Abstract
Mucus hypersecretion by airway epithelium and plugging of the airways are primary reasons of mortality in asthma patients and major causes of asthma disease progression and exacerbation. MUC5AC protein is a major component of airway mucus. MicroRNAs (miRNAs), a class of small noncoding RNAs, have emerged as moderators of MUC5AC production and secretion and are implicated in the pathogenesis of asthma. Recently, miR-330 has been reported to be downregulated in the blood of asthmatic patients, acting as a biomarker for asthma. The role of miR-330 in asthma, however, is unclear. Here, we showed that interleukin (IL)-13 induced MUC5AC secretion and inhibited miR-330 expression in a concentration-dependent manner in human bronchial epithelial cells (HBE16). Upregulation of miR-330 in HBE16 cells inhibited IL-13-induced MUC5AC secretion while, conversely, depletion of endogenous miR-330 exacerbated MUC5AC secretion. Munc18b (Syntaxin-Binding Protein 2; STXBP2) is a limiting component of the exocytic machinery of airway epithelial cells. We identified and validated that Munc18b was a direct target of miR-330 and miR-330 regulated MUC5AC secretion in HBE16 cells by acting directly on the 3’UTR of Munc18b mRNA. Collectively, these data reveal that miR-330 inhibits IL-13-induced MUC5AC secretion in human bronchial epithelial cells by targeting Munc18b, encouraging us to further explore the potential of manipulating miR-330 in treatment of airway diseases with mucus hypersecretion.
Keywords: Asthma, miR-330, MUC5AC, Munc18b
Introduction
Asthma is a chronic airway inflammatory disease that affects an estimated 300 million people in the world [1]. Recent epidemical studies have indicated that incidence, morbidity, and mortality caused by asthma are increasing worldwide despite important advances in treatment of this disease [2,3]. Airway mucus hypersecretion and plugging has long been observed in patients that die from asthma [4]. Airway mucus is a viscoelastic gel and consists of water, ions, cellular debris, and gel-forming mucins. Mucins, heavily glycosylated proteins with high molecular weight, are the major macromolecular components of airway mucus and are responsible for its viscoelastic, rheological, and clearance properties. In healthy airways, mucus is continuously secreted at a low basal rate and presents a first line of defense against inhaled pathogens, particulates, and environmental toxicants. When oversecreted, however, mucus contributes significantly to mortality related with certain human respiratory diseases such as asthma. In human airways, MUC5AC and MUC5B are the major mucins secreted by goblet cells of the epithelial surface and submucosal glands, respectively. MUC5B is the principal gel-forming mucin at baseline and, therefore, likely performs most homeostatic clearance functions and plays a protective role [5]. In contrast, MUC5AC is the principal gel-forming mucin upregulated in airway inflammation and has been thought to play a pathologic role [6]. In fact, many studies have confirmed MUC5AC hypersecretion in various airway diseases, including asthma [4,7,8]. MUC5AC has been shown to be upregulated in respiratory epithelial cells by various stimuli implicated in the pathogenesis of asthma, including neutrophil elastase, cytokines (interleukin (IL)-13 and IL-9), and air pollutants [9]. Mechanisms governing MUC5AC hypersecretion, nevertheless, are not well understood.
MicroRNAs (miRNAs) are a class of small noncoding RNAs with a length of 18-22 nucleotides. It has been verified that miRNAs play critical roles in posttranscriptional regulation of genes, typically by base pairing to the 3’-untranslated region (UTR) of mRNAs and causing mRNA degradation and/or translational inhibition [10]. miRNAs regulate a wide range of physiologic and pathologic processes including cell maintenance, proliferation, differentiation, aging, apoptosis, immune responses, and development [10,11]. Over 1,000 miRNA are predicted to exist in the human genome and can regulate as many as 30% of protein-encoding genes, which also underscores the potential influence of miRNAs on almost all biological processes [12,13]. Previous studies have also confirmed that some miRNAs are involved in MUC5AC production and secretion, such as miR-146a, miR-145, miR-218, miR-21, and miR-143 [14-18]. miR-330, a cancer-related miRNA, has been widely reported to repress initiation and development of various cancers including cancers of the respiratory tract, human nasopharyngeal carcinoma, and lung cancer [19,20]. Recently, several studies have linked the deregulation of miR-330 to asthma. For example, Panganiban et al. [21] found that miR-330 was significantly decreased in the blood of asthmatic patients compared with the blood of nonasthmatic patients with allergic rhinitis and nonallergic nonasthmatic subjects, possibly acting as a biomarker for asthma. However, the role of miR-330 in asthma remains unclear.
In this study, we performed cell experiments to investigate whether miR-330 functions by regulating MUC5AC secretion and investigated specific molecular mechanisms.
Materials and methods
Cell culture
Human bronchial epithelial cells HBE16 were obtained from the Institute of Biochemistry and Cell Biology (Shanghai, China) and were grown in DMEM supplemented with 10% fetal calf serum (FCS; Life Technologies, Rockville, MD, USA), 1% weight per volume penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA), and 2 mM L-glutamine (Invitrogen) in a 5% CO2-humidified atmosphere at 37°C.
Cell treatment and transfection
For treatment with IL-13, HBE16 cells with 5×105 cells/well were plated in a 6-well plate and allowed to grow to 90% confluency. Then, the cells were incubated with different concentrations (0, 10, 20, 40, 60 and 80 ng/mL) of IL-13 for 12 hours. After treatment, the cells and culture supernatants were harvested for qRT-PCR, Western blot, or ELISA detection.
For overexpression or knockdown of miR-330 or Munc18b, total RNA of HBE16 cells were isolated with TRIzol Reagent (Invitrogen) and reverse-transcribed into cDNA using a Reverse Transcription Kit (Takara, Dalian, China). Munc18b cDNA was amplified and inserted into pcDNA3.1 vectors (pcDNA-Munc18b). miR-330 mimic, miR-330 inhibitor, and specific small-interfering RNAs targeting Munc18b (si-Munc18b) and their respective controls were purchased from Sangon Biotechnology, Co., Ltd. (Shanghai, China). All transfection reactions were performed using Lipofectamine 2000 (Invitrogen). After transfection, cells were directly incubated in the fresh medium with a concentration of 60 ng/mL IL-13 for 12 hours. Culture supernatants were collected and used in ELISA detection.
RNA extraction and qRT-PCR
Total RNA was prepared using TRIzol Reagent (Invitrogen) under the guidance of operation instructions. First strand cDNA was synthesized with a Reverse Transcription Kit (Takara, Dalian, China). Expression levels of miR-330 were quantified by stem-loop RT-PCR and U6 snRNA was used as a loading control. The primer sequences for miR-330 and U6 snRNA were as follows: miR-330 forward: 5’-TTTGGCGATCACTGCCTCTC-3’; miR-330 reverse: 5’-CTCTCTGCAGGCCGTG-TG-3’; U6 snRNA forward: 5’-CTCGCTTCGGCAGCACA-3’; and U6 snRNA reverse: 5’-CTCGCTTCGGCAGCACA-3’. qRT-PCR was carried out using SYBR Premix Dimer Eraser (Takara) on a 7500HT system. Primer sequences for Munc18b and β-actin gene expression were as follows: Munc18b forward: 5’-AGATCTTATGGGATGTATTAAATCAAAAAGGAA-3’; Munc18-b reverse: 5’-CCGCGGTCCTGGCTGCTGCTGATACTGC-3’; β-actin forward: 5’-TGGAATCCTGTGGCATCCATGAAAC-3’; and β-actin reverse: 5’-TAAAACGCAGCTCAGTAACAGTCCG-3’. Relative Munc18b mRNA level was normalized to β-actin. Fold changes of gene expression were calculated using 2-ΔΔCt method.
ELISA
MUC5AC protein levels in culture supernatants were determined with Human MUC5AC ELISA kit (USCNK, Wuhan, China), according to instructions provided by the manufacturer.
Western blot assay
Cells were lysed in a lysis buffer containing 50 mmol/l Tris (pH 7.4), 1 mmol/l ethylene diamine tetraacetic acidsodium (EDTA), 0.2% sodium deoxycholate, 1% Triton X-100, and complete protease inhibitors. Protein concentrations were determined by Bio-Rad assays. Equal amounts of cell lysate were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon Hybond-C membranes (Thermo Fisher Scientific, Pittsburgh, PA, USA), and probed with appropriate primary and secondary antibodies. Blots were visualized by Pierce ECL substrate Western blot detection system (Thermo Fisher Scientific). Integrated density of the blots was measured using Image J software (National Institutes of Health, MD, USA). Primary antibodies against Munc18b (Abcam, Cambridge, MA, USA), β-actin (Abcam), and secondary antibody peroxidase-conjugated anti-IgG (Abcam) were employed in this assay.
Luciferase reporter assay
HBE16 cells were co-transfected with miR-330 mimic or mimic control and pMIR-report luciferase vector containing Munc18b gene fragments with wild-type (WT) or mutant (MUT) binding sites of miR-330 by Lipofectamine 2000, under the guidance of operation instructions. Forty-eight hours later, cells were collected and luciferase activity was determined with a Dual Luciferase Reporter gene assay kit (BioVision, Milpitas, CA, USA).
Statistical analysis
All statistical analyses were carried out on SPSS17.0 software (SPSS, Chicago, IL, USA). Values are expressed as mean ± standard deviation (SD). Comparisons between groups were performed via Student’s t-test or ANOVA. P values of less than 0.05 denoted the presence of a statistically significant difference.
Results
IL-13 treatment induces MUC5AC secretion and inhibits miR-330 expression
To further confirm if IL-13 can induce MUC5AC secretion, HBE16 cells were treated with different concentrations (0, 10, 20, 40, 60 and 80 ng/mL) of IL-13 for 12 hours and then culture supernatants were collected for MUC5AC detection by ELISA. The results showed that MUC5AC levels increased with IL-13 concentration increasing from 0 to 60 ng/mL, with no difference at concentrations of 60 and 80 ng/mL (Figure 1A). In addition, after 12 hours of treatment with different concentrations of IL-13, cells were also collected for miR-330 detection by qRT-PCR assay. These results showed that IL-13 suppressed miR-330 expression in a concentration-dependent manner (Figure 1B). Taken together, these data suggest that IL-13 induces MUC5AC secretion and inhibits miR-330 expression.
Figure 1.
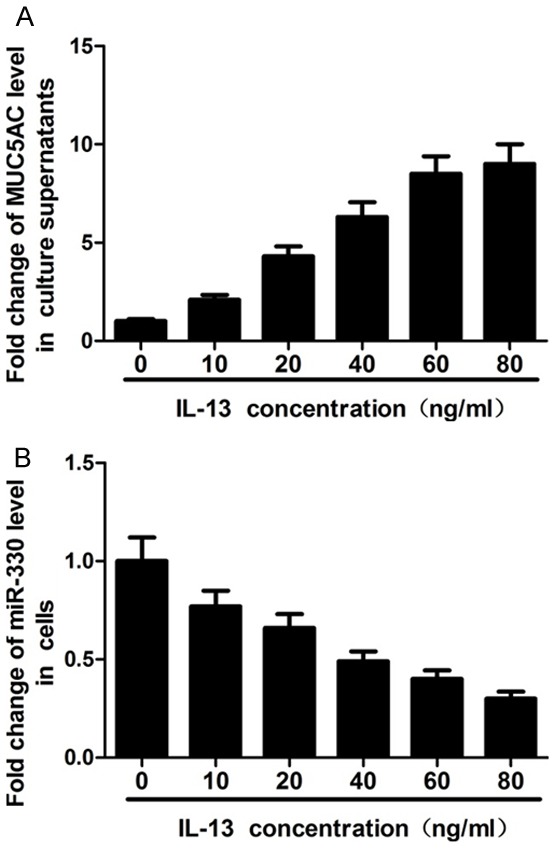
Effects of IL-13 treatment on MUC5AC secretion and miR-330 expression in HBE16 cells. A: ELISA shows that MUC5AC levels increase with IL-13 concentration increasing from 0 to 60 ng/ml, with no difference at the concentration of 60 and 80 ng/ml. B: qRT-PCR shows that IL-13 suppresses miR-330 expression in a concentration-dependent manner.
miR-330 regulates MUC5AC secretion induced by IL-13
Next, the effects of miR-330 expression on IL-13-induced MUC5AC secretion were explored. HBE16 cells were transfected with miR-330 mimic, miR-330 inhibitor, or their respective controls. After transfection, miR-330 levels were detected by qRT-PCR assay. Our results showed that miR-330 mimic transfection significantly increased miR-330 levels compared with mimic control and miR-330 inhibitor obviously decreased miR-330 expression compared with inhibitor control, confirming effective transfection (Figure 2A and 2B). These transfected cells were then exposed to IL-13 (60 ng/mL) for 12 hours and culture supernatants were collected for MUC5AC level detection. Results showed that increase of miR-330 inhibited MUC5AC secretion induced by IL-13, while decrease of miR-330 exacerbated IL-13-induced MUC5AC secretion (Figure 2C and 2D). These data indicate that miR-330 negatively regulates IL-13-induced MUC5AC secretion.
Figure 2.
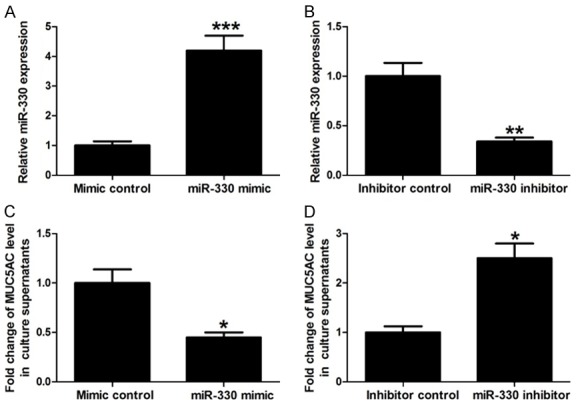
Association between miR-330 expression and MUC5AC secretion induced by IL-13. A and B: qRT-PCR shows miR-330 expression in HBE16 cells transfected with miR-330 mimic, miR-330 inhibitor, or their respective controls. C and D: ELISA shows that the increase of miR-330 inhibits MUC5AC secretion induced by IL-13, while the decrease of miR-330 exacerbates IL-13-induced MUC5AC secretion. *P < 0.05, **P < 0.01 and ***P < 0.001.
miR-330 targets Munc18b
To further investigate the molecular mechanism whereby miR-330 regulates IL-13-induced MUC5AC secretion, miRNA target analysis tool TargetScan was employed to explore potential targets of miR-330. Multiple targets were identified. In these targets, Syntaxin-Binding Protein 2 (STXBP2; Munc18b) was selected for further exploration since this gene had been reported to be a limiting component of the exocytic machinery of airway epithelial cells (Figure 3A). The effects of IL-13 on Munc18b mRNA and protein expression were determined by qRT-PCR and Western blot assay. These results showed that IL-13 promoted Munc18b mRNA and protein expression in a concentration-dependent manner (Figure 3B and 3C). Furthermore, the increase of miR-330 obviously suppressed Munc18b mRNA and protein expression and decrease of miR-330 significantly enhanced Munc18b mRNA and protein expression (Figure 3D-G). To further confirm that miR-330 modulates Munc18b expression by base pairing to the 3’-UTR of its mRNA, luciferase reporter assay was performed. These results showed that overexpression of miR-330 significantly repressed luciferase activity of the wild-type reporter gene but it had less effect on the mutant reporter (Figure 3H). These results indicate that miR-330 regulates Munc18b expression by binding to the 3’-UTR of its mRNA.
Figure 3.
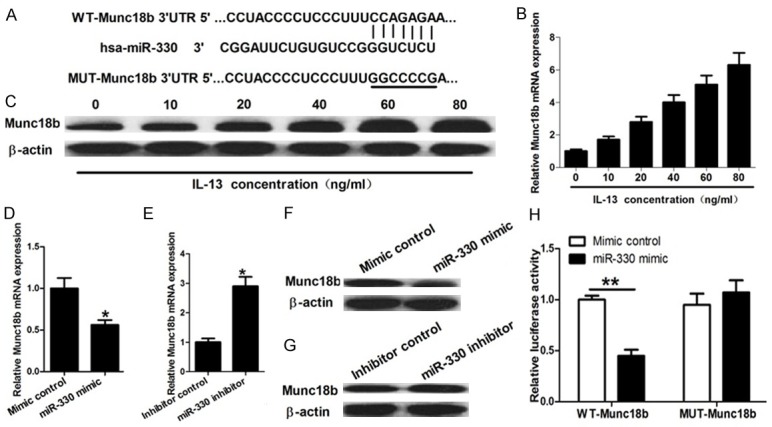
miR-330 targets Munc18b. A: Bioinformatics-based target prediction analysis shows that Munc18b is a potential target gene of miR-330 and the binding site is on the 3’UTR of Munc18b. B and C: qRT-PCR and Western blot assay show that IL-13 promotes Munc18b mRNA and protein expression in a concentration-dependent manner. D-G: qRT-PCR and Western blot assay show that the increase of miR-330 obviously suppresses Munc18b mRNA and protein expression and decrease of miR-330 significantly enhances Munc18b mRNA and protein expression. H: Luciferase reporter assay shows that overexpression of miR-330 significantly represses the luciferase activity of the wild-type reporter gene but it has less effect on the mutant reporter. *P < 0.05 and **P < 0.01.
miR-330 regulates IL-13-induced MUC5AC secretion by targeting Munc18b
To further explore whether miR-330 functions by targeting Munc18b, HBE16 cells were transfected with pcDNA-Munc18b, si-Munc18b, or their respective controls. After transfection, Munc18b mRNA and protein expression were detected by qRT-PCR and Western blot assay. Results showed that the si-Munc18b group had lower Munc18b mRNA and protein levels than si-control group and the pcDNA-Munc18b group had significantly higher Munc18b mRNA and protein levels than pcDNA-control group, indicating that HBE16 cells with Munc18b knockdown or overexpression were successfully established (Figure 4A-D). These cells were then treated with IL-13 (60 ng/mL) for 12 hours and culture supernatants were collected for MUC5AC detection. These results showed that Munc18b knockdown significantly inhibited IL-13-induced MUC5AC secretion and Munc18b overexpression obviously aggravated MUC5AC secretion induced by IL-13 (Figure 4E and 4F). Collectively, these data suggest that miR-330 inhibits IL-13-induced MUC5AC secretion by targeting Munc18b.
Figure 4.
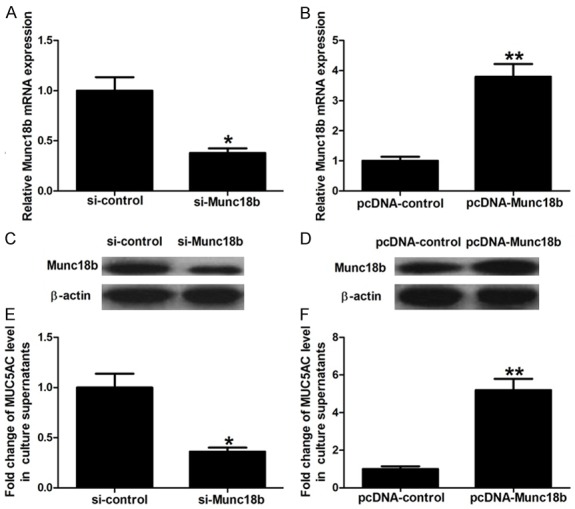
Function of Munc18b. A-D: qRT-PCR and Western blot assay show Munc18b mRNA and protein levels in HBE16 cells transfected with si-Munc18b, pcDNA-Munc18b, or their respective controls. E and F: ELISA shows that Munc18b knockdown significantly inhibits IL-13-induced MUC5AC secretion, and Munc18b overexpression obviously aggravates MUC5AC secretion induced by IL-13. *P < 0.05 and **P < 0.01.
Discussion
Excessive mucus secretion is a hallmark of patients with chronic inflammatory airway diseases such as asthma, chronic bronchitis, cystic fibrosis, and chronic obstructive pulmonary disease, causing airway obstruction and gas-exchange impairment. In fact, it has long been recognized that mucus hypersecretion by airway epithelium and plugging of the airways are primary reasons of mortality in asthma patients and major causes of asthma disease progression and exacerbation. MUC5AC, a major mucin secreted by human airway epithelial cells, has been reported to be upregulated in various chronic inflammatory airway diseases, including asthma. For example, Caramori et al. [7] reported that MUC5AC expression is increased in bronchial submucosal glands of stable chronic obstructive pulmonary disease patients. Henke et al. [8] reported that MUC5AC increases in cystic fibrosis airway secretions during pulmonary exacerbation and Groneberg et al. [4] found that MUC5AC is the main mucin in mild-to-moderate asthma and increases in fatal asthma.
IL-13, the primary stimulatory factor implicated in the pathogenesis of asthma, is the most extensively researched cause in mucus hypersecretion. It has been documented that IL-13 can induce MUC5AC secretion in airway epithelia [22-24]. Our previous studies have also showed that IL-13 can induce MUC5AC production and secretion in HBE16 cells [25]. In this study, we further confirmed a dose-dependent induction of MUC5AC secretion by IL-13 in HBE16 cells.
miRNAs have formed a novel field of biology with increasing evidence suggesting that they play a critical role in almost every biological process, including MUC5AC production and secretion. For example, Zhong et al. [14] reported that miR-146a negatively regulates neutrophil elastase-induced MUC5AC secretion from HBE16 cells. Cheng et al. [15] reported that miR-145 alleviates airway remodeling by downregulating MUC5AC and inhibits cytokine expression by targeting EGFR. Lampe et al. [17] illustrated that miR-21 decreases MUC5AC secretion by downregulating expression of Myristoylated Alanine-Rich C Kinase Substrate, an important regulator of airway mucin secretion. Xu et al. [16] found that miR-218 inhibits cigarette smoke extract-induced MUC5AC hyper-production and inflammation by targeting tumor necrosis factor receptor 1-mediated activation of NF-κB. Liu et al. [18] reported that miR-143 inhibits IL-13-induced inflammatory cytokine and MUC5AC production in nasal epithelial cell from allergic rhinitis patients partly by targeting IL-13 receptor α1 chain. miR-330 has been widely reported to be involved in various diseases. For example, miR-330 regulates lipopolysaccharide (LPS)-induced disseminated intravascular coagulation by targeting aquaporin 5; miR-330 regulates proliferation of mouse epidermal keratinocytes by inhibiting Srpr expression; miR-330 suppresses cell proliferation and invasion in cutaneous malignant melanoma by regulating tyrosinase and PDIA3 expression; miR-330 is a putative modulator of neoadjuvant chemoradiotherapy sensitivity in esophageal adenocarcinoma; and miR-330 functions as a tumor suppressor by downregulating MUC1 mucin in pancreatic cancer cells [26-30]. Recently, several studies have reported the deregulation of miR-330 in chronic airway diseases. For example, Yang et al. [31] reported that miR-330 was decreased in serums of idiopathic pulmonary fibrosis patients compared to healthy controls and Panganiban et al. [21] reported that miR-330 was significantly downregulated in the blood of asthmatic patients and might serve as a biomarker of asthma. Little is known about the role of miR-330 in asthma, however. Here, we first investigated the effects of IL-13 on miR-330 expression and found that IL-13 inhibits miR-330 expression in a concentration-dependent manner. We then investigated the effects of miR-330 on IL-13-induced MUC5AC secretion and found that ectopic expression of miR-330 inhibits IL-13-induced MUC5AC secretion while, conversely, knockdown of this miRNA causes a significant increase in MUC5AC secretion.
Sec1/Munc18 (SM) family is a critical component of the eukaryotic trafficking machinery. In one model of SM-SNARE interaction, SM protein binding to the t-SNARE Syntaxin prevents SNARE complex formation and suppresses exocytosis but simultaneously induces a conformational change in Syntaxin, which is necessary to interaction of bopenQ Syntaxin and other SNARE proteins. Munc18 proteins are mammalian SM proteins specialized for exocytosis [32]. Munc18 has three isoforms, Munc18a, Munc18b, and Munc18c [33]. Kim et al. [34] reported that Munc18b is an essential gene in mice and its expression is limiting for secretion by airway epithelial and mast cells. Our previous study also demonstrated that Munc18b knockdown significantly inhibits IL-13-induced MUC5AC secretion in HBE16 cells [25]. In this study, we identified and confirmed that Munc18b is a target of miR-330 via a series of experiments. Furthermore, we also further confirmed the role of Munc18b that Munc18b is limiting for IL-13-induced MUC5AC secretion in HBE16 cells. Collectively, these data suggest that miR-330 controls IL-13-induced MUC5AC secretion by targeting Munc18b in bronchial epithelial cells.
In conclusion, this study first uncovered that miR-330 inhibits MUC5AC secretion induced by IL-13 in human bronchial epithelial cells via downregulation of Munc18b. These findings encourage us to further explore the potential of manipulating miR-330 in treatment of airway diseases with mucus hypersecretion.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant No. 81400024), Natural Science Foundation of Shandong Province of China (grant No. ZR2014HM105), and Qingdao Outstanding Health Professional Development Fund.
Disclosure of conflict of interest
None.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy. 2004;59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Keller M, Lowenstein S. Epidemiology of asthma. Semin Respir Crit Care Med. 2002;23:317–29. doi: 10.1055/s-2002-34327. [DOI] [PubMed] [Google Scholar]
- 3.Buist A. Worldwide trends in asthma morbidity and mortality. Bull Int Union Tuberc Lung Dis. 1991;66:77–8. [PubMed] [Google Scholar]
- 4.Groneberg D, Eynott P, Lim S, Oates T, Wu R, Carlstedt I, Roberts P, McCann B, Nicholson A, Harrison B, Chung K. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology. 2002;40:367–73. doi: 10.1046/j.1365-2559.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- 5.Wickström C, Davies J, Eriksen G, Veerman E, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J. 1998;334:685–93. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hovenberg H, Davies J, Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. Biochem J. 1996;318:319–24. doi: 10.1042/bj3180319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caramori G, Casolari P, Di Gregorio C, Saetta M, Baraldo S, Boschetto P, Ito K, Fabbri L, Barnes P, Adcock I, Cavallesco G, Chung K, Papi A. MUC5AC expression is increased in bronchial submucosal glands of stable COPD patients. Histopathology. 2009;55:321–31. doi: 10.1111/j.1365-2559.2009.03377.x. [DOI] [PubMed] [Google Scholar]
- 8.Henke M, John G, Germann M, Lindemann H, Rubin B. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med. 2007;175:816–21. doi: 10.1164/rccm.200607-1011OC. [DOI] [PubMed] [Google Scholar]
- 9.Rose M, Voynow J. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–78. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 10.Pillai R. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–61. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal A, Kasinski A. Animal models to study MicroRNA function. Adv Cancer Res. 2017;135:53–118. doi: 10.1016/bs.acr.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk R, Cuppen E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell. 2005;120:21–4. doi: 10.1016/j.cell.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Lewis B, Burge C, Bartel D. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Zhong T, Perelman J, Kolosov V, Zhou X. MiR-146a negatively regulates neutrophil elastase-induced MUC5AC secretion from 16HBE human bronchial epithelial cells. Mol Cell Biochem. 2011;358:249–55. doi: 10.1007/s11010-011-0975-2. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Z, Dai L, Wang X, Jia L, Jing X, Li P, Liu M, Wang H, An L. MicroRNA-145 down-regulates mucin 5AC to alleviate airway remodeling and targets EGFR to inhibit cytokine expression. Oncotarget. 2017;8:46312–46325. doi: 10.18632/oncotarget.17933. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Xu H, Sun Q, Lu L, Luo F, Zhou L, Liu J, Cao L, Wang Q, Xue J, Yang Q, Yang P, Lu J, Xiang Q, Liu Q. MicroRNA-218 acts by repressing TNFR1-mediated activation of NF-κB, which is involved in MUC5AC hyper-production and inflammation in smoking-induced bronchiolitis of COPD. Toxicol Lett. 2017;280:171–180. doi: 10.1016/j.toxlet.2017.08.079. [DOI] [PubMed] [Google Scholar]
- 17.Lampe WR, Fang S, Yin Q, Crews AL, Park J, Adler KB. Mir-21 regulation of MARCKS protein and mucin secretion in airway epithelial cells. Open Journal of Respiratory Diseases. 2013;3:89–96. [Google Scholar]
- 18.Teng Y, Zhang R, Liu C, Zhou L, Wang H, Zhuang W, Huang Y, Hong Z. miR-143 inhibits interleukin-13-induced inflammatory cytokine and mucus production in nasal epithelial cells from allergic rhinitis patients by targeting IL13Rα1. Biochem Biophys Res Commun. 2015;457:58–64. doi: 10.1016/j.bbrc.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 19.Song P, Yin S. Long non-coding RNA EWSAT1 promotes human nasopharyngeal carcinoma cell growth in vitro by targeting miR-326/-330-5p. Aging (Albany NY) 2016;8:2948–2960. doi: 10.18632/aging.101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Shi H, Liu B, Li J, Liu Y, Yu B. miR-330-3p controls cell proliferation by targeting early growth response 2 in non-small-cell lung cancer. Acta Biochim Biophys Sin (Shanghai) 2015;47:431–40. doi: 10.1093/abbs/gmv032. [DOI] [PubMed] [Google Scholar]
- 21.Panganiban R, Wang Y, Howrylak J, Chinchilli V, Craig T, August A, Ishmael F. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 2016;137:1423–32. doi: 10.1016/j.jaci.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Laoukili J, Perret E, Willems T, Minty A, Parthoens E, Houcine O, Coste A, Jorissen M, Marano F, Caput D, Tournier F. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest. 2001;108:1817–24. doi: 10.1172/JCI13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang D, Li Q, Kolosov V, Zhou X. The inhibition of aldose reductase on mucus production induced by interleukin-13 in the human bronchial epithelial cells. Int Immunopharmacol. 2012;12:588–93. doi: 10.1016/j.intimp.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Qin Y, Jiang Y, Sheikh A, Shen S, Liu J, Jiang D. Interleukin-13 stimulates MUC5AC expression via a STAT6-TMEM16A-ERK1/2 pathway in human airway epithelial cells. Int Immunopharmacol. 2016;40:106–114. doi: 10.1016/j.intimp.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Hao W, Wang J, Zhang Y, Wang Y, Sun L, Han W. Leptin positively regulates MUC5AC production and secretion induced by interleukin-13 in human bronchial epithelial cells. Biochem Biophys Res Commun. 2017;493:979–984. doi: 10.1016/j.bbrc.2017.09.106. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Chen M, Zhang Y, Peng P, Li J, Xin X. miR-96 and miR-330 overexpressed and targeted AQP5 in lipopolysaccharide-induced rat lung damage of disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 2014;25:731–7. doi: 10.1097/MBC.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 27.Kim B, Yoo H, Choi K, Lee A, Yoon S. Regulation of Srpr expression by miR-330-5p controls proliferation of mouse epidermal keratinocyte. PLoS One. 2016;11:e0164896. doi: 10.1371/journal.pone.0164896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su B, Zhou S, Gan C, Zhang X. MiR-330-5p regulates tyrosinase and PDIA3 expression and suppresses cell proliferation and invasion in cutaneous malignant melanoma. J Surg Res. 2016;203:434–40. doi: 10.1016/j.jss.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Correction: MicroRNA-330-5p as a putative modulator of neoadjuvant chemoradiotherapy sensitivity in oesophageal adenocarcinoma. PLoS One. 2015;10:e0137155. doi: 10.1371/journal.pone.0137155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tréhoux S, Lahdaoui F, Delpu Y, Renaud F, Leteurtre E, Torrisani J, Jonckheere N, Van Seuningen I. Micro-RNAs miR-29a and miR-330-5p function as tumor suppressors by targeting the MUC1 mucin in pancreatic cancer cells. Biochim Biophys Acta. 2015;1853:2392–403. doi: 10.1016/j.bbamcr.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 31.Yang G, Yang L, Wang W, Wang J, Wang J, Xu Z. Discovery and validation of extracellular/circulating microRNAs during idiopathic pulmonary fibrosis disease progression. Gene. 2015;562:138–44. doi: 10.1016/j.gene.2015.02.065. [DOI] [PubMed] [Google Scholar]
- 32.Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Südhof T, Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 1999;18:4372–82. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nigam R, Sepulveda J, Tuvim M, Petrova Y, Adachi R, Dickey B, Agrawal A. Expression and transcriptional regulation of Munc18 isoforms in mast cells. Biochim Biophys Acta. 2005;1728:77–83. doi: 10.1016/j.bbaexp.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Kim K, Petrova Y, Scott B, Nigam R, Agrawal A, Evans C, Azzegagh Z, Gomez A, Rodarte E, Olkkonen V, Bagirzadeh R, Piccotti L, Ren B, Yoon J, McNew J, Adachi R, Tuvim M, Dickey B. Munc18b is an essential gene in mice whose expression is limiting for secretion by airway epithelial and mast cells. Biochem J. 2012;446:383–94. doi: 10.1042/BJ20120057. [DOI] [PMC free article] [PubMed] [Google Scholar]


