-related (hepatitis B or C virus for hepatocellular carcinoma) infections or autoimmune diseases (inflammatory bowel disease for colon cancer) [1]. These infectious organisms and/or mechanical injury can trigger chronic inflammation which is related to the development of bladder cancer, one of the most common urological and highly immunogenic malignancies. Bladder cancer ranks 9th among the most common cancers worldwide, comprising about 3% of all malignancies. Males are affected more often than females. The incidence of bladder cancer is rare before the age of 50 but increases sharply after the age of 65 [2]. Once chronic inflammation develops, it can mediate bladder cancer pathogenesis by stimulating cancer cell growth, invasion and metastasis through the recruitment of inflammatory cells and signaling molecules [3].
Oncogenesis is associated with chronic inflammation in approximately 20% of cancers. Cytokines including Interleukin-1 (IL-1), IL-6, IL-11, IL-8 Tumor necrosis factor-alpha (TNF-α), GCSF, and GM-CSF play an important role in both acute and chronic inflammation. This latter group can be subdivided into cytokines mediating humoral responses such as IL-4, IL-5, IL-6, IL-7, and IL-13, and those mediating cellular responses such as IL-1, IL-2, IL-3, IL-4, IL-7, IL-9, IL-10, IL-12, interferons, transforming growth factor-beta (TGF-β), TNF-α and TNF-β [4]. Interleukins are known to exert numerous functions related to cell growth, survival, differentiation and apoptosis in a number of diseases [5].
IL6, a phosphorylated glycoprotein containing 185 amino acids, is a pleiotropic and multifunctional cytokine, especially involved in chronic immune inflammatory response [6]. It initiates several different intracellular pathways like JAK/STAT, MAPK, PI3K that activate the expression of other genes, resulting in persistent inflammation and development of bladder cancer [7]. A common single nucleotide polymorphism at position -174 (-174G/C, rs1800795) of the IL-6 gene promoter region is thought to influence the binding of the glucocorticoid receptor and thus repress transcriptional activation [8]. Several studies have found an association between IL-6 and the risk of bladder cancer. Leibovici (2005) found that IL-6 variant genotype (C/C) was associated with an increased risk of bladder cancer with an odds ratio of 1.77 [9]. Ahirwar et al. (2008) suggested that the -174G>C single-nucleotide polymorphism may lead to decreased IL6 production and low-producing variant C/C in IL-6 might be a risk factor for bladder cancer [10]. Guey et al. (2010) examined evidence for the contribution of germline genetic variation to bladder cancer heterogeneity and found that genetic susceptibility loci might be correlated with the molecular diversity of bladder cancer. Ebadi et al. (2014) further found that IL-6 (-174G>C) genotype is significantly associated with an increased risk of bladder cancer in the Iranian population and showed a possible role of gene-environment interaction at the -174 position for IL-6 gene and predisposition of a specific genotype at this region to bladder cancer in exposure to these risk factors such as smoking habits or working at high risk jobs [11]. However, one Indian study in 2016 showed that IL-6 (-174G>C) substitution confers significant protection against the risk of urinary bladder cancer in the study population [12].
Due to some possible reasons such as different races, regions, sample size, the findings of correlation of polymorphism in the IL-6 gene and bladder cancer between these published papers are inconsistent. We thus conducted a meta-analysis to evaluate the relation between IL-6 -174G/C polymorphism and bladder cancer.
Materials and methods
Search strategy and study selection
Eligible literature regarding the association of IL-6 rs1800795 (-174G/C) polymorphism and bladder cancer was chosen from a literature search on PubMed, Embase, and Web of Science published from 2001 through July 2017. There was no language restriction. Public databases were searched by the following keywords: “interleukin-6”, “IL-6” and “gene polymorphism” or “genotype” or “rs1800795” or “promoter”; and “bladder cancer” or “urothelial carcinoma”. The inclusion criteria must meet the following rules: (1) studies on human subjects; (2) studies regarding the association between IL-6 rs1800795 (-174G/C) polymorphism and bladder cancer; (3) case-control study design; (4) sufficient information for evaluating genotype frequency or odds ratio (OR) with 95% confidence interval (CI). Following the above search strategy, we collected 188 studies, of which 6 were eligible for evaluation in this meta-analysis (Figure 1).
Figure 1.
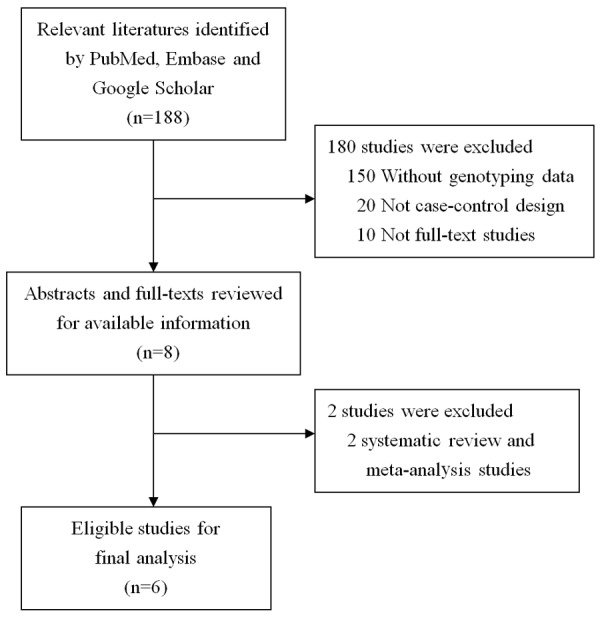
Flow diagram of the inclusion and exclusion of eligible studies.
Data extraction
The data extraction was performed independently by two of the authors (Y.T. Chiang and C.W. Sung), and discrepancies were initially resolved by discussion. If there was no consensus, another independent investigator (C.C. Wu) was consulted to make a final decision. Information recorded from each study included first author’s name, year of publication, country where this study was conducted, the ethnicity of participants, the distribution of genotype frequency of IL-6 rs1800795 (-174G/C) polymorphism for cases and controls, minor allele frequency, source of control groups, and genotyping method. For individual study, the examination of Hardy-Weinberg equilibrium (HWE) in the control group was evaluated using the goodness-of-fit Chi-square test.
Statistical analysis
The strength of the association between the IL-6 rs1800795 (-174G/C) polymorphism and risk of bladder cancer was calculated as a measure of the pooled OR and its corresponding 95% CI. We performed the Cochran Q-statistic test and an I 2 test to evaluate the heterogeneity across the different studies (I 2 < 25%, low heterogeneity; I 2 = 25-50%, moderate heterogeneity; I 2 > 50%, obvious heterogeneity). Based on the heterogeneity, the pooled OR was estimated by a fixed-effect model (Mantel-Haenszel method) or a random-effect model (DerSimonian and Laird method).
The sensitivity analysis was conducted to assess the influence of each study on the pooled OR by omitting the individual study. Publication bias was evaluated by funnel plot, and the asymmetric plot implied a publication bias. The asymmetry was tested by the Egger’s linear regression. All statistical analyses were performed using the Comprehensive Meta-Analysis version 3.0 (Biostat, Englewood, NJ, USA).
Results
Characteristics of selected studies
After a literature search under the inclusion criteria, a total of six eligible studies regarding the association between IL-6 rs1800795 (-174G/C) polymorphism and risk of bladder cancer were included in the present meta-analysis. Table 1 shows major characteristics of these selected studies. The eligible literatures were published between 2005 and 2016. Among these selected studies, one Indian study conducted by Gautam et al., has an extremely different pattern with the other 5 literatures. As shown in Table 2, it shows that the C allele frequency is significantly different from the others, which may be due to the selection bias of the control group in this Indian study. Therefore, we only included a total of 5 literatures in the subsequent analyses. A total of 2,179 cases and 2,810 controls was included in the present study. The genotype frequency among cases and controls for IL-6 rs1800795 (-174G/C) polymorphism were shown in Table 2. The distributions of genotype in the control group were in HWE for most of studies (P > 0.05).
Table 1.
Basic characteristics of eligible studies in the present meta-analysis
| First author | Year | Country | Ethnicity | Sample size (case/control) | Genotyping method |
|---|---|---|---|---|---|
| Leibovici et al. | 2005 | United States | Caucasian | 465/450 | TaqMan |
| Ahirwar et al. | 2008 | India | Asian | 136/200 | ARMS-PCR* |
| Guey et al. | 2010 | Spain | Caucasian | 1,017/1,065 | TaqMan |
| Wu et al. | 2013 | Taiwan | Asian | 300/594 | PCR-RFLP |
| Ebadi et al. | 2014 | Iran | Asian | 261/251 | PCR-RFLP |
| Gautam et al. | 2016 | India | Asian | 232/250 | Sequencing |
ARMS-PCR: Amplification refractory mutation system-polymerase chain reaction.
Table 2.
Frequency of the IL-6 rs1800795 (-174G/C) polymorphism among selected studies
| First author | Year | Genotype frequencies for cases | Genotype frequencies for controls | HWE (P-value) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| G/G | G/C | C/C | C allele (%) | G/G | G/C | C/C | C allele (%) | |||
| Leibovici et al. | 2005 | 112 | 222 | 110 | 49.8 | 164 | 211 | 68 | 39.2 | 0.56 |
| Ahirwar et al. | 2008 | 86 | 24 | 26 | 27.9 | 130 | 56 | 14 | 21.0 | 0.68 |
| Guey et al. | 2010 | 470 | 438 | 109 | 32.3 | 450 | 495 | 120 | 34.5 | 0.59 |
| Wu et al. | 2013 | 121 | 109 | 24 | 31.0 | 350 | 111 | 46 | 20.0 | 0.19 |
| Ebadi et al. | 2014 | 154 | 89 | 18 | 23.9 | 160 | 79 | 12 | 20.5 | 0.55 |
| Gautam et al. | 2016 | 95 | 109 | 28 | 35.6 | 66 | 125 | 59 | 51.4 | 0.78 |
Main findings of meta-analysis
No heterogeneity existed between these five eligible studies under the dominant model or the recessive model. In Figure 2 (the dominant model), compared with subjects carrying the G/G genotype of IL-6 rs1800795 (-174G/C) polymorphism, those with the C/C and G/C genotypes had higher significant bladder cancer risks under the fixed effects model (OR = 1.202, 95% CI = 1.065-1.356, P = 0.003), but there was no significance in the random effects model (OR = 1.367, 95% CI = 0.892-2.094, P = 0.151). In Figure 3 (the recessive model), compared with subjects carrying the G/C and G/G genotypes of IL-6 rs1800795 (-174G/C) polymorphism, those with the C/C genotype had significantly increased bladder cancer risks under the fixed effects model (OR = 1.300, 95% CI = 1.082-1.562, P = 0.005). However, in the random effects model, the significance disappeared (OR = 1.300, 95% CI = 1.082-1.562, P = 0.005).
Figure 2.
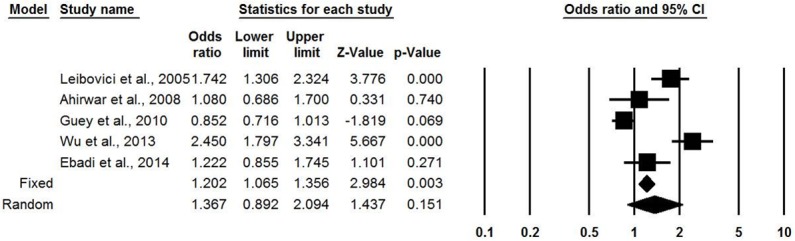
Forest plot for the association between the IL-6 -174G>C polymorphism and bladder cancer under the dominant model. (C/C+G/C vs. G/G genotypes).
Figure 3.
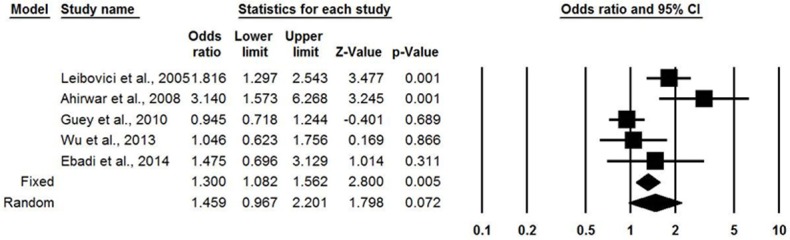
Forest plot for the association between the IL-6 -174G>C polymorphism and bladder cancer under the recessive model. (C/C vs. G/C+G/G genotypes).
Stratified by ethnicity, 3 eligible studies are Asian and the others are Caucasian. In the Asian group, the risk of bladder cancer significantly increased in both dominant and recessive models in fixed model (OR = 1.63, 95% CI = 1.32-2.00, P = 0.001 in dominant model; OR = 1.54, 95% CI = 1.07-2.21, P = 0.021 in recessive model). In the random effect model, however, no significance was found in two models. On the other hand, in the Caucasian subgroup, there was no significance between IL-6 rs1800795 (-174G/C) polymorphism and risk of bladder cancer in dominant or recessive model in both fixed and random model (Table 3).
Table 3.
The pooled ORs and 95% CIs for IL-6 rs1800795 (-174G/C) polymorphism and bladder cancer risk stratified by ethnicity
| No. | Model | C/C+G/C versus G/G (Dominant model) | C/C versus G/C+G/G (Recessive model) | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| Total | 5 | Fixed | 1.20 (1.07-1.36) | 0.003 | 1.30 (1.08-1.56) | 0.005 |
| Random | 1.37 (0.89-2.09) | 0.151 | 1.46 (0.97-2.20) | 0.072 | ||
| Ethnicity | ||||||
| Asian | 3 | Fixed | 1.63 (1.32-2.00) | 0.001 | 1.54 (1.07-2.21) | 0.021 |
| Random | 1.50 (0.89-2.55) | 0.132 | 1.65 (0.85-3.19) | 0.138 | ||
| Caucasian | 2 | Fixed | 1.03 (0.89-1.19) | 0.700 | 1.23 (0.99-1.52) | 0.059 |
| Random | 1.21 (0.59-2.43) | 0.600 | 1.30 (0.69-2.47) | 0.421 | ||
Sensitivity analysis and publication bias
Sensitivity analysis was generally conducted by omitting of the individual study and the cumulative statistics showed that the pooled OR of IL-6 rs1800795 (-174G/C) polymorphism was not significantly influenced by any individual study under the dominant or recessive model. No significant publication bias and the heterogeneity is acceptable in this study.
Discussion
Inflammation is considered to play a key role in the development of various cancers including bladder cancer. IL-6 is one of the inflammatory cytokines. Several studies have explored the relationship between IL-6 -174G/C polymorphism (rs1800795) and bladder cancer, however, these results were inconsistent [6,9-11]. In the present study, we conducted a meta-analysis to examine the association between IL-6 -174G/C polymorphism and bladder cancer risk. Because one study conducted by Gautam et al. had a extremely different finding from the other five studies, we included a total of five eligible studies in the subsequent analyses.
In the present study, we observed that subjects carrying the IL-6 -174 C/C and G/C genotypes had a significant bladder cancer risk (OR = 1.2) comparing to those with the G/G genotype. Moreover, a significantly increased risk of bladder cancer (OR = 1.3) was found for subjects with the C/C genotype of IL-6 -174 G/C polymorphism under the recessive model. A previous study showed that the -174C allele has a poor binding activity to the glucocorticoid receptor and contributes to lower plasma IL-6 levels [8]. Another study also reported that subjects who carried the C/C genotype of IL-6 -174G/C polymorphism had a higher risk of bladder cancer, which is consistent with our findings [13]. Regarding the potential effect of IL-6 -174G/C polymorphism, a study found that -174G/C polymorphism may affect the binding affinity of certain transcription factors such as GATA1 to the promoter region of IL-6 gene and plays an important role in several cancers [14]. However, Tindall et al. reported that -174G>C polymorphism was not associated with the risk and survival of prostate cancer [15]. These previous findings indicated that the -174G/C polymorphism of IL-6 gene not only regulated chronic inflammation but also play different roles in the development of various malignancies.
Various single nucleotide polymorphisms (SNPs) of IL-6 gene are associated with different cancer types, therefore, individual SNPs such as the -174G/C polymorphism may have specific risk for different cancer types among various populations [16,17]. In the present study, the frequency of the C/C genotype is higher in bladder cancer cases than that in the controls which is in consistent with other studies. Because the ethnic variation may contribute to the differences in genotype frequencies, we further performed a stratification analysis to evaluate the effect of -174G/C polymorphism of IL-6 gene among various populations. As shown in Table 3, significantly greater bladder cancer risks of 1.63 and 1.54 were found for the Asian population under the dominant (C/C+G/C vs. G/G) model and the recessive (C/C vs. G/C+G/G) model, respectively. However, no significant findings emerged for the Caucasian population. A study conducted by Joshi et al. reported that the relationships existed between IL-6 -174G/C polymorphism and genitourinary malignancies risk in the Indian population [18]. A meta-analysis study also showed that the IL-6 -174G/C polymorphism was significantly associated with the risk of various malignancies including colorectal cancer, breast cancer and leukemia [13].
There were some limitations of the present study. First, we only evaluated a single polymorphism (-174G/C) located in the promoter which cannot represent for the entire function of IL-6. Therefore, more functional SNPs of IL-6 gene are suggested to be included in a future study to replicate our findings. Second, since environmental risk factors including cigarette smoking, chronic arsenic, and occupational exposures were adjusted in a part of these included studies, the gene-environment interaction cannot be investigated in the present study. Therefore, based on the aforementioned potential limitations in our study, we have to pay attention to present these findings cautiously.
In conclusion, we performed a study to investigate the association between the IL-6 -174G/C polymorphism and bladder cancer risk and found that subjects who carried the C/C genotype of IL-6 gene had a significantly increased risk of bladder cancer. The major finding was that the highest significant risk of bladder cancer found for the Asian population. Further studies with a larger sample size are needed to validate the effects of IL-6 polymorphisms on bladder cancer risk.
Acknowledgements
This study was supported by grants from the National Science Council (Grant No: MOST 104-2314-B-038-079) and Taipei Medical University (Grant No: TMU103-AE1-B08), Taiwan.
Disclosure of conflict of interest
None.
References
- 1.Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, Sica A. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214:761–77. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Nesi G, Nobili S, Cai T, Caini S, Santi R. Chronic inflammation in urothelial bladder cancer. Virchows Arch. 2015;467:623–33. doi: 10.1007/s00428-015-1820-x. [DOI] [PubMed] [Google Scholar]
- 4.Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 5.Brumatti G, Salmanidis M, Ekert PG. Crossing paths: interactions between the cell death machinery and growth factor survival signals. Cell Mol Life Sci. 2010;67:1619–30. doi: 10.1007/s00018-010-0288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CC, Huang YK, Chung CJ, Huang CY, Pu YS, Shiue HS, Lai LA, Lin YC, Su CT, Hsueh YM. Polymorphism of inflammatory genes and arsenic methylation capacity are associated with urothelial carcinoma. Toxicol Appl Pharmacol. 2013;272:30–6. doi: 10.1016/j.taap.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Tawara K, Oxford JT, Jorcyk CL. Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Manag Res. 2011;3:177–89. doi: 10.2147/CMR.S18101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–76. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leibovici D, Grossman HB, Dinney CP, Millikan RE, Lerner S, Wang Y, Gu J, Dong Q, Wu X. Polymorphisms in inflammation genes and bladder cancer: from initiation to recurrence, progression, and survival. J. Clin. Oncol. 2005;23:5746–56. doi: 10.1200/JCO.2005.01.598. [DOI] [PubMed] [Google Scholar]
- 10.Ahirwar D, Kesarwani P, Manchanda PK, Mandhani A, Mittal RD. Anti- and proinflammatory cytokine gene polymorphism and genetic predisposition: association with smoking, tumor stage and grade, and bacillus Calmette-Guerin immunotherapy in bladder cancer. Cancer Genet Cytogenet. 2008;184:1–8. doi: 10.1016/j.cancergencyto.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Ebadi N, Jahed M, Mivehchi M, Majidizadeh T, Asgary M, Hosseini SA. Interleukin-12 and interleukin-6 gene polymorphisms and risk of bladder cancer in the Iranian population. Asian Pac J Cancer Prev. 2014;15:7869–73. doi: 10.7314/apjcp.2014.15.18.7869. [DOI] [PubMed] [Google Scholar]
- 12.Gautam KA, Muktanand T, Sankhwar SN, Goel A, Sankhwar PL, Rajender S. Functional polymorphisms in the IL6 gene promoter and the risk of urinary bladder cancer in India. Cytokine. 2016;77:152–6. doi: 10.1016/j.cyto.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Xu B, Niu XB, Wang ZD, Cheng W, Tong N, Mi YY, Min ZC, Tao J, Li PC, Zhang W, Wu HF, Zhang ZD, Wang ZJ, Hua LX, Feng NH, Wang XR. IL-6 -174G>C polymorphism and cancer risk: a meta-analysis involving 29,377 cases and 37,739 controls. Mol Biol Rep. 2011;38:2589–96. doi: 10.1007/s11033-010-0399-1. [DOI] [PubMed] [Google Scholar]
- 14.Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci U S A. 2010;107:5681–6. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tindall EA, Severi G, Hoang HN, Southey MC, English DR, Hopper JL, Giles GG, Hayes VM Australian Prostate Cancer BioResource. Interleukin-6 promoter variants, prostate cancer risk, and survival. Prostate. 2012;72:1701–1707. doi: 10.1002/pros.22557. [DOI] [PubMed] [Google Scholar]
- 16.Zhai K, Yang Y, Gao ZG, Ding J. Interleukin-6 -174G>C gene promoter polymorphism and prognosis in patients with cancer. Oncotarget. 2017;8:44490–44497. doi: 10.18632/oncotarget.17771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang K, Zhang L, Zhou J, Hao Z, Fan S, Yang C, Liang C. Association between interleukin-6 polymorphisms and urinary system cancer risk: evidence from a meta-analysis. Onco Targets Ther. 2016;9:567–77. doi: 10.2147/OTT.S94348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi N, Kannan S, Kotian N, Bhat S, Kale M, Hake S. Interleukin 6 -174G>C polymorphism and cancer risk: meta-analysis reveals a site dependent differential influence in Ancestral North Indians. Hum Immunol. 2014;75:901–8. doi: 10.1016/j.humimm.2014.06.018. [DOI] [PubMed] [Google Scholar]


