Abstract
Background: A secondary inflammatory response is the most important mechanism of injury after intracerebral hemorrhage (ICH). Previous studies found microRNAs (miRs) expressed abnormally in the perihematomal tissue and blood of patients with ICH and demonstrated that miRs were related to pathophysiological changes and prognosis after ICH, and the development of inflammation. Methods: We induced a microglial inflammatory response by lipopolysaccharide (LPS) to construct a microglial inflammatory model. MiR-21/miR-146a overexpression adenovirus was used to infect microglia to increase miR-21/miR-146a expression. MiR-21, miR-146a, IRAK1, MMP-9, TNF-α, TIMP3 and other inflammatory factors were analyzed. Then, miR-21/miR-146a overexpression adenovirus was injected into rats with ICH to modulate the expression. Inflammation, brain edema, and neurological scores were assessed. Results: For in vitro and vivo experiments, overexpression of miR-21/miR-146a decreased the expression of IL-1β, IL-6, IL-8, IRAK1, MMP-9 and TNF-α, meanwhile increased the expression of TIMP3 significantly (P<0.001), compared with the negative control group. Additionally, miR-21 and miR-146a reduced brain edema and improved the neurological function in ICH rats. Conclusion: Our study proved that miR-21 and miR-146a could negatively regulate the inflammatory response of microglia after ICH and provided a new theoretical basis for the treatment of secondary inflammatory injury after ICH in humans.
Keywords: Intracerebral hemorrhage, inflammation, microglia, microRNA-21, microRNA-146a
Introduction
As a subtype of stroke, intracerebral hemorrhage (ICH) harm to human health is serious, characterized by high incidence, mortality and disability. The brain damage caused by ICH, includes primary hematoma compression and secondary injury which result from hematoma decomposition products. Studies have demonstrated that secondary injury after ICH is the critical factor in the prognosis of patients with ICH. The mechanism of secondary injury after ICH is complicated, including acute inflammatory reaction, local free oxygen release, edema effect, apoptosis, and autophagy. Secondary inflammation is considered to be the most important mechanism of secondary injury after ICH [1,2], but the detail of its mechanism of action has not been clearly known.
Microglia play an important role in the initial inflammatory response after spontaneous ICH. As an important inflammatory effector cell in the nerve system, microglia are activated first, after ICH, and release a series of inflammatory mediators and biological activity factors: such as TNF-α, IL-1β, IL-6, IL-8, and so on, through the process of cell morphological and physiological changes, which lead to occurrence of an inflammatory response after ICH and result in further secondary injury [2,3].
MicroRNAs (miRNAs, miRs) are a group of small non-coding RNAs with a length of 18-25 nucleotides, that can accumulate in cells and down-regulate the expression of target genes, by binding the special sequence on the 3’ untranslated region (3’UTR) of mRNA, and have the effect of regulating gene transcription [4]. miRs play a very important role in maintaining the biological activity of cells. Studies showed that miRs are expressed abnormally in perihematomal tissue and blood of patients with ICH and relate to pathophysiological changes and prognosis after ICH, and the development of inflammation [5,6]. Literature review disclosed two miRs, miR-21 and mi-146a, that have an anti-inflammatory effect in some diseases. MiR-21 can regulate the Akt and/or ERK/MAPK pathways [7] and can also negatively regulate the expression of Toll-like 4 receptor (TLR4) to regulate inflammatory responses [8]. MiR-146a targets 3’UTRs of interleukin 1 receptor associated kinase-1 (IRKA1) and TNF receptor associated factor 6 (TRAF6) to suppress NF-κB pathway [9] and can regulate inflammatory infiltration by targeting TRAF6 and affecting IL-17/Intercellular Adhesion Molecule 1 (ICAM-1) pathway [10]. However, their anti-inflammatory effect after ICH has not been well studied.
In the present study, we report that miR-21 and miR-146a negatively regulated the inflammatory response of microglia and diminished cell death after ICH. These results indicate that miR-21 and miR-146a are novel inflammatory regulators and potential therapy for ICH.
Materials and methods
Microglial culture and treatment
Primary microglial cells were prepared from neonatal Sprague-Dawley (SD) rats (less than 24 h old), according to standard protocols [11], with minor modifications. Briefly, after removal of the meninges, choroid plexus, brainstem and cerebellum, the cortices of the cerebral hemispheres were digested by 0.25% trypsin/0.002% EDTA (10 min, 37°C), mechanically dissociated, and centrifuged at 1000× g for 10 min. The supernatant was discarded, and the cell were cultured in DMEM medium supplemented with 10% fetal bovine serum both from GIBCO-Invitrogen (Grand Island, NY, USA) and 1% penicillin & streptomycin (Sigma, St-Louis, MO, USA). Cells were maintained at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air. The microglia cell purity was more than 90% as determined by immunohistochemistry test, using CD11b antibody (Santa Cruz USA).
Cells were treated with lipopolysaccharide (LPS, Sigma-Aldrich, Saint Louis, MO, USA) with different concentrations (1 ng/ml, 5 ng/ml, 10 ng/ml, and 100 ng/ml), and maintained at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air for 12 h or 24 h.
Adenovirus and adenovirus infection
miR-146a-5p and miR-21-5p over-expression adenovirus and negative control adenovirus were purchased from the Shanghai R&S Biotechnology Co., Ltd. The adenovirus vector (pAd/CMV/V5-DEST) of miR-146a-5p or miR-21-5p was co-transfected into human embryonic kidney 293T cells, according to standard protocols [12]. The supernatant was collected at 48 h after transfection, and fresh medium was added to the culture flask. After the cells were cultured for another 24 h, the supernatant was collected again. The mixture of the supernatants collected from 48 and 72 h was centrifuged at 2500× g for 15 min at 4°C, then the liquid was filtered by a 0.45-µm filter membrane, and the acquired virus was stored at -80°C until use. The titers of the adenovirus were 1010 PFU/ml.
Microglial cells were seeded into 12-well plates (2×105 cells/well) one day before adenovirus infection. The next day, adenovirus was added into wells with a multiplicity of infection (MOI) of 50 to infect cells. The infection efficiency was detected by fluorescence microscopy analysis of GFP at 48 h after infection and the efficiency was ensured higher than 90%.
Intracerebral hemorrhage model
Male SD rats (weighing 280-320 g) were anesthetized with pentobarbital (45 mg/kg) intraperitoneally. A feedback-controlled heating pad was used for maintaining core temperature at 37°C. The right femoral artery was catheterized for whole blood collection, continuous blood pressure and blood gas monitoring. The rats were positional in a stereotactic frame Model 500, Kopf Instruments, Tujunga, CA, USA and a cranial burr hole (1 mm) was drilled in the right coronal suture (coordinates 0.2 mm anterior, 5.5 mm ventral, 3.5 mm lateral to the bregma). Either 100 µL autologous blood and 10 µL adenovirus (miR-146a-5p, miR-21-5p over-expression adenovirus or negative control adenovirus, 1×1010 PFU/ml) (as a model of ICH treated with miRNA) or 100 µL autologous blood (as a model of control) into the right basal ganglia. After injection, the needle was removed and the skin incision sutured closed [13]. The animal ethics committee of the First Affiliated hospital, Zhejiang University, School of Medicine approved the protocol for this study and all animal experiments were performed in accordance with National Research Council guide for the care and use of laboratory animal.
Rats were divided into five groups. Rats received only intracerebral needle insertion in Sham group orhad intracerebral infusion of 100 µL autologous blood in Control group, or infusion of 100 µL autologous blood and 10 µL adenovirus (miR-21-OE group received miR-21-5p over-expression adenovirus, miR-146a-OE group received miR-146a over-expression adenovirus, NC group received negative control adenovirus). Rats were killed after 12 h, 24 h or 72 h after surgery.
RNA extraction and quantitative real-time qPCR (qRT-PCR)
Total RNA was extracted from cell lines or brain tissues using Trizol reagent (Invitrogen, USA). The qRT-PCR reactions were performed using All-in-One™ miRNA qRT-PCR Detection Kit (GeneCopoeia Inc, USA), and iQ-5 (Bio-Rad) was used to monitor the PCR in real-time. The qPCR cycling profile was denatured at 95°C for 10 min, followed by 40 cycles of annealing at 95°C for 15 seconds and extension at 60°C for 20 seconds with a final extension at 72°C for 20 seconds. The endogenous U6 was chosen as the internal control for miRs and GAPDH for IL-1β, IL-8, and TNF-α. The average cycle threshold (CT), from triplicate assays, was used for further calculations. Relative expression levels were normalized to control. The 2-ΔΔCt method was used to quantify the relative amount of miR-146a, miR-21, IL-1β, IL-6, and TNF-α. The sequences of the primers are listed in Table 1.
Table 1.
RT and qPCR primers used in this study
| Name | Sequence (5’→3’) |
|---|---|
| U6 | RT: CGCTTCACGAATTTGCGTGTC |
| Forward: TCGCTTCGGCAGCACATATAC | |
| Reverse: GCGTGTCATCCTTGCGCAG | |
| miR-21 | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGTCAG |
| Forward: GTTGACTGTTGAATCTCATGGCAACA | |
| Reverse: ATCCAGTGCAGGGTCCGAGG | |
| miR-146a | RT: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACACGATG |
| Forward: ACTGAATTCCATGGGTTGTGTCAGT | |
| Reverse: ATCCAGTGCAGGGTCCGAGG | |
| GAPDH | Forward: CCCCAATGTATCCGTTGTG |
| Reverse: CTCAGTGTAGCCCAGGATGC | |
| TNF-α | Forward: CTCTTCTGTCTACTGAACTTCGGG |
| Reverse: ACGTGGGCTACGGGCTTGT | |
| IL-1β | Forward: TGCCACCTTTTGACAGTGATG |
| Reverse: TGTGCTGCTGCGAGATTTG | |
| IL-8 | Forward: TGGGTGAAGGCTACTGTTGG |
| Reverse: TGGAAAGGGAAATATTCTCTGT |
ELISA assay
To test the inflammatory response after LPS inducement, IL-1β, IL-6, IL-8, MMP-9, IRAK1, TIMP3 and TNF-α of cells supernatant activity were measured by ELISA. IL-1β, IL-6, MMP-9 and TNF-α were assayed using assay Kits purchased from Cloud-Clone Corp (TX, USA) according to manufacturer’s instruction. IL-8, IRAK1 and TIMP3 activity were measured with assay Kits purchased from R&D Systems (MN, USA) in accordance with the manufacturer’s instruction.
Histology
Rats were immediately sacrificed by cervical dislocation and their brain tissues were collected, weighed, and frozen at -20°C until analysis. Edema and hemorrhage were used as indicators of acute inflammation.
For histological observation, paraffin-embedded basal ganglia sections (18 μm thick) were prepared. Slides were deparaffinized and the sections were stained with hematoxylin-eosin (H&E) for histological examination (Nikon ECLIPSE TS2000-0, Japan).
Brain water content measurement
Brain water content was measured after ICH as described previously [14]. In brief, rats were anesthetized by intraperitoneal injection with pentobarbital (60 mg/kg). Then, the cerebral tissues were removed, and the surface water on the cerebral tissues was blotted with filter paper. Brain samples were divided into five parts: ipsilateral and contralateral basal ganglia, ipsilateral and contralateral cortex, and cerebellum. These samples were immediately weighed on an electric analytic balance to obtain the wet weight and then dried at 100°C for 24 h to obtain the dry weight. Brain water content was calculated using the following formula: brain water content (%) = (wet weight-dry weight)/wet weight ×100%.
Evaluation of neurological scores
Animals were tested before and after surgery and scored by investigators who were blind to neurological and treatment condition. Two behavioral assessments were used: corner turn and forelimb use asymmetry tests, as described before [15].
Statistical analysis
SPSS 22.0 software was used for all statistical analyses. Data are presented as means ± standard deviations (SD). Significant differences in the mean values of two groups were evaluated by Student’s unpaired t-test. Data needing multiple comparisons were evaluated by one-way ANOVA with Bonferroni correction. P-value of 0.05 or less was considered to be statistical significant.
Results
LPS induces microglia inflammation
LPS with different concentrations was used to stimulate microglia for 12 h or 24 h and the relative expression of IL-1β, IL-8 and TNF-α were measured by qPCR to evaluate inflammatory response. LPS induced IL-1β, IL-8, and TNF-α expression in a dose-dependent increase (Figure 1A-C). Compared with control group (LPS 0 ng/ml), 1-100 ng/ml of LPS significantly increased expression of IL-1β, IL-8 and TNF-α at 12 h or 24 h (P<0.001). The expression of IL-1β and IL-8 peaked at 24 h after treatment of LPS with the concentration of 10 ng/ml, while TNF-α peaked at 24 h after treatment of LPS with the concentration of 100 ng/ml. But the expression of TNF-α is much higher than control group at 24 h after treatment of 10 ng/ml LPS.
Figure 1.
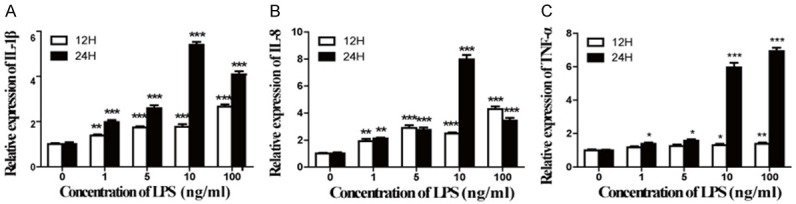
Acute inflammation of microglia was induced by LPS. Microglia were treated with LPS of different concentrations (0 ng/ml, 1 ng/ml, 5 ng/ml, 10 ng/ml, 100 ng/ml) for 12 h or 24 h. The relative expression of IL-1β (A), IL-8 (B), and TNF-α (C) was assessed by qPCR, with data represented as mean ± SD, n=6. *P<0.05, **P<0.01, ***P<0.001 versus control (0 ng/ml of LPS).
MiR-21/miR-146a attenuates inflammation response of microglia induced by LPS
MiR-21/miR-146a overexpression adenovirus was used to infect microglia. The relative expression of miR-21/miR-146a was measured by qPCR. The expression of IL-1β, IL-6, IL-8, IRAK1, MMP-9, TNF-α, and TIMP3 was measured by ELISA. The relative expression of miR-21 in microglia treated with LPS (10 ng/ml) and infected by miR-21 overexpression adenovirus was 1.53 fold and miR-146a expression was 2.10 fold more than cells infected by negative control adenovirus (NG) and treated with LPS (Figure 2A, 2B). The IL-1β, IL-6, IL-8, IRAK1, MMP-9 and TNF-α levels of microglia in the supernatant were significantly up-regulated (P<0.001) and TIMP3 levels down-regulated (P<0.001) after being treated with LPS which confirmed the relative expression of IL-1β, IL-8, and TNF-α measured by qPCR. Overexpression of miR-21 or miR-146a significantly decreased the levels of IL-1β, IL-6, IL-8, IRAK1, MMP-9 and TNF-α and increased the levels of TIMP3 (P<0.001) compared with that of negative control cells (Figure 2C-I).
Figure 2.
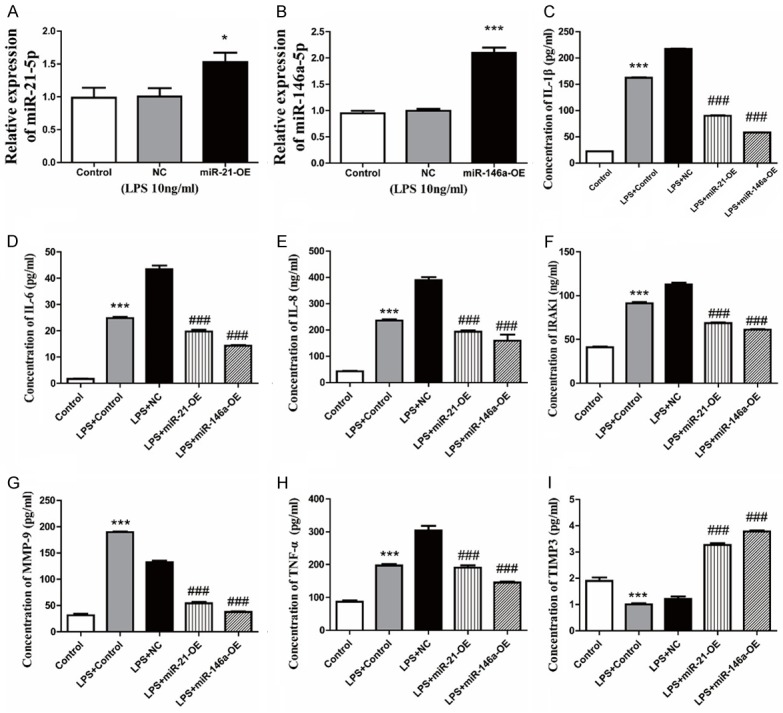
miR-21 and miR-146a attenuate the inflammatory response of microglia induced by LPS. After infection of miR-21 overexpression adenovirus (miR-21-OE), miR-146a overexpression adenovirus (miR-146a-OE), or negative control adenovirus (NG), microglia were treated by LPS (10 ng/ml) at 24 h after infection. The relative expression of miR-21-5p (A) or miR-146a-5p (B) was measured by qPCR, with data represented as mean ± SD, n=6. *P<0.05, **P<0.01, ***P<0.001 versus NC. The concentration of IL-1β (C), IL-6 (D), IL-8 (E), IRAK1 (F), MMP-9 (G), TNF-α (H) and TIMP3 (I) were assayed by ELISA, with data represented as mean ± SD, n=6. ***P<0.001 versus control, ###P<0.001 versus LPS+NC group.
Intracerebral hematoma induces brain tissue inflammation
H&E was used for histological observation of perihematomal tissue (Basal ganglia). We used qPCR to detect the relative expression of IL-1β, IL-6, IL-8, IRAK1, TNF-α, miR-21, and miR-146a in the perihematomal tissue at 12 h, 24 h and 72 h post-ICH. Representative sections from perihematomal tissues (Model) and normal contralateral cerebral tissues (Normal) of intracerebral hemorrhage rats are shown in Figure 3A. Hemorrhage distribution tended to disperse from 12 h to 24 h, and decrease at 72 h. Results of qPCR indicated that the relative expression of IL-1β, IL-6, IRAK1, and TNF-α was significantly increased in the perihematomal tissues compared with normal contralateral cerebral tissues (P<0.001) (Figure 3B, 3C, 3E, 3F), whereas IL-8 expression began to increase significantly at 24 h (Figure 3D). The expression of IL-1β, IL-6, and IRAK1 peaked at 12 h, and began to decrease at 24 h after ICH. However, the expression of IL-8 and TNF-α peaked at 24 h and decreased at 72 h.
Figure 3.
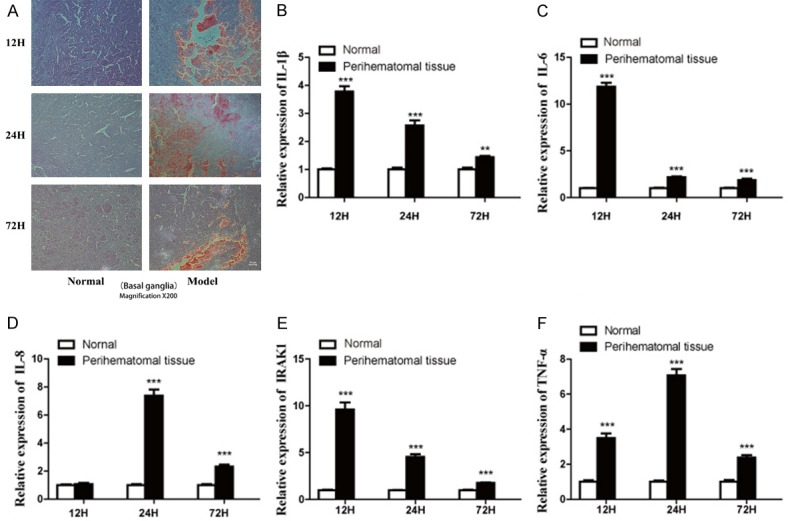
Intracerebral hematoma induces brain tissue inflammation. (A) H&E staining for histological observation of perihematomal tissues (Model) and normal contralateral cerebral tissues (Normal) was performed at 12 h, 24 h and 72 h after ICH (Original magnification ×200). Hemorrhage distribution tended to disperse from 12 h to 24 h and decrease at 72 h. There is no obvious change in the normal tissue. Relative expression of IL-1β (B), IL-6 (C), IL-8 (D), IRAK1 (E) and TNF-α (F) in the perihematomal tissue and normal contralateral cerebral tissues at 12 h, 24 h and 72 h post-ICH was measured by qPCR. Relative expression levels were normalized to endogenous GAPDH, with data represented as mean ± SD, n=6. **P<0.01, ***P<0.001 versus Normal group. The expression of IL-1β, IL-6, and IRAK increased at all time-tested points and peaked at 12 h in the perihematomal tissue. IL-8 expression started to increase at 24 h and peaked at 24 h. The expression of TNF-α also increased at all time-tested points and peaked at 24 h.
MiR-21 and miR-146a downregulate inflammation response in vivo
The relative expression of IL-1β, IL-6, IL-8, IRAK1, MMP-9, TNF-α, TIMP3, miR-21, and miR-146a in brain tissues of rat at 72 h after ICH or ICH and injection with adenovirus (miR-21-OE, miR-146a-OE or NG adenovirus) were also measured by qPCR. The results indicate that miR-21/miR-146a expression of perihematomal tissue was significantly increased after ICH and injection with miR-21/miR-146a overexpression adenovirus (P<0.001) (Figure 4A, 4B). The relative expression of IL-1β, IL-6, IL-8, IRAK1, MMP-9 and TNF-α in perihematomal tissues (Model) were significantly upregulated (P<0.05) and TIMP3 levels downregulated (P<0.01) after ICH or ICH and injection negative control adenovirus (NC) which confirmed the relative expression of IL-1β, IL-6, IL-8, IRAK1, MMP-9 and TNF-α measured by qPCR and showed in Figures 3, 4C-I. The expression of IL-1β, IL-6, IL-8, IRAK1, MMP-9, and TNF-α of hematoma tissues after ICH and injection miR-21 or miR-146a overexpression adenovirus (miR-21 or miR-146a-OE) was significantly decreased (P<0.01) and the expression of TIMP3 was increased (P<0.01) compared with the negative control (Figure 4C-I). This result matches our observations in rat microglial cells treated with LPS and infected by adenovirus.
Figure 4.
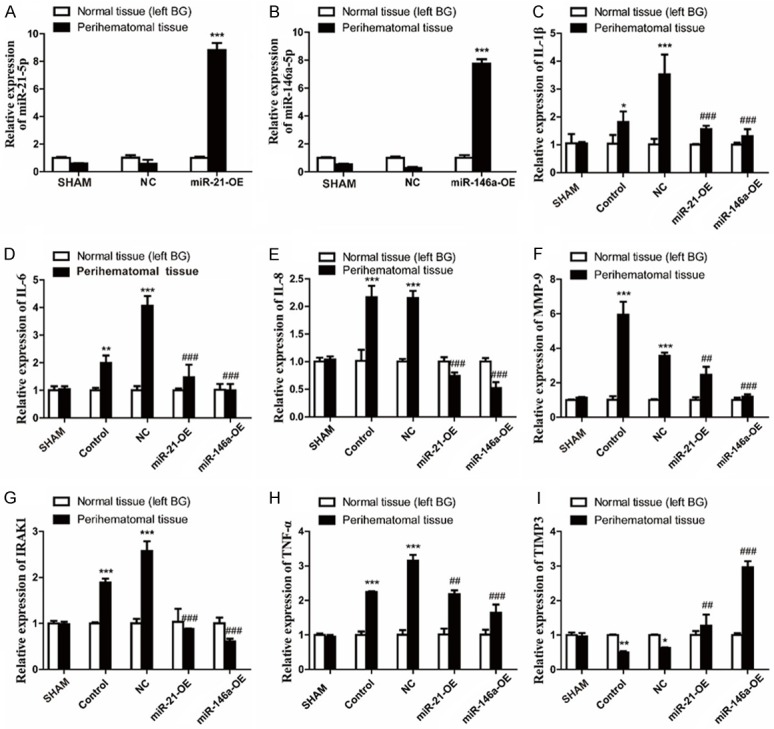
miR-21 and miR-146a downregulate inflammatory response in vivo. The relative expression of miR-21 (A) and miR-146a (B) in perihematomal tissue and normal contralateral cerebral tissues at 72 h after ICH or ICH and injection with adenovirus (miR-21-OE, miR-146a-OE or NG adenovirus) were also measured by qPCR. Relative expression levels were normalized to U6, with data represented as mean ± SD, n=6. The relative expression of miR-21/miR-146a in perihematomal tissues significantly increased after injection with miR-21/miR-146a overexpression adenovirus (miR-21/miR-146a-OE) compared with negative control adenovirus (NC) (***P<0.001). The relative expression of IL-1β (C), IL-6 (D), IL-8 (E), MMP-9 (F), IRAK1 (G), TNF-α (H) and TIMP3 (I) were measured by qPCR. Relative expression levels were normalized to endogenous GAPDH, with data represented as mean ± SD, n=6. Overexpression of miR-21/miR-146a decreased the expression of IL-1β, IL-6, IL-8, IRAK1, TNF-α and increased the expression of TIMP3 in perihematomal tissue at 72 h after ICH. *P<0.05, **P<0.01, ***P<0.001, perihematomal tissues versus normal contralateral cerebral tissues. ##P<0.01, ###P<0.001, perihematomal tissues in miR-21/miR-146a-OE group versus NC group.
miR-21/miR-146a reduces brain damage and improves neurological function
Brain water content was measured in rat cerebral tissues at 72 h after ICH. The results shown that the water content of ipsilateral basal ganglia tissue was significantly reduced after injection of miR-21 OE/miR-146a OE, compared with control and NC groups (Figure 5A). The behavioral test was performed before surgery and 72 h after surgery. Neurological scores in the miR-21 or miR-146a group was also significantly higher than NC group (Figure 5B, 5C).
Figure 5.
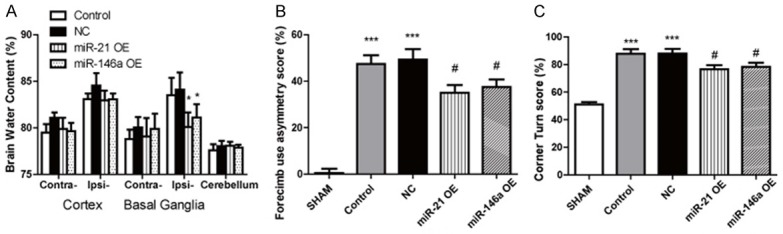
miR-21/miR-146a reduces brain damage and improves neurological function. Brain water content (A) was measured in rat cerebral tissues at 72 h after ICH, with data represented as mean ± SD, n=6. *P<0.05, miR-21/miR-146a-OE group versus NC group. Forelimb use asymmetry (B) and corner turn test (C) at 72 h after ICH or sham control. Data represented as mean ± SD, n=10. ***P<0.001 versus SHAM group, #P<0.05 versus NC group.
Discussion
Several mechanisms have been proposed for secondary injury after ICH, but there is no breakthrough in prevention and treatment of ICH clinically. Inflammation response is considered to be the most important mechanism of brain injury after ICH. It is a hot spot to study intracerebral hemorrhage therapy for inflammation, but further mechanisms still need exploration. miRs are a group of small non-coding RNAs newly discovered and studied much in recently years. They can regulate the expression of target genes, by binding to the target gene, and regulate the formation of proteins. The role of miRs in spontaneous ICH have gotten much recent attention. miR-132 has been proven to ameliorate brain edema, lessen blood-brain barrier (BBB) integrity, and attenuate the inflammatory response after ICH in mice [16]. miR-223 has been shown to be acrucial regulator of microglial activation, inflammation, and neuron injury after ICH by directly targeting HLRP3 [17].
Literature review disclosed two miRs, miR-21 and mi-146a, which have an anti-inflammatory effect in some diseases. miR-21 can target the 3’-untranslated regions (3’UTRs) of PTEN and Smad7 to downregulate PI3K/Akt pathway in human umbilical vein endothelial cells [18], and miR-21 can target Smad7 to regulate the Akt and/or ERK/MAPK pathways, contributing to renal fibrosis [7]. miR-21 can also negatively regulate the expression of Toll-like 4 receptor (TLR4) via targeting of the proinflammatory tumor suppressor programmed cell death protein 4 (PDCD4) to regulate inflammatory responses [8]. MiR-146a targets 3’UTRs of IRKA1 and TNF receptor associated factor 6 (TRAF6) to suppress the NF-κB pathway [9] and can regulate inflammatory infiltration by targeting TRAF6 and affecting the IL-17/Intercellular Adhesion Molecule 1 (ICAM-1) pathway in polymyositis/dermatomyositis [10]. However, their anti-inflammatory effect after ICH has not been well studied.
In the present study, we proved that miR-21 and miR-146a can negatively regulate the secondary inflammatory response of microglia after intracerebral hemorrhage, in vitro and in vivo. It has been reported that LPS could activate microglia and induce the expression of pro-inflammatory mediators [19]. Therefore, we used LPS at different doses to stimulate rat microglia to conduct a model of brain inflammation in vitroto explore ICH inflammation-related protein expression. Results demonstrated that the effect of LPS on microglia cells was dose-dependent (0-10 ng/ml) and time-dependent (12-24 h). We increased the expression of miR-21 and miR-146a in microglia by adenovirus infection, and found miR-21 and miR-146a can both attenuate the expression of IL-1β, IL-6, IL-8, IRAK1, MMP-9 and TNF-α and increase the expression of TIMP3. Based on experimental results in vitro, we injected autologous whole-blood into a rat ICH model and injected miR-21/miR-146a overexpression adenovirus to upregulate the expression of miR-21/miR-146a. As reported, the hematoma developed produces the desired brain injury and neurologic deficits [20], and a high level of inflammatory cytokines was significantly correlated with cerebral hematoma [21]. Our study also demonstrated that the expression of IL-1β, IL-6, IL-8, IRAK1, and TNF-α in the perihematomal tissues significantly increased after ICH. miR-21 and miR-146a could downregulate the expression of these inflammatory cytokines and upregulate TIMP3 expression. Furthermore, our results indicated that miR-21 and miR-146a reduce the edema and improves neurological function after ICH. As described above, the target genes of miR-21 include PTEN, Smad7, and PDCD4, and those of miR-146a include IRKA1 and TRAF6. In further research, we will confirm the target genes of these miRs in ICH, by constructing vectors of target gene 3’UTR construction and luciferase reporter assays.
Acknowledgements
This study was supported by National Natural Science Foundation of China (NSFC) (Grant No. 81371336 awarded to Shu Wan), Project of Medical and Health Technology Development Program of Zhejiang Province (Grant No. 2013KYA082 awarded to Shu Wan and Grant No. 2018KY044 awarded to Ming Wang) and Scientific Research Project of Traditional Chinese Medicine Administration in Zhejiang Province (Grant No. 2010ZA066 awarded to Shu Wan).
Disclosure of conflict of interest
None.
References
- 1.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral ha-emorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- 3.Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, Aronowski J. Cell death in experimental intracerebral hemorrhage: the “black hole” model of hemorrhagic damage. Ann Neurol. 2002;51:517–524. doi: 10.1002/ana.10160. [DOI] [PubMed] [Google Scholar]
- 4.Damiani D, Tiribelli M, Franzoni A, Michelutti A, Fabbro D, Cavallin M, Toffoletti E, Simeone E, Fanin R, Damante G. BAALC overexpression retains its negative prognostic role across all cytogenetic risk groups in acute myeloid leukemia patients. Am J Hematol. 2013;88:848–852. doi: 10.1002/ajh.23516. [DOI] [PubMed] [Google Scholar]
- 5.Guo D, Liu J, Wang W, Hao F, Sun X, Wu X, Bu P, Zhang Y, Liu Y, Liu F, Zhang Q, Jiang F. Alteration in abundance and compartmentalization of inflammation-related miRNAs in plasma after intracerebral hemorrhage. Stroke. 2013;44:1739–1742. doi: 10.1161/STROKEAHA.111.000835. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Zhu Y, Jin F, Tang L, He Z, He Z. Differential expression of circulation microRNAs in blood and haematoma samples from patients with in-tracerebral haemorrhage. J Int Med Res. 2016;44:419–432. doi: 10.1177/0300060516630852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu G, Xi G, Hua Y, Sagher O. T2* magnetic resonance imaging sequences reflect brain tissue iron deposition following intracerebral hemorrhage. Transl Stroke Res. 2010;1:31–34. doi: 10.1007/s12975-009-0008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang G, Yang Y, Wu G, Hua Y, Keep RF, Xi G. Early erythrolysis in the hemotoma after experimental intracerebral hemorrhage. Transl Stroke Res. 2017;8:174–182. doi: 10.1007/s12975-016-0505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan B, Shen H, Lin L, Su T, Zhong L, Yang Z. MicroRNA367 negatively regulates the inflammatory response of microglia by targeting IRAK4 in intracerebral hemorrhage. J Neuroinflamm. 2015;12:206. doi: 10.1186/s12974-015-0424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral test after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Han B, He Y, Li D, Ma X, Liu Q, Hao J. Micro-RNA-132 attenuates neurobehavioral and neuropathological changes associated with intracerebral hemorrhage in mice. NeurochemIn. 2017;107:182–190. doi: 10.1016/j.neuint.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z, Zhong LN, Xian RH, Yuan BQ. MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Mol Immunol. 2015;65:267–276. doi: 10.1016/j.molimm.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Rippe C, Blimline M, Magerko KA, Lawson BR, LaRocca TJ, Donato AJ, Seals DR. MicroRNA changes in human arterial endothelial cells with senescence: relation to apoptosis, eNOS and inflammation. Exp Gerontol. 2012;47:45–51. doi: 10.1016/j.exger.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loboda A, Sobczak M, Jozkowicz A, Dulak J. TGF-β1/Smads and miR-21 in renal fibrosis and inflammation. Mediators Inflamm. 2016;2016:8319283. doi: 10.1155/2016/8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheedy FJ, Palsson-Mcdermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, Johnson DS, Chen Y. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 16.Zhong X, Jiang YZ, Liu P, He W, Xiong Z, Chang W, Zhu J, Cui Q. Toll-like 4 receptor/NFκB inflammatory/miR-146a pathway contributes to the ART-correlated preterm birth outcome. Oncotarget. 2016;7:72475–72485. doi: 10.18632/oncotarget.11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin Y, Li F, Shi J, Li S, Cai J, Jiang Y. MiR-146a regulates inflammatory infiltration by macrophages in polymyositis/dermatomyositis by targeting TRAF6 and affecting IL-17/ICAM-1 pathway. Cell Physiol Biochem. 2016;40:486–498. doi: 10.1159/000452563. [DOI] [PubMed] [Google Scholar]
- 18.Defaux A, Zurich MG, Braissant O, Honegger P, Monnet-Tschudi F. Effects of the PPAR-beta agonist GW501516 in an in vitro model of brain inflammation and antibody-induced demyelination. J Neuroinflammation. 2009;6:15. doi: 10.1186/1742-2094-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–477. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frati A, Salvati M, Mainiero F, Ippoliti F, Rocchi G, Raco A, Caroli E, Cantore G, Delfini R. Inflammation markers and risk factors for recurrence in 35 patients with a posttraumatic chronic subdural hematoma: a prospective study. J Neurosurg. 2004;100:24–32. doi: 10.3171/jns.2004.100.1.0024. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Yang Q, Chen G, Zhang JH. An update on inflammation in the acute phase of intracerebral hemorrhage. Tansl Stroke Res. 2015;6:4–8. doi: 10.1007/s12975-014-0384-4. [DOI] [PubMed] [Google Scholar]


