Abstract
Background: Fibrin and cytokines in platelet-rich fibrin (PRF) can be combined into a powerful biological scaffold, which is an integrated reservoir of growth factors involved in tissue regeneration. Insulin-like growth factor-1 (IGF-1) is a kind of effective mitogenic protein, which can enhance osteogenic differentiation of periodontal ligament fibroblasts. However, whether PRF and IGF-1 can stimulate the osteogenic differentiation and osteogenesis of human periodontal ligament stem cells (PDLSCs) remains unclear. This study aims to investigate the osteogenic capability of PDLSCs in vitro and in vivo after being separated from human PDL tissues, purified with STRO-1 and treated with PRF and IGF-1. Methods: The proliferative capabilities of PDLSCs under different conditions were analyzed via methyl thiazolyl tetrazolium (MTT), growth curve, alkaline phosphatase activity, reverse transcription-polymerase chain reaction (RT-PCR) and Western blotting, respectively. Results: PRF and IGF-1 significantly promoted the growth, proliferation and differentiation of PDLSCs, up regulated the expressions of Runt-related transcription factor 2 (RUNX2), osterix (OSX) and osteocalcin (OCN), phosphorylated extracellular signal-regulated kinase (ERK) and phosphorylated c-Jun N-terminal kinase (JNK) in stem cells. Conclusion: Our data indicate that PRF and IGF-1 facilitate the proliferation of alveolar osteoblast via the activation of the mitogen-activated protein kinase (MAPK) signaling pathway.
Keywords: PRF, IGF-1, osteoblast, proliferation, molecular mechanism
Introduction
Periodontitis represents a global medical problem resulting in irreversible damage to periodontal support tissues, namely the alveolar bone, the periodontal ligament (PDL), and the tooth root cementum. According to the database of the World Health Organization (WHO), it is estimated that severe periodontal disease affects 5-20% population worldwide. It is the main cause of tooth loss, and is also associated with many systemic diseases, such as diabetes mellitus and hypertension. In modern clinical practice, a wide range of treatment methods have been adopted, such as barrier membrane, autografts, demineralized lyophilized bone allografts, bovine-derived xenografts and a combination of membrane and fillers. However, few of them are considered real regeneration techniques, while limited success and unpredictable results frequently occur [1].
Since the discovery of adult periodontal ligament stem cells (PDLSCs) in 2004, periodontal tissue regeneration based on stem cells has become a hot topic in dentistry [2]. In addition to the ecological niches of stem cells and biomimetic materials, appropriate insulin-like growth factor (IGF) is also required for functional periodontal tissue regeneration [3,4]. In general, platelet-rich fibrin (PRF) and IGF-1 bind to receptors on the cell surface to mediate their effects, activate the intrinsic tyrosine kinase activity in receptors, and internalize the receptor ligand complex. It therefore induces a signaling cascade and ultimately affects gene expression and protein synthesis. Many studies have proved that IGF-1 is a key protein in the development and growth of many tissues [5-9], among which, a multifunctional peptide regulates cell growth, differentiation and extracellular matrix protein expression. Moreover, IGF-1 is considered as a key mediator in wound healing and mesenchymal cell proliferation. Raja et al reported that IGF-1 enhanced cell survival in PDL fibroblasts by up-regulating anti-apoptotic molecules and down-regulating pro-apoptotic molecules [10]. Local and controlled release of IGF-1 from the glucan co-gelatin microspheres can improve periodontal regeneration [11]. Fibrin gels utilize the final stage of the coagulation cascade, among which fibrinogen molecules self-assemble into highly-biocompatible three-dimensional fiber networks. Fibrin and cytokines in PRF are combined into a powerful biological scaffold, which is an integrated reservoir of growth factors used for tissue regeneration [12]. The applicability of PRF as a bioactive platform has been proved in many studies that clarify the proliferation and differentiation of osteoblasts and gingival fibroblasts [13,14]. Clinical studies have shown that PRF promotes soft tissue and bone regeneration [15,16], as well as periodontal tissue regeneration [17,18]. The capacity of PRF to enhance and regenerate damaged tissues may be associated with the combination with bone substitutes, such as Bio-Oss or autologous bone [19,20].
In this study, we aimed to determine the effect of exogenous PRF and IGF-1 on the osteogenesis of PDLSCs.
Materials and methods
Materials
Type I collagenase was bought from Sigma (Temecula, CA, USA). The dispase was provided by Roche, (Indianapolis, IN, USA). α-minimum Eagle’s medium (MEM) was obtained by Gibco (Waltham, MA, USA). The ascorbic acid 2-phosphate was purchased from Sigma (Temecula, CA, USA). The glutamine and double antibodies were obtained from Gibco (Waltham, MA, USA). The rabbit anti-STRO-1 antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Goat anti-rabbit immunoglobulin G (IgG) Dynabeads was provided from Dynal Biotech (Oslo, Norway). The cytokeratin and vimentin antibodies were acquired from Bioworld Technology (Atlanta, GA, USA). The IGF-1 powder was from Peprotech (Offenbach, Germany). The methyl thiazolyl tetrazolium (MTT) was purchased from Sigma (Temecula, CA, USA). The automatic enzyme-linked immunosorbent assay (ELISA) tester ELX800 was from BioTek Instruments Inc. (Norcross, GA, USA). The SYBR®Premix Ex Taq™ kit was provided by Takara (Kusatsu, Shiga, Japan). Primers were all synthesized by Sangon Biotech (Shanghai, China) Runt-related transcription factor 2 (RUNX2) antibody, extracellular signal-regulated kinase 1/2 (ERK1/2) antibodies, phosphorylated-ERK1/2, c-Jun N-terminal kinase 1/2/3 (JNK1/2/3), phosphorylated-JNK1/2/3, P38 and phosphorylated-P38 Osterix (OSX) and osteocalcin (OCN) antibodies were purchased from Abcam (Cambridge, MA, USA).
Cell separation and culture
A total of 15 subjects aged 18-25 years old with normal, non-carious third molars were collected from the Department of Stomatology, Wuhan University Stomatological Hospital, Hubei Province. PDL was gently separated from the root surface and digested. PDL samples from different individuals were put together, and cells were filtered through a 70 μm filter to obtain the single cell suspension. PDLSCs derived from multiple colonies were separated in the α-MEM containing 10% fetal bovine serum (FBS), 100 μmol/L ascorbic acid 2-phosphate, 2 mmol/L glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin and cultured under 5% CO2 at 37°C. These cells were purified using rabbit anti-STRO-1 antibody and goat anti-rabbit IgG Dynabeads. To determine their mesenchymal origin, immunostaining was performed for PDLSCs with cytokeratin and vimentin antibodies. After passage 2-4 times, the cells were used for subsequent experiments.
Preparation of PRF conditioned medium: 10 mL precaval venous blood was collected from a sow aged 3.1 months old on average and put into a 10 mL glass-coated tube without anticoagulant. The whole blood platelet count was 105/μL. The sample was immediately centrifuged at 2100 rpm for 12 min using a Beckman centrifuge. The PRF clot was concentrated in the middle of the centrifuge tube, separated, and gently compressed using gauze to drain the remaining fluid. The PRF membrane was immersed into 3 mL fresh Dulbecco’s modified Eagle medium (DMEM), and the medium was collected once every 48 h as the PRF conditioned medium.
MTT assay
IGF-1 powder was dissolved in sterile distilled water for standby application. The proliferation of PDLSCs was detected via an MTT assay based on the mitochondrial succinate dehydrogenase method of proliferating cells, in which MTT was reduced to the insoluble purple formazan reaction product and could be detected via a colorimetric assay. Briefly, cells were cultured in a 96-well plate until they covered 60% of the bottom at an initial density of 3×103 cells/well using the non-conditioned medium (α-MEM containing 10% FBS) and PRF conditioned medium, and then starved with serum for 24 h. IGF-1 (25, 50, 100 and 200 ng/mL) was added into the α-MEM containing 10% FBS in the experimental group, respectively. After 7 d, MTT (5 mg/mL) was added for incubation at 37°C for 4 h, and the mixture was washed with 10 mM phosphate buffered saline (PBS) for 3 times. The absorbance at 490 nm was read using the automatic ELISA tester. In the experiment, cells were divided into 4 groups: non-treated medium group (Control), PRF-treated medium group (PRF), IGF-1 + non-treated medium group (IGF-1), and IGF-1 + PRF-treated medium group (IGF-1 + PRF). The MTT results were presented as mean ± standard deviation (SD), and the experiment was repeated 3 times.
Cell growth curve
To analyze the growth kinetics of PRF + IGF-1-treated PDLSCs, cells were inoculated into the 96-well plate (3×103 cells/well), and PRF medium and non-treated medium were set. After serum starvation, the IGF-1-treated group was also set. 100 ng/mL IGF-1 was added to the PRF-treated medium and the non-treated medium, respectively. The medium was replaced once every 2 d. Cells were collected and counted after trypan blue staining every day for 9 consecutive days. The independent growth kinetics experiment was performed 3 times on each group. The curves were drawn based on the average cell count, and the population doubling time (PDT) was calculated according to the Patterson formula (Patterson 1979).
Alkaline phosphatase (ALP) activity
To evaluate the osteogenic differentiation of these stem cells, PDLSCs untreated and treated with IGF-1 were inoculated into a 24-well plate at a density of 1×104 cells/well, and the PRF conditioned medium and non-conditioned medium were set, respectively. Then cells were incubated in the α-MEM containing 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L L-glutamine, 50 mg/L ascorbic acid, 10 mmol/L β-glycerophosphate and 10 nmol/L dexamethasone (Sigma). ALP activity and calcium deposition were evaluated at different time points. Another 24-well plate inoculated with PDLSCs at the same density was used to detect the protein concentrations in different groups at different time points. The ALP activity was determined using the ALP kit as previously described [21]. Six parallel replicates were analyzed in each group.
Detection of RUNX2, OSX and OCN Messenger ribonucleic acid (mRNA) expressions via real-time reverse transcription-polymerase chain reaction (RT-PCR)
PDLSCs untreated and treated with PRF and IGF-1 were co-cultured with the osteogenic medium. After culture for 3 d, 7 d, and 14 d, total RNA was extracted using TRIzol reagent and its concentration was determined. Complementary deoxyribonucleic acid (cDNA) was synthesized via real-time RT-PCR using the SYBR®Premix Ex Taq™ kit in the quantitative PCR system (ABI 7300, Waltham, MA, USA). Real-time RT-PCR conditions were as follows: 95°C for 30 s, 95°C for 5 s, 40 cycles, and 60°C for 30 s finally. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The relative expressions were calculated based on the ratio of the copy number of target genes (RUNX2, OSX and OCN) in each sample to GAPDH. Primers of RUNX2, OSX and OCN are shown in Table 1.
Table 1.
Primers used in real-time RT-PCR
| Gene | Primer sequence |
|---|---|
| RUNX2 | TCTTAGAACAAATTCTGCCCTTTTGCTTTGGTCTTGAAATCACA |
| OSX | CCTCCTCAGCTCACCTTCTCGTTGGGAGCCCAAATAGAAA |
| OCN | AGCAAAGGTGCAGCCTTTGTGCGCCTGGGTCTCTTCACT |
| GAPDH | GAAGGTGAAGGTCGGAGTCGAGATGGTGATGGGATTTC |
Western blotting
PDLSCs untreated and treated with IGF-1 were cultured for 3 d, 7 d, and 14 d in the osteogenic medium, and the non-treated and PRF-treated groups were set. The mitogen-activated protein kinase (MAPK) pathway was evaluated: PDLSCs were inoculated into the 60 mm culture dish, incubated for 24 h, starved with serum for 48 h, and then treated with 100 ng/mL IGF-1 for 30, 60, and 90 min. Cells in different groups were collected, washed twice with cold PBS and lysed in the radioimmunoprecipitation assay (RIPA) lysis buffer containing 1 mM phenylmethylsulfonyl fluoride and phosphatase inhibitor. Cell debris was removed via centrifugation at 12000 rpm at 4°C for 15 min. The protein concentration was determined via the Bradford protein assay. 40 μg protein was loaded onto the 10% gel for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a 0.22 μm polyvinylidene fluoride (PVDF) membrane at 300 mA in a blotter and sealed with 5% skim milk powder at room temperature for 2 h. Then the primary antibodies of RUNX2, OSX, OCN, ERK1/2, phosphorylated-ERK1/2,1, JNK1/2/3, phosphorylated-JNK1/2/3, P38, phosphorylated-P38 and β-actin were diluted at 1:1000 at 4°C overnight. The membrane was washed with PBS with Tween-20 (PBST) for 3 times, and horseradish peroxidase (HRP)-labeled secondary antibody (diluted at 1:10000) was added for incubation at room temperature for 1 h. Finally, SuperSignal West Pico chemiluminescent substrate was used for observation.
Immunohistochemistry
Paraffin-embedded tissue sections (5 μm) were dewaxed in xylene, and then rehydrated with the ethanol solution in gradient concentration. For antigen epitope retrieval, sections were heated via conventional microwave for 10 min in 0.01 M citrate buffer (0.01 M sodium citrate and 0.01 M citric acid, pH 6.0). 100 μL 3% H2O2 was added to the top of section at room temperature (22°C) to inhibit the endogenous peroxidase activity for 10 min. Then sections were sealed with 5% normal goat serum for 1 h and incubated with primary antibodies (RUNX2, 1:200. OSX, 1:100. OCN, 1:100) at 4°C overnight. PBS was used to replace the primary antibody for incubation as the negative control. Finally, sections were rinsed at room temperature (22°C), incubated with biotinylated secondary antibody for 45 min, washed with PBST for 3 times, incubated with strept avidin-biotin complex (SABC) for 30 min, and stained with 100 μL pigment solution. When there was brown color, the glass slide was re-stained with hematoxylin for 1 min and observed under a lighted microscope. The percentage of positive area or cells in each sample was calculated using the Image-Pro Plus 5.0 software.
Statistics
Data in this study were presented as mean ± standard deviation; Statistical Product and Service Solutions (SPSS) 19.0 software (SPSS Inc., Chicago, IL, USA) was used for data processing; a t test was used for the intergroup comparison, and a chi-square test was used for the enumeration data. Continuous data from multiple groups were analyzed by using one-way ANOVA, with Tukey’s post hoc test. P-values <0.05 were considered statistically significant.
Results
PRF and IGF-1 increased the proliferation of PDLSCs
The proliferation of PDLSCs cultured in non-treated medium and PRF-treated medium could be promoted by 25-200 ng/mL IGF-1. The proliferation efficacy of PDLSCs cultured in PRF-treated medium was increased under the IGF-1 concentration of 100 ng/mL (Figure 1A, P<0.01). Therefore, 100 ng/mL IGF-I was used for subsequent experiments, and the cell growth curves are shown in Figure 1B.
Figure 1.
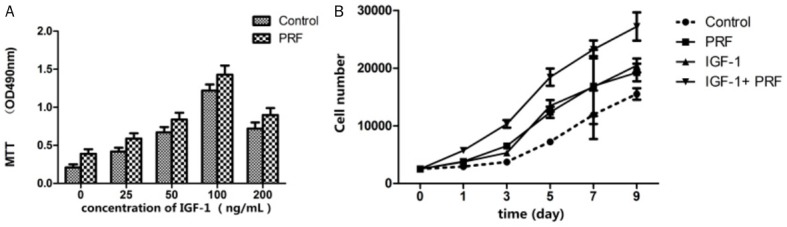
Effects of PRF and IGF-1 on in-vitro proliferation of PDLSCs (n=6).
PRF and IGF-1 enhanced the osteogenic differentiation of PDLSCs
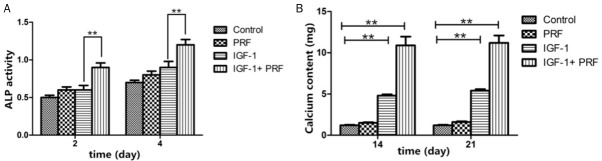
Compared with that in the non-treated group, PDLSCs cultured in the osteogenic medium in the PRF-treated group showed higher ALP activity at 3 d (P<0.01) and 4 d (P<0.01) (Figure 2A). The ALP activity of PDLSCs cultured in the osteogenic medium added with 100 ng/mL IGF-1 in PRF-treated group was further increased at 3 d (P<0.01) and 4 d (P<0.01) compared to cells solely treated with IGF-1 (Figure 2A). The calcium content in osteoblasts was the highest at 14 d and 21 d (P<0.01, Figure 2B).
Figure 2.
ALP staining of PDLSCs under different culture conditions. **P<0.01, n=6.
Detection of RUNX2, OSX and OCN mRNA expressions via real-time RT-PCR
Results further showed that RUNX2, OSX, and OCN mRNA expressions were significantly increased in PDLSCs treated with PRF and IGF-1 at 14 d, compared with those in the non-treated medium group (P<0.01, Figure 3).
Figure 3.
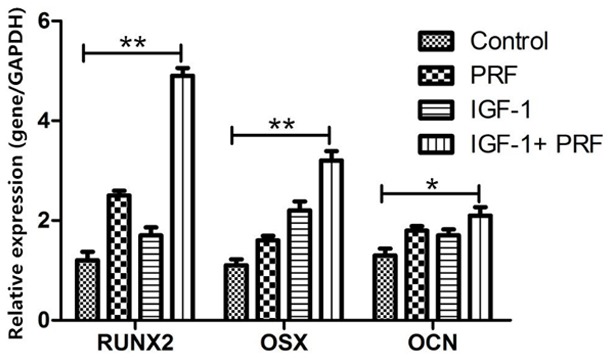
Analysis of RUNX2, OSX and OCN mRNA expressions via real-time RT-PCR. GAPDH is used as an internal control, and the results are described as the fold change relative to the GAPDH. Data are presented as mean ± SD. **P<0.01, *P<0.05, n=6.
Detection of RUNX2, OSX and OCN protein expressions via Western blotting
Western blotting data indicated that RUNX2, OSX and OCN protein levels were obviously increased at 14 d in PRF + IGF-1-treated groups, compared with those in the non-treated group (Figure 4).
Figure 4.
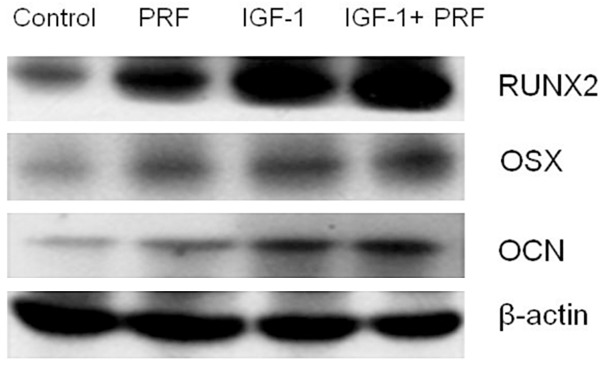
Analysis of RUNX2, OSX and OCN protein expressions under different conditions via Western blotting.
Detection of percentages of RUNX2, OSX and OCN positive cells via Immunohistochemistry
We further used an immunohistochemistry assay and found that the percentages of RUNX2, OSX and OCN positive cells in treated implants were significantly higher than those in untreated implants (P<0.01). These findings further confirmed the osteogenic effects of PRF and IGF-1 on PDLSCs (Figure 5A-C).
Figure 5.
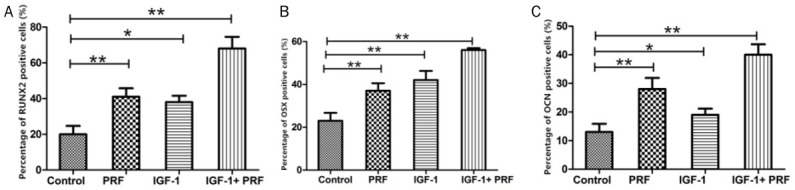
Percentages of RUNX2, OSX and OCN positive cells in treated group and non-treated group. **P<0.01, *P<0.05, n=20.
PRF and IGF-1 could activate ERK and JNK pathways in PDLSCs
To clarify the reason why PRF and IGF-1 could promote the function of PDLSCs, the effect of the MAPK pathway in PDLSCs was investigated. Of note, the levels of phosphorylated-ERK1/2 and phosphorylated-JNK were up-regulated in cells treated with PRF and IGF-1 (Figure 6). The phosphorylated-ERK1/2 level was increased by about 8 times at 30 min, by 7 times at 60 min, and by 5 times at 90 min. Compared with that in the non-treated group, the phosphorylated-JNK level in the PRF + IGF-1-treated group was statistically increased by about 4 times at 60 and 90 min (P<0.01).
Figure 6.
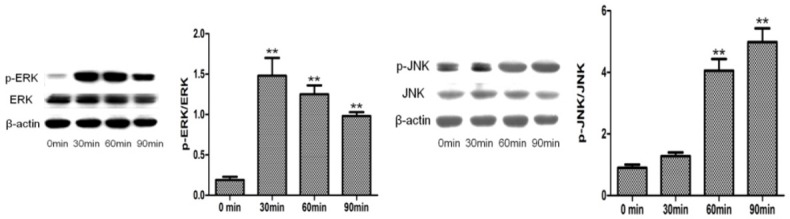
Effects of PRF and IGF-1 on MAPK pathway in PDLSCs.
Discussion
During tooth root morphogenesis, various growth factors mediate the interaction among the fibroblast growth factor, the transforming growth factor, the bone morphogenetic protein, the IGF and other adjacent mesenchymal tissues [21,22]. Previous findings showed that IGF-1 stimulated the proliferation of PDL cells, promoted the PDL wound healing, maintained the periodontal homeostasis, and even extended its root system [23]. Therefore, it is reasonable that IGF-1 may contribute to a certain influence on the differentiation of PDLSCs.
In this study, IGF-1 could significantly promote the proliferation of PDLSCs, reduce the nucleus/cytoplasm ratio, and change the in vitro ultrastructure of PDLSCs in a dose-dependent manner. Under the concentration of 100 ng/mL, the proliferation rate of PDLSCs reached the peak of the curve with the highest proliferative index. In addition, PRF and IGF-1 participated in the regulation on the ALP activity, calcium deposition and up-regulation of osteoblast markers (RUNX2, OSX and OCN) in PDLSCs, indicating that PRF and IGF-1 can enhance the osteogenic differentiation of these periodontal stem cells. PDLSCs treated with PRF and IGF-1 showed higher gene/protein expression levels of RUNX2, OSX and OCN at 3 d and 7-14 d, indicating that PRF and IGF-1 can trigger the early osteogenic differentiation of PDLSCs and maintain the late osteogenic differentiation in vitro. The transcription factors (RUNX2 and OSX) are required for osteogenic differentiation. RUNX2 guides the differentiation of mesenchymal stem cells into osteoblast lineages, and then it prevents osteoblasts from maturing and transferring to osteocytes. In addition, it has been found that RUNX2 regulates the expressions of other mineralization-related genes. As a downstream gene of RUNX2, OSX is the second transcription factor, which is required for osteogenic differentiation and is specifically expressed in all developing bones.
OSX is involved in osteogenic differentiation in the early and late osteogenesis. Genetic studies have shown that the formation of cortical bone and bone trabecula in OSX-knockout mice is eliminated, and OSX may play a role in bone regeneration and control the osteogenic differentiation involved in regenerative bone formation. In addition, OSX plays an important role in the mechanical stress-induced osteogenic differentiation and alveolar bone remodeling during orthodontic treatment. OCN is generally considered as a marker of advanced mineralized bone, and it is normally activated during the differentiation of PDLSCs into osteoblastic lineages. In this study, levels of RUNX2, OSX and OCN were increased in PDLSCs treated with PRF and IGF-1 at different time points, suggesting that PRF and IGF-1 play important roles in not only the early stage, but also the late stage of bone formation. Moreover, PRF and IGF-1 increased the expressions of phosphorylated-ERK1/2 and phosphorylated-JNK, indicating that the MAPK pathway is activated during osteogenic differentiation of PDLSCs treated with PRF and IGF-1.
Conclusion
PRF and IGF-1 can promote the osteogenic differentiation of PDLSCs and enhance their osteogenic mineralization through the regulation of the MAPK pathway, suggesting that PRF and IGF-1 play important roles in the osteogenic differentiation process and provide theoretical leads for periodontal tissue regeneration and alveolar bone remodeling.
Acknowledgements
This work was supported by Shenzhen Science and Innovation Committee Technology Research and Development Fund (JCYJ20170307141759582).
Disclosure of conflict of interest
None.
References
- 1.Bosshardt DD, Sculean A. Does periodontal tissue regeneration really work? Periodontology. 2009;51:208. doi: 10.1111/j.1600-0757.2009.00317.x. [DOI] [PubMed] [Google Scholar]
- 2.Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, An Y, Zhang C, Wang S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. 2010;28:1829–1838. doi: 10.1002/stem.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen FM, Jin Y. Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng Part B Rev. 2010;16:219. doi: 10.1089/ten.TEB.2009.0562. [DOI] [PubMed] [Google Scholar]
- 4.Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31:7892–927. doi: 10.1016/j.biomaterials.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Zeng YF, Huang YD. Insulin-like growth factor 1 and its roles in the development of tissues and organs. Life Science Research. 2015 [Google Scholar]
- 6.Hadem IK, Sharma R. Age- and tissue-dependent modulation of IGF-1/PI3K/Akt protein expression by dietary restriction in mice. Horm Metab Res. 2015;48:201–206. doi: 10.1055/s-0035-1559770. [DOI] [PubMed] [Google Scholar]
- 7.Reckenbeil J, Kraus D, Stark H, Rath-Deschner B, Jager A, Wenghoefer M, Winter J, Gotz W. Insulin-like growth factor 1 (IGF1) affects proliferation and differentiation and wound healing processes in an inflammatory environment with p38 controlling early osteoblast differentiation in periodontal ligament cells. Arch Oral Biol. 2017;73:142–150. doi: 10.1016/j.archoralbio.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Vassilakos G, Philippou A, Koutsilieris M. Identification of the IGF-1 processing product human Ec/rodent Eb peptide in various tissues: evidence for its differential regulation after exercise-induced muscle damage in humans. Growth Horm IGF Res. 2016;32:22–28. doi: 10.1016/j.ghir.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Tahimic CG, Long RK, Kubota T, Sun MY, Elalieh H, Fong C, Menendez AT, Wang Y, Vilardaga JP, Bikle DD. Regulation of ligand and shear stress-induced insulin-like growth factor 1 (IGF1) signaling by the integrin pathway. J Biol Chem. 2016;291:8140–8149. doi: 10.1074/jbc.M115.693598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raja S, Byakod G, Pudakalkatti P. Growth factors in periodontal regeneration. Int J Dent Hyg. 2009;7:82–89. doi: 10.1111/j.1601-5037.2009.00380.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen FM, Zhao YM, Wu H, Deng ZH, Wang QT, Zhou W, Liu Q, Dong GY, Li K, Wu ZF, Jin Y. Enhancement of periodontal tissue regeneration by locally controlled delivery of insulin-like growth factor-I from dextran-co-gelatin microspheres. J Control Release. 2006;114:209. doi: 10.1016/j.jconrel.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Chen FM, Zhang M, Wu ZF. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 13.Shivashankar VY, Johns DA, Maroli RK, Sekar M, Chandrasekaran R, Karthikeyan S, Renganathan SK. Comparison of the effect of PRP, PRF and induced bleeding in the revascularization of teeth with necrotic pulp and open apex: a triple blind randomized clinical trial. J Clin Diagn Res. 2017;11:ZC34–ZC39. doi: 10.7860/JCDR/2017/22352.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto NR. Leukocyte-platelet rich fibrin membrane (L-PRF) alone or associated with a biomimetic implant surface act as a biological scaffold for hard and soft tissue regeneration [C] International Conference on Tissue Science and Regenerative Medicine. 2015 [Google Scholar]
- 15.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Peck MT, Marnewick J, Stephen L. Alveolar ridge preservation using leukocyte and platelet-rich fibrin: a report of a case. Case Rep Dent. 2011;2011:345048. doi: 10.1155/2011/345048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A, Pradeep AR. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: a randomized controlled clinical trial. J Periodontol. 2011;82:1705. doi: 10.1902/jop.2011.110075. [DOI] [PubMed] [Google Scholar]
- 18.Manojkumar T, Pradeep AR, Borse P. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: a controlled clinical trial. J Clin Periodontol. 2011;38:925. doi: 10.1111/j.1600-051X.2011.01760.x. [DOI] [PubMed] [Google Scholar]
- 19.Inchingolo F, Tatullo M, Marrelli M, Inchingolo AM, Scacco S, Inchingolo AD, Dipalma G, Vermesan D, Abbinante A, Cagiano R. Trial with Platelet-Rich Fibrin and Bio-Oss used as grafting materials in the treatment of the severe maxillar bone atrophy: clinical and radiological evaluations. Eur Rev Med Pharmacol Sci. 2010;14:1075–1084. [PubMed] [Google Scholar]
- 20.Zhang Y, Tangl S, Huber CD, Lin Y, Qiu L, Rausch-Fan X. Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. J Craniomaxillofac Surg. 2012;40:321. doi: 10.1016/j.jcms.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Catón J, Tucker AS. Current knowledge of tooth development: patterning and mineralization of the murine dentition. J Anat. 2009;214:502. doi: 10.1111/j.1469-7580.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres CB, Alves JB, Silva GA, Goes VS, Nakao LY, Goes AM. Role of BMP-4 during tooth development in a model with complete dentition. Arch Oral Biol. 2008;53:2–8. doi: 10.1016/j.archoralbio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Kheralla Y, Götz W, Kawarizadeh A, Rath-Deschner B, Jäger A. IGF-I, IGF-IR and IRS1 expression as an early reaction of PDL cells to experimental tooth movement in the rat. Arch Oral Biol. 2010;55:215–222. doi: 10.1016/j.archoralbio.2010.01.002. [DOI] [PubMed] [Google Scholar]