Abstract
Objective: This study aimed to evaluate whether exposure to bisphenol A (BPA) affects the ovarian reserve. Methods: Follicular fluid (FF) was collected from diminished ovarian reserve (DOR) and non-DOR patients who underwent in vitro fertilization or intracytoplasmic sperm injection. ELISA was used to detect the BPA and hormones levels in 54 cases of DOR and 67 cases of non-DOR. A total of 64, five-week-old SPF C57BL/6 mice were randomly divided into four groups, of which three were exposed to 5, 50, and 500 µg/kg/day of BPA solution, and one was exposed to con oil only as the control. The weight and estrus of each mouse were recorded daily, and the E2 hormone and anti-Müllerian hormone (AMH) in the serum were detected by ELISA. The expression levels of AMH mRNA and protein were also detected. Results: The BPA levels in the FF of DOR patients were significantly higher than those of non-DOR patients (234.048±81.736 ng/L vs. 193.300±67.225 ng/L, P<0.01); The AMH and E2 levels in the FF of DOR patients were lower than those of non-DOR patients ([555.689+74.224] pg/ml vs. [587.178+77.731] pg/ml, P<0.05, [209.720+31.556] pg/ml vs. [221.845+32.632] pg/ml, P<0.05). The BPA concentration was correlated with the AMH and E2 levels in the FF (rBPA & AMH=-0.312, P<0.05; rBPA & E2=-0.290, P<0.05); in the animal experiment, the levels of serum AMH and E2 as well as the expression levels of the AMH gene and protein in the BPA treatment group displayed downward trends. The concentrations of the 5 and 500 µg/kg/day groups decreased significantly (P<0.05). Conclusion: The increased BPA in the FF may promote the pathogenesis of DOR. BPA did not present a single-dose effect on the mouse ovary. Sub-chronic exposure to a low dose of BPA can reduce the ovarian reserve in female mice.
Keywords: Bisphenol A (BPA), follicular fluid, decreased ovarian reserve
Introduction
Bisphenol A (BPA) is an estrogen-like chemical that is extensively used in polycarbonate plastics and epoxy resins, and exposure to BPA is considered ubiquitous worldwide. This issue has raised concerns because of the increased use and continuous release of BPA into the environment. The global consumption of BPA in 2011 reached 5.5 million metric tons. In the United States, BPA was detected in 95% of urine samples with a median concentration of 1.28 μg/L [1-4]. Humans are directly exposed via interactions with consumer products containing this compound. In China, BPA has been detected in approximately 50% of blood samples and 90% of urine samples collected from the general population [5]. In 1988, the US Environmental Protection Agency declared for the first time that 50 µg/kg/day was the safe reference dose for humans, and this standard has been followed to date [6]. Whether this dose is safe for all tissues and organs is worth probing. BPA is suspected to cause problems relating to reproduction development and function. Considerable evidence from studies on laboratory animals, wildlife, and humans suggests that the increased incidence of female reproductive disorders, such as menstrual disorders, endometriosis, premature ovarian failure, and polycystic ovary syndrome (PCOS), is associated with various endocrine-disrupting chemicals (EDCs) [7-10].
Ovarian reserve refers to the ability to form fertilized oocytes in the cortex of the ovary. It includes the number of follicles and the quality of oocytes, making it an indicator of female fertility. Diminished ovarian reserve (DOR) refers to the decrease in the number of raised follicles and the quality of oocytes in the ovary. DOR manifests as menstrual disorders, menstrual thinning, amenorrhea, infertility, and other symptoms, all of which lead to the reduced fertility of women. The clinical indicators of DOR include decreased levels of estrogen and anti-Müllerian hormone (AMH) and a reduced number of antral follicles (antral follicle count, AFC). The incidence of DOR accounts for 10% of infertility cases, and DOR typically develops into premature ovarian failure within 1-6 years. Although in vitro fertilization (IVF) and embryo transplantation have become routine methods for addressing infertility, DOR patients generally present a lower number of oocytes, a low pregnancy rate, a high cycle cancelation rate, a high abortion rate, and increased aneuploidy risk, all of which severely affect women’s reproductive health. The etiology of DOR remains unclear to date. DOR may be related to genetic and metabolic abnormalities, autoimmune diseases, iatrogenic injury, infection, and environment. AMH is a glycoprotein produced by the granulosa cells of preantral and small antral follicles. Its expression is flanked by two major regulatory steps of folliculogenesis, first appearing in the granulosa cells of primary follicles and being the strongest in preantral and small antral follicles. AMH can be directly used to reflect the state of the ovary and assess the ovarian function [11,12]. Associated with the growth and development of follicular fluid (FF), AMH can be used to accurately assess the ovarian reserve capacity.
In recent years, the incidence of DOR has been increasing among the younger population. The influence of environmental factors on reproductive health has been extensively studied. Studies have shown that poor environmental factors can accelerate the decline of the ovarian reserve in females. However, few studies have explored the relationship between BPA and DOR. Thus, we chose FF as an ideal biological specimen. The correlation between BPA and DOR was investigated by detecting the concentration of BPA and hormone levels in FF. The findings were validated on animal specimens. We intend to provide insight into and clinical evidence for the mechanism of the occurrence and development of DOR.
Materials and methods
Ethical approval
This study was approved by the Ethics Committee of the Zhongnan Hospital of Wuhan University. All patients provided informed consent before we collected the specimens. During the entire experiment, the mice were humanely treated. Before being sacrificed, all mice were anesthetized, and all operations were designed to minimize suffering. All experimental procedures were approved by the A3 Animal User, Wuhan University.
Study objects and sampling
A total of 54 DOR patients and 67 non-DOR patients who underwent IVF or intracytoplasmic sperm injection (ICSI) were enrolled in the reproductive medicine center of Zhongnan Hospital of Wuhan University from November 2015 to November 2016.
Incision and exclusion criteria
For the experimental group, the inclusion criteria included patients who met the DOR diagnostic criteria and provided informed consent. The exclusion criteria included patients who used hormone drugs within the past three months and those who have hyperprolactinemia, PCOS, endometriosis, ovarian surgery, and thyroid or adrenal endocrine diseases.
For the control group, the exclusion criteria included patients in the experimental group who used hormone drugs in the past three months and have hyperprolactinemia, PCOS, endometriosis, ovarian surgery, and thyroid or adrenal endocrine diseases. The inclusion criterion was patients who did not receive received IVF/ICSI treatment.
Collected samples and outcome index
Oocytes were collected by vaginal puncture under the guidance of a transvaginal ultrasound. Clear FF without blood was collected using pickling glass bottles. The supernatant was centrifuged and stored at 80°C for testing. The serum levels of basic hormones (AMH and E2) were examined by the laboratory of our hospital. The BPA and AMH levels in the FF were detected by using an ELISA Kit (Wuhan Hualian Biological Corporation), and the operation was conducted in accordance with the kit’s instructions.
Collection of mouse samples
Five-week-old SPF C57BL/6 female mice were purchased and housed at the A3 animal experiment center of Wuhan University. The mice were raised in a climate-controlled (21±2°C) animal room under a constant 12 h light/dark cycle, with unlimited access to mice chow. The mice were allowed to acclimate to the facility for one week before disposal. During this period, the vaginal opening of the female mice was checked, and the normalcy of their sexual cycle was verified. A total of 64 mice were randomly divided into four groups, of which three were given different doses of BPA (5, 50, and 500 µg/kg/day) and one was exposed to corn oil only as the control, under continuous lavage treatment for 28 days. All BPA concentrations were first dissolved in the same dose of ethanol and then diluted with corn oil.
Estrous cycles of each group
The estrous cycles of the mice were determin-ed by daily examinations for vaginal smears (between 08:00 and 10:00 AM). Vaginal secretion was collected using a pipetting device filled with 10 µL of normal saline (NaCl 0.9%). The tip of the pipette was inserted into the vagina at a depth of 2-5 mm. Then, normal saline was flushed into the vagina and returned into the pipette by gently squeezing and releasing the bulb of the pipette. These steps were repeated for three times before the sample was collected. Subsequently, a drop of the cell suspension was smeared onto a labeled glass slide, which was then dyed with a pap dyeing liquid and observed under a light microscope.
Collection of serum samples and hormone assays
Blood samples were collected from the angular vein after anesthesia and stored at 4°C static. After 24 h, each blood sample was centrifuged at 4°C and 3000 r/min for 15 min to separate the serum components. Serum E2 and P4 were detected by using an ELISA reagent kit in accordance with the manufacturer’s instructions. All samples were measured in duplicate to ensure that the data had no statistical difference in the same group.
Tissue collection and pathological section
After the blood was collected, the animals were sacrificed. The ovary was immediately stripped, and the fat and connective tissues surrounding this organ were removed. Half of the ovary was fixed in 4% paraformaldehyde, and the other half was snap-frozen in liquid nitrogen. After 24 h, the ovarian tissue was removed from the paraformaldehyde, transferred to 70% ethanol, cleared in xylene, embedded in paraffin, and sectioned. The ovary tissue was serially sectioned into 5 μm slices, mounted onto glass slides, and stained with hematoxylin and eosin.
RNA isolation and real-time quantitative polymerase chain reaction (PCR)
RNA was extracted using TRIzol (Invitrogen), and the concentration was determined using an ND-1000 spectrophotometer (λ=260/280 nm; NanoDrop). Messenger RNA (mRNA: 2 ng) was reverse-transcribed to cDNA and subjected to a quantitative real-time PCR by using the CFX96 Real-Time PCR Detection System (Bio-Rad Inc.) and accompanying software (CFX Manager Software). Primers were designed by BYE Biotechnology Company. The regular cycling program consisted of a 15 min hold at 95°C and 45 cycles of denaturing at 95°C for 15 s, annealing at 58°C for 15 s, and an extension at 72°C for 20 s, at which point the data were acquired. All samples were run in triplicate, and the mean value was used for determining the mRNA levels. Each sample was normalized to B-actin prior to the quantification. The fold-change in the gene expression was quantified by 2-ΔΔCt method (Table 1).
Table 1.
Primer sequence of fluorescent quantitative PCR
| Primer | Primer sequence | Bp | |
|---|---|---|---|
| AMH | M | 5’-AGC CAG TTT CCG CAT CTA CC-3’ | 244 |
| R | 5’-GTC AGG TAG CGG TTG AAA TGG-3’ | ||
| B-actin | M | 5’-TGA AGG GTG GAG CCA AAA G-3’ | 150 |
| R | 5’-AGT CTT CTG GGT GGC AGT GAT-3’ |
Immunohistochemistry
The paraffin blocks were cut into sections and mounted on slides. The sections were incubated with the polyclonal AMH antibody (1:100 dilution; Sigma, St Louis, MO, USA) at 4°C overnight. A biotinylated secondary antibody and horseradish peroxidase (HRP) conjugated streptavidin were added onto the ovary sections after the slides were washed twice with TBS. The expression was visualized with 3,3-diaminobenzidine (DAB) substrate and observed under microscopy (OLYMPUS BX51). The result was analyzed based on the area of stained cells and the intensity of the cells to realize a comprehensive evaluation. The scoring criteria for the dyeing area were 0: <5% of the stained cells; 1: 5%-25%; 2: 25%-50%; 3: 50%-75%; and 4: >75%. The standards for cell staining intensity were no color: 0 points; light yellow: 1 point; brown: 2 points; and dark brown: 3 points. Comprehensive score = the score of staining cell area × the score of cells staining intensity.
Statistical analysis
SPSS 17.0 statistical software was used for data analysis. The data were expressed as means ± SEM. The Pearson analysis was adopted to study the correlation among the continuous variables. A one-way analysis of variance with the LSD test was applied for multiple-group comparisons. All statistical tests were two-sided, and the level of statistical significance was set to P<0.05.
Results
Comparison of BPA and hormone levels in FF
As shown in Table 2, the BPA levels in the FF of the DOR patients were significantly higher than those of the non-DOR patients (234.050±81.736 ng/L vs. 193.300±67.225 ng/kg/L, P<0.01). The levels of AMH and E2 in the FF of the DOR patients were lower than those of the non-DOR patients (555.690±74.224 pg/mL vs. 587.180±77.731 pg/mL and 209.720±31.556 pg/mL vs. 221.850±32.632 pg/mL P<0.05).
Table 2.
Analysis of hormone concentration in follicular fluid
| Hormone | Non-DOR | DOR | P |
|---|---|---|---|
| BPA (ng/L) | 193.300±67.225 | 234.050±81.736 | <0.01 |
| E2 (pg/mL) | 221.850±32.632 | 209.720±31.556 | <0.05 |
| AMH (pg/mL) | 587.180±77.731 | 555.690±74.224 | <0.05 |
Correlation analysis of BPA level and hormone concentration in FF
As shown in Table 3, the BPA concentration was negatively correlated with the AMH (r=-0.290) and E2 concentrations (r=-0.312) in the FF of the DOR patients.
Table 3.
Analysis of hormone concentration in follicular fluid
| Hormone | R | 95% CI | P |
|---|---|---|---|
| E2 (pg/mL) | -0.290 | 0.308-0.824 | <0.05 |
| AMH (pg/mL) | -0.312 | 0.196-0.634 | <0.05 |
Effect of BPA on the estrous cyclicity of the mice
The estrus of the mice is presented in Figure 1. Compared with the control group, the experimental groups spent a longer time in diestrus but less time in estrus and proestrus. The cyclicity of the 5 and 500 µg/kg/day of the BPA exposure groups was significantly decreased (P<0.05). The changes of estrus cycle are shown in Table 4.
Figure 1.
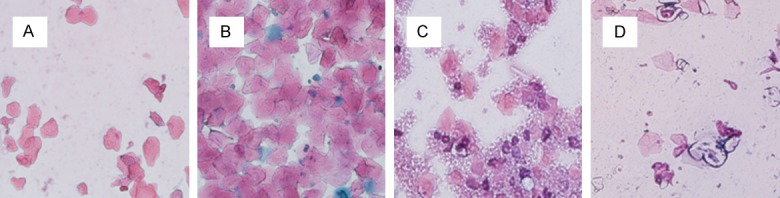
Effects of chronic exposure to BPA on the estrous cycle in mice. Picture magnification 20×. A: Proestrus: much nucleated epithelial cells, and less keratin epithelial cells; B: Estrus: keratin epithelial cells from scattered to agglomerate, less nucleated epithelial cells; C: Metaestrus: keratin epithelial cell accumulation, many nucleated epithelial cells and white blood cells; D: Diestrus: white blood cells and less mucus.
Table 4.
Effects of BPA on estrous cycle in female rat
| Groups | n | Proestrus | Estrus | Metestrus | Diestrus |
|---|---|---|---|---|---|
| Control | 16 | 0.7±0.67 | 2.7±0.67 | 2.3±0.48 | 4.2±1.32 |
| 5 ug/kg/d | 16 | 0.2±0.42* | 2.1±0.57* | 2.2±0.63 | 5.8±1.03* |
| 50 ug/kg/d | 16 | 0.6±0.42 | 2.6±0.32 | 2.1±0.32 | 4.3±0.63 |
| 500 ug/kg/d | 16 | 0.1±0.32* | 1.4±0.84** | 2.1±0.88 | 6.2±1.14** |
P<0.05 VS. control;
P<0.01 VS. control.
Effects of BPA on the levels of serum hormone in mice
The concentration of serum AMH and E2 in the BPA-treated groups was lower than that of the control group. The levels of serum hormones in the 5 and 500 µg/kg/day of BPA exposure groups were significantly decreased (Figure 2). The main serum hormones are shown in Table 5.
Figure 2.
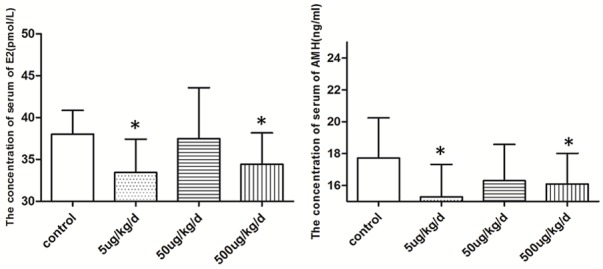
Comparison of E2 and AMH levels between the 4 groups (*P<0.05 vs. control). The serum estrogen and AMH were decreased obviously, the 5 ug/kg/d and 500 ug/kg/d of BPA expose decreased obviously compared with control.
Table 5.
Effects of BPA on serum hormone levels
| Group | E2 | P | AMH | P |
|---|---|---|---|---|
| Control | 38.02±2.84 | 17.72±2.53 | ||
| 5 ug/kg/d | 33.47±3.96* | <0.05 | 15.29±2.04* | <0.05 |
| 50 ug/kg/d | 37.50±6.07 | >0.05 | 16.30±2.28 | >0.05 |
| 500 ug/kg/d | 34.42±3.75* | <0.05 | 16.09±1.92* | <0.05 |
P<0.05 Vs. control.
Effects of BPA on the pathological changes of ovarian tissues
In the experimental group, the structure of the granulosa cells in the ovary displayed various changes, such as loosening, degeneration, a widening of the gap between the granulosa cells and the membrane cells, degeneration of the edema, nuclear pyknosis, nuclear fragmentation, and other pathological changes. The ovarian tissue in the control group had a normal structure and did not display the pathological changes of inflammation and edema (Figure 3).
Figure 3.
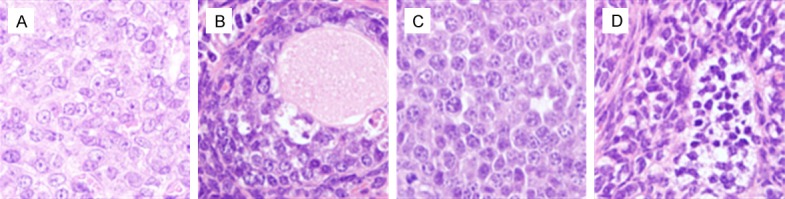
Effects of Pathological changes of ovary by chronic exposure to BPA in mice. A: Control; B: 5 ug/kg/d BPA; C: 50 ug/kg/d BPA; D: 500 ug/kg/d BPA; Picture magnification 20×.
Effects of BPA on changes in the mRNA expression of AMH
In the experimental group, the expression of AMH mRNA was significantly decreased (Figure 4) in the 5 and 500 µg/kg/day of BPA exposure groups (P<0.05) (Table 6).
Figure 4.
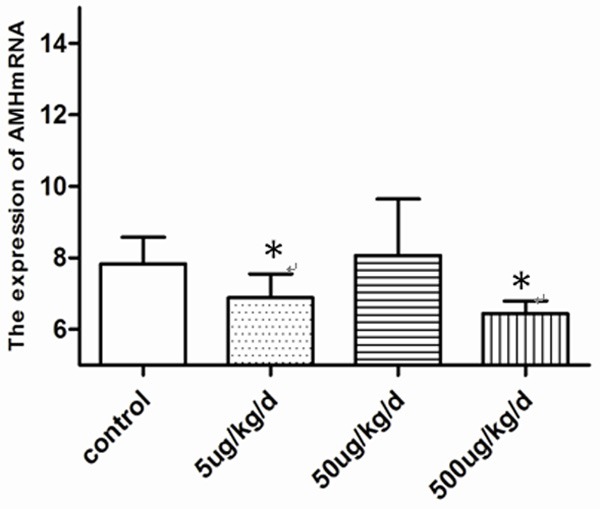
Effects of BPA on Changes in the mRNA expression of AMH, the 5 ug/kg/d and 500 ug/kg/d of BPA expose group was decrease obviously compared with control (*P<0.05 vs. control).
Table 6.
Effects of BPA on expression of AMH
| Group | Num | AMH mRNA | P | AMH protein | P |
|---|---|---|---|---|---|
| Control | 16 | 7.84±0.74 | 6.96±2.09 | ||
| 5 ug/kg/d | 16 | 6.89±0.67* | <0.05 | 5.43±1.86* | <0.05 |
| 50 ug/kg/d | 16 | 8.07±1.58 | >0.05 | 6.53±1.74 | >0.05 |
| 500 ug/kg/d | 16 | 6.45±0.35* | <0.05 | 5.35±1.92* | <0.05 |
P<0.05 Vs. control.
Effects of BPA on the changes in the protein expression of AMH
As shown in Figure 5, the experimental group had a lower expression of AMH protein than the control. The expression levels in the 5 and 500 µg/kg/day BPA exposure groups were significantly lower than those of the control (P<0.05) (Table 6).
Figure 5.
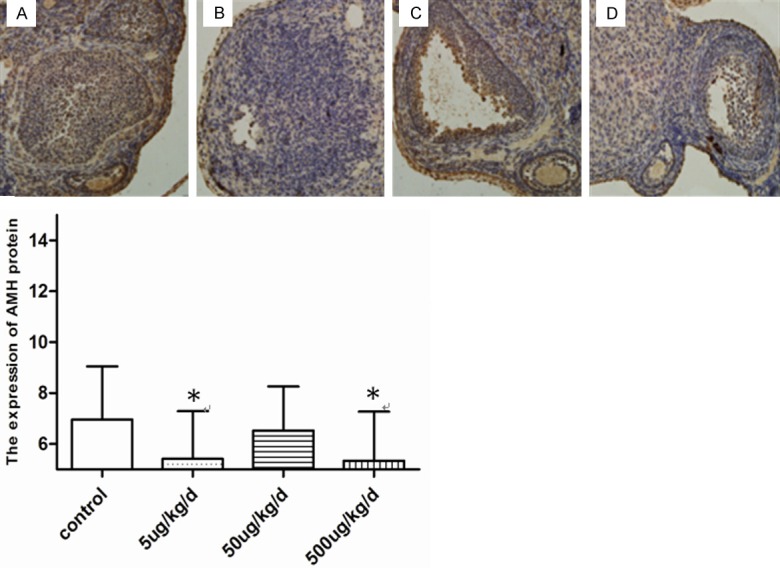
The protein expression of AMH in ovary exposed to BPA. A: Control; B: 5 ug/kg BPA; C: 50 ug/kg BPA; D: 500 ug/kg BPA. Picture magnification 20×. The expression at the 5 ug/kg/d and 500 ug/kg/d BPA exposure was obviously lower than the control (*P<0.05 vs. control).
Discussion
In view of the widespread use of plastic products, the general population is exposed to BPA. Epidemiology shows that BPA can be detected in more than 90% of collected urine samples. Severe exposure to BPA affects the female reproductive system. Considerable attention has been devoted to BPA because of its severe toxic effects. We found that BPA can be detected in female FF, and the decline in the ovarian reserve is associated with an increased BPA concentration. The E2 and AMH levels in the FF of the DOR patients were low. This finding was verified by the animal exposure experiments, particularly at the concentration of 5 and 500 µg/kg/day of BPA. We know that the synthesis of AMH originated from the granulosa cells of preantral follicles and small antral follicles. We hypothesized that BPA may reduce the ovarian granulosa cell activity and accelerate its apoptosis, leading to the decreased synthesis of AMH and the diminished AMH levels in the serum and FF. The DOR patients had a lower number of oocytes and oocyte recovery. We speculated that the high BPA level in the FF may influence the onset of DOR. The animal experiment revealed that BPA exposure hindered the development of the ovarian reserve when ovarian function just begins, and these effects were severe even at low doses. Based on previous studies on the exposure to high doses of BPA, we inferred that BPA exerts a non-monotonic dose effect. That is, a BPA dose lower than the safety reference dose of BPA can also affect the organism. This effect could be intensified, and its role differed from that of high-dose BPA. The effect of BPA on ovarian reserve function did not display a single-dose effect but an obvious “U” type. BPA had different mechanisms in the entire dose range, and the type of mechanism may have a special biological effect of hormone receptor mediated or antagonized at low doses. When the dose of BPA increased to a certain extent, its action and hormone receptor reached saturation and then showed an acute toxicity mechanism at a higher dose. As early as 2004 [13], Zala had pointed out that in the toxicological studies of endocrine-disrupting chemicals, this non-monotonic effect reltionship is a rule rather than a special case.
Most of the follicles in the ovary cannot mature. Only the dominant follicle can ultimately develop into a mature follicle and complete the ovulation process. We observed the ovarian tissue and found that BPA induced serious ovarian toxicity. BPA exposure impaired the structure of the ovarian tissue. The structure of the ovarian tissue was normal in the control group. By contrast, upon BPA exposure, the structure of the granular cells exhibited loosening, edema, degeneration, nuclear pyknosis, and nuclear fragmentation. The gap between the granular cells and the membrane cells widened. The structure of the corpus luteum cells became loose. Then, the plasma cells and eosinophils infiltrated. The follicle is the main functional unit of the ovary and is under the control of the pituitary gonadotropin and sex hormones. Follicles are present in different stages of development, such as corpus luteum and atresia. Follicularis is developed in the process of dynamic development.
By accurately observing the estrous cycle changes of the female mice with a normal sexual cycle, we found that chronic exposure to a low dose of BPA decreased the estrus and proestrus of the mice but significantly prolonged the diestrus. Wang’s [14] research found that exposure to a low dose of BPA in utero shortened the estrus and extended the metestrus and the diestrus. BPA was speculated to destroy the germ cell nest, which diminished the fertility of the mice. Their results were similar to our findings. The normal sexual cycle of a female animal is 4-5 days, which is typically divided into proestrus, estrus, metestrus, and diestrus. Examination of the vulva state combined with vaginal smears can effectively determine the estrous cycle of mice. The estrous cycle was also related to serum hormone levels, which can affect the development of follicles, ultimately hampering the ovulation. BPA may inhibit the secretion of estrogen before ovulation by changing the morphology and function of the ovarian granulosa cells. Consequently, the feedback of FSH increased again, and it could not stimulate the appearance of the LH peak, leading to the atrophy of the granulosa cells and the small size of the follicles. Exposure to BPA reduced the activity of the granulosa cells, and more granular cells were arrested at the G2-M phase. The production of steroid hormone synthetase was inhibited, and the synthesis of E2 hormones was ultimately reduced. Therefore, BPA decreased the E2 concentration by affecting the function of the granulosa cells, which in turn influenced the growth of follicular cells and the maturation of oocytes, ultimately reducing the ovarian reserve.
AMH is sex specific and time dependent in the development of mammals. The defects in AMH expression during the embryonic period can result in hermaphroditism. It plays an important role in the growth and development of follicular cells. AMH is highly expressed in follicular cells, and it decreases with the disappearance of germ cells. The expression of AMH was low in atresia follicles, indicating that the development of primordial follicles into growth follicles was affected. Sub-chronic exposure to BPA reduced the expression of AMH in the ovary of mice, implying that BPA can accelerate the apoptosis of granulosa cells and follicle atresia. Durlinger’s work [15] indicated that AMH-gene-deficient mice had nearly three times smaller follicles than those of wild-type mice born in the same litters. The primordial follicles in the ovary were depleted with the growth of the mouse. BPA can affect the secretion hormones of granulosa cells and the meiotic process to accelerate the apoptosis of ovarian granulosa cells and follicular atresia. BPA may accelerate the apoptosis of granulosa cells and the atresia of follicles by interfering with the meiosis of germ cells, which reduces the expression of AMH. With the lack of AMH, primordial follicles would be increased at a faster rate, leading to the premature maturation of the follicular pool and a shortened reproductive lifespan.
In summary, our study determined that BPA is involved in the reduction of the ovarian reserve. Even sub-chronic exposure to low doses of BPA can decrease the ovarian reserve. However, the effects of BPA on the number of small antral follicles and the composition of follicles at all levels require further study.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of china (Grant No.81370707).
Disclosure of conflict of interest
None.
References
- 1.Ganesan S, Keating AF. Bisphenol a-induced ovotoxicity involves DNA damage induction to which the ovary mounts a protective response indicated by increased expression of proteins involved in DNA repair and xenobiotic biotransformation. Toxicol Sci. 2016;52:169–80. doi: 10.1093/toxsci/kfw076. [DOI] [PubMed] [Google Scholar]
- 2.Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol a exposure on the ovaries in multiple generations of mice. Reprod Toxicol. 2016;60:39–52. doi: 10.1016/j.reprotox.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flint S, Markle T, Thompson S, Wallace E. Bisphenol a exposure, effects, and policy: a wildlife perspective. J Environ Manage. 2012;15:19–34. doi: 10.1016/j.jenvman.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol a and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–5. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang T, Sun HW, Kannan K. Blood and urinary bisphenol a concentrations in children, adults and pregnant women from China: partitioning between blood and urine and maternal and fetal cord blood. Environ Sci Technol. 2013;47:4686–94. doi: 10.1021/es303808b. [DOI] [PubMed] [Google Scholar]
- 6.EPA. Health assessment information on bisphenol a (CASRN80-05-7) 1988. http://cfpub.epa.gov/ncea/iris/index.cfm?fuseaction=iris.showQuickview&substance_nmbr=0356#reforal (1988)
- 7.Forte M, Mita L, Cobellis L, Merafina V, Specchio R, Rossi S, Mita DG, Mosca L, Castaldi MA, De Falco M, Laforgia V, Crispi S. Triclosan and bisphenol a affect decidualization of human endometrial stromal cells. Mol Cell Endocrinol. 2016;15:74–83. doi: 10.1016/j.mce.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Bruner-Tran KL, Gnecco J, Ding T, Glore DR, Pensabene V, Osteen KG. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: translating lessons from murine models. Reprod Toxicol. 2017;68:59–71. doi: 10.1016/j.reprotox.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanley JA, Arosh JA, Burghardt RC, Banu SK. A fetal whole ovarian culture model for the evaluation of crvi-induced developmental toxicity during germ cell nest breakdown. Toxicol Appl Pharmacol. 2015;289:58–69. doi: 10.1016/j.taap.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vahedi M, Saeedi A, Poorbaghi S, Sepehrimanesh M, Fattahi M. Metabolic and endocrine effects of bisphenol a exposure in market seller women with polycystic ovary syndrome. Environ Sci Pollut Res Int. 2016;23:23546–23550. doi: 10.1007/s11356-016-7573-5. [DOI] [PubMed] [Google Scholar]
- 11.Ota T, Asahina H, Park SH, Huang Q, Minegishi T, Auersperg N, Leung PC. HOX cofactors expression and regulation in the human ovary. Reprod Biol Endocrinol. 2008;6:49. doi: 10.1186/1477-7827-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristensen SG, Ebbesen P, Andersen CY. Transcriptional profiling of five isolated sizematched stages of human preantral follicles. Mol Cell Endocrinol. 2015;5:189–201. doi: 10.1016/j.mce.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Zala SM, Penn DJ. Abnormal behaviours induced by chemical pollution: a review of the evidence and new challenges. Anim Behav. 2004;68:649–664. [Google Scholar]
- 14.Wang W, Hafner KS, Flaws JA. In utero bisphenol a exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276:157–164. doi: 10.1016/j.taap.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. control of primordial follicle recruitment by antimullerian hormone in mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]


