Abstract
As a complex pathophysiological event, myocardial ischemia/reperfusion injury (IRI) can cause heart failure, which has been associated with pyroptosis, a pro-inflammatory programmed cell death. Small endogenous non-coding RNAs have been shown to be involved in myocardial IRI. In the present study, we aimed to investigate whether miR-424 modulated pyroptosis in response to myocardial IRI and determine its underlying regulatory mechanism. An in vivo mouse model of cardiac IRI was established, and contractile function was evaluated by echography. The serum and heart tissue were harvested 24 h after reperfusion to assess the status of pyroptosis. For the in vitro study, H9C2 cells (a rat heart cell line) were subjected to 6 h of hypoxia, followed by 18 h of reoxygenation. The gene expressions at the mRNA level were assessed by real-time PCR, and the expressions at the protein level were examined by western blotting, immunofluorescence staining, and enzyme-linked immunosorbent assay (ELISA). Bioinformatic analysis was applied to predict miR-424 targets, which were then confirmed by a luciferase reporter assay. We found that the expressions of pyroptosis-related proteins, including caspase-1, caspase-11, IL-1β, and IL-18, were significantly increased upon myocardial IRI. Similarly, hypoxia/reoxygenation injury (HRI) also induced pyroptosis in H9C2 cells. Furthermore, our study revealed that the miR-424 expression was substantially increased in I/R heart tissue and H/R-challenged H9C2 cells. In addition, we found that exogenous expression of miR-424 directly targeted cysteine-rich secretory protein LCCL domain-containing 2 (CRISPLD2) and up-regulated the expressions of caspase-1 and the pro-inflammatory cytokines IL-1β and IL-18. Taken together, our findings provided a new signaling pathway of miR-424/CRISPLD2 in cardiac pyroptosis under IRI conditions.
Keywords: Myocardial ischemic/reperfusion injury, pyroptosis, miR-424, CRISPLD2
Introduction
The best way to limit infarct size for patients with acute myocardial infarction is by an early reperfusion of the occluded coronary artery using primary percutaneous coronary intervention, which contributes to preserving left ventricular (LV) contraction and preventing the onset of heart failure. Although reperfusion can salvage the myocardium, reperfusion itself paradoxically induces further cardiomyocyte death, which is generally known as myocardial ischemia/reperfusion injury (IRI) [1-4]. However, cardiac IRI triggers pronounced tissue-disruptive and sterile proinflammatory responses, which compromise outcome. Numerous strategies have been used to ameliorate myocardial IRI in animal models [5-8]. However, the translation of these beneficial effects to the clinical setting is disappointing. Therefore, it is beneficial to determine the mechanisms underlying myocardial IRI and identify novel therapeutic targets that can effectively suppress this adverse process.
As a class of endogenous, small-sized (~22 nucleotides), non-coding single-stranded RNAs, microRNAs (miRNAs) can anneal to nearly complementary sequences in the 3’ untranslated regions (3’-UTRs) of target mRNAs to either facilitate their degradation or repress the translation process [9]. Much evidence has shown that miRNAs are involved in a wide variety of biological processes, including cell proliferation, differentiation, metastasis, apoptosis, and immune responses [10-13]. Moreover, they also function as prognostic markers in the development and progression of ischemic cardiac disease by targeting pertinent genes [14-20].
As a pro-inflammatory programmed cell death [21], pyroptosis has the biochemical and morphologic characteristics of necrosis and apoptosis. However, pyroptosis, unlike apoptosis or necrosis [22], results in the release of cytokines that activate pro-inflammatory immune cell mediators [23-26]. Caspase-1 is activated during pyroptosis by a large supramolecular complex known as the pyroptosome, and it subsequently processes the inactive precursors of inflammatory cytokines interleukin (IL)-1β and IL-18 into their active forms to trigger or aggravate inflammatory responses [27-29]. Therefore, pyroptosis may not only cause cell death but also play an important role in the cascade of reactions that lead to damaged tissues. Several studies have indicated that pyroptosis contributes to infectious diseases, nervous system disorders and atherosclerosis [30-32]. During the development and progression of IRI, cardiomyocyte death and inflammation may affect the severity and prognosis of IRI [33-35]. In addition, miRNA-9 has been reported to inhibit hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. However, whether pyroptosis is involved in cardiomyocyte death upon IRI and how pyroptosis is correlated with inflammatory cytokines remain largely unexplored. Collectively, it is necessary to assess the underlying mechanism of pyroptosis during the development of IRI. Moreover, few studies have focused on the participation of miRNAs in pyroptosis in ischemic heart disease.
In the present study, we aimed to elucidate the essential role of miRNAs in regulating ischemic heart diseases and the underlying mechanisms. Our data demonstrated that miR-424 promoted cardiomyocyte pyroptosis by directly targeting cysteine-rich secretory protein LCCL domain-containing 2 (CRISPLD2), a GC and developmentally regulated gene encoding a secreted mesenchymal protein in lung and other organs [36,37]. Based on our findings, we verified that miR-424 played a crucial role in the pathogenesis of cardiomyocyte pyroptosis, suggesting that miR-424 could be used as a potential therapeutic target in the treatment of ischemic heart diseases.
Material and methods
Animals
Male C57BL/6 mice (6-7 weeks of age; 20-30 g of weight) were obtained from the Slac Laboratory Animal Center (Shanghai Slac Laboratory Animal Co., Ltd., China). The experimental protocols were approved by the Institutional Animal Care and Use Committee of the Second Military Medical University.
Cardiac IRI
Animals were anesthetized with inhaled isoflurane using an endotracheal tube, and positive-pressure ventilation was carried out with a constant-volume ventilator operating on the Starling principle (HSE MiniVent, Harvard Apparatus GmbH). After the thoracic cavity was opened by left thoracotomy, an 8-0 prolene suture was passed under the left anterior descending (LAD) coronary artery at the inferior edge of the left atrium and tied to produce an occlusion. Body temperature was maintained at 37°C using a heating pad, and the temperature was monitored using a rectal thermometer. After 60 min of ischemia, the ligature was released to reperfuse the LAD coronary artery. Reperfusion was confirmed by the visible restoration of color in the ischemic myocardium and inversion of the T wave on the electrocardiogram. The chest was closed with continuous 6-0 prolene sutures. The endotracheal tube was removed until spontaneous respiration was resumed. A sham operation including all procedures was carried out as a control, except for the ligation of LAD coronary artery.
Echocardiography
Echocardiography was performed by Vevo2100 (VisualSonics, Ontario, Canada) as previously described. Parameters were measured from M-mode images acquired from the parasternal short-axis view at the papillary muscle level, including the percentages of fractional shortening (FS%) and ejection fraction (EF%), LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV).
Cell culture and HRI
Rat heart cell line H9C2 was obtained from American Type Culture Collection (Manassas, VA, USA). Cells were seeded in 100-mm culture dishes and maintained in Dulbecco’s modified Eagle’s medium (ThermoFisher, USA) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 2 mM glutamine, 100 μg/mL streptomycin and 1 mM HEPES at 37°C in humidified air containing 5% CO2. The culture medium was replaced every other day. For the H/R group, cells were exposed to hypoxia (5% CO2, 1% O2 and 94% N2) for 24 h, followed by 12 h of reoxygenation (5% CO2, 21% O2 and 74% N2). To determine the effect of miR-424, cells were transfected with either miR-424 mimic or inhibitor (Sigma-Aldrich, MO, USA) using PureFection reagents (System Biosciences, CA, USA) at 12 h prior to the H/R treatment.
Measurement of malondialdehyde (MDA) content and superoxide dismutase (SOD) activity
Oxidative stress in H9C2 cells was assessed by measuring the MDA content and SOD activity. The intracellular concentration of MDA was determined with commercial kits (Beyotime) by the thiobarbituric acid method, and measurement was performed at a wavelength of 535 nm. The results were reported as μmol per milligram of extracted protein. The SOD activity in the kidney was detected using a Total Superoxide Dismutase Assay Kit (Beyotime) and reported as U/mg protein.
Cell viability assay
At the end of the indicated time, H9C2 cells were treated with CCK-8 reagent (10 μL/well, Sigma, USA) for an additional 2 h, and then the absorbance at a wavelength of 450 nm was recorded using a microplate absorbance reader (Tecan, Safire II, Switzerland).
Western blotting analysis
Cells were lysed in ice-cold RIPA lysis buffer (Solarbio, Beijing, China) containing 0.1 mM PMSF and a protease inhibitor cocktail (Roche) for 30 min on ice. Samples were subjected to 12% SDS-PAGE and then transferred onto nitrocellulose membranes. Blots were probed with primary antibodies against caspase-1 (Abcam, Cambridge, UK), caspase-11 (Abcam), CRISPLD2 (Abcam) and GAPDH (Santa Cruz Biotechnology). After four washes with PBS containing 0.1% Tween-20, blots were then incubated with horseradish peroxidase-conjugated secondary antibodies. The immunoreactive bands were detected with Pierce® ECL Western blotting substrate (Pierce, Rockford, IL, USA) according to the manufacturer’s instructions and exposed on X-ray films (Kodak, Rochester, NY, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from rat kidneys with TRIzol reagent (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. Purified RNA (1 μg) was reversely transcribed into cDNA by Superscript II reverse transcriptase (Invitrogen) and random primer oligonucleotides (Invitrogen). qRT-PCR was performed on the 7900 HT Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), and GAPDH was selected as a housekeeping gene. Gene-specific TaqMan miRNA assay probes (Applied Biosystems) were used to analyze the expressions of miRNAs. Briefly, 1 μg total RNA was reversely transcribed into cDNA using AMV reverse transcription (Takara, Kyoto, Japan) and a stem-loop RT primer (Applied Biosystems). The expression of miRNA in cells and tissues was normalized to U6 snRNA. All experiments were performed in triplicate. The relative expressions of target genes were calculated by 2-ΔΔCt method.
ELISA
Blood samples were collected to measure the serum concentrations of IL-1β and IL-18 using ELISA kits according to the manufacturer’s instructions (uscn-SEA064R and uscn-SEA563Ra, respectively).
Luciferase assay
CRISPLD2 3’-UTRs containing conserved miR-424 binding sites as well as 3’-UTRs with mutated sites were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) and amplified by PCR. The PCR fragments were subcloned into the XhoI and NotI sites downstream from the luciferase gene in the psi-CHECK2 vector (Promega Biotech Co., Ltd., Madison, WI, USA). The 3’-UTR of luciferase vector (150 ng) was co-transfected into H9C2 cells with either miR-424 mimic or inhibitor using Lipofectamine 2000 (ThermoFisher), and 20 ng of Renilla luciferase reporter was used as an internal control. After 48 h, the cells were collected and lysed. A luciferase activity assay was performed using the Dual-Luciferase Reporter Assay System (Promega Biotech Co., Ltd.) according to the manufacturer’s instructions.
Statistical analysis
Continuous variables were presented as the mean ± S.E.M. Analysis of variance (ANOVA) and post-hoc Bonferroni analysis were conducted for multiple comparisons by GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). P values <0.05 were considered significant.
Results
IRI induces cardiac pyroptosis and inflammation as well as elevated miR-424 expression
A mouse model of cardiac IRI was used to investigate the effects of IRI on cardiac pyroptosis and inflammation, and mice were subjected to either sham surgery or IRI. Echocardiography (Figure 1A) was performed 24 h after reperfusion. Data showed that EF% and FS% were significantly decreased (Figure 1B), while LVEDD, LVESD, LVEDV and LVESV were markedly decreased (Figure 1B). These results indicated that IRI impaired the cardiac function in mice. The concentrations of serum IL-1β and IL-18 were significantly increased in mice with IRI (Figure 1C). qRT-PCR showed the similar changes in terms of IL-1β and IL-18 expressions (Figure 1D). Western blotting revealed that the protein levels of cleaved caspase-1 and caspase-11 were markedly increased after IRI (Figure 1E). These results suggested that IRI induced dramatic cardiac pyroptosis and inflammation. Moreover, the expression of miR-424 was increased in IRI heart tissues.
Figure 1.
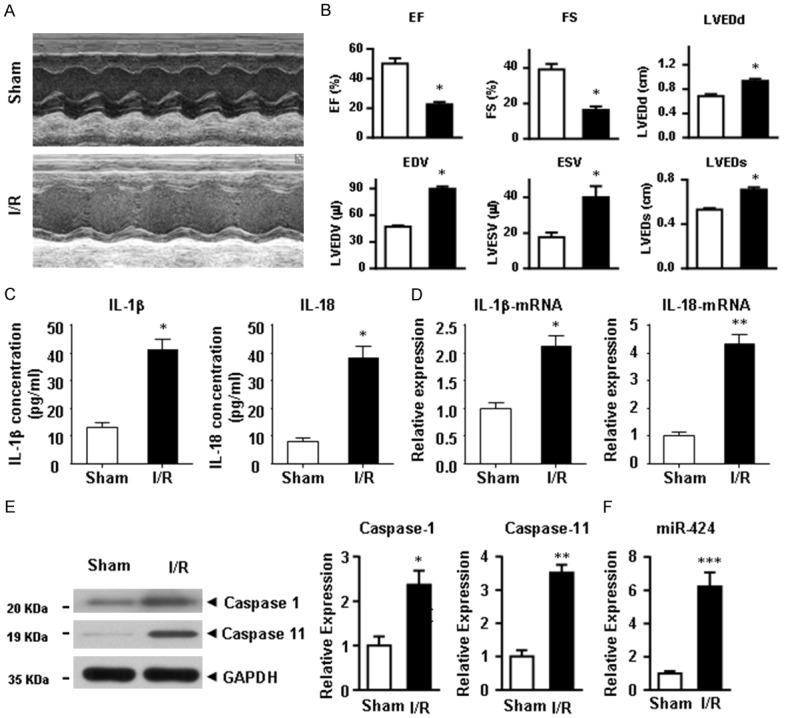
IRI induces cardiac pyroptosis, inflammation, and miR-424 up-regulation. Hearts from individual mice were reperfused for 24 h. Control mice underwent a sham surgery (sham group) without IRI. (A) The evaluation of cardiac function was detected by echocardiography. (B) The parameters of cardiac function, including EF% and FS%, were significantly decreased (B), and LVEDD, LVESD, LVEDV and LVESV were analyzed. (C) The expressions of IL-1β and IL-18 at the serum and mRNA levels (D) in heart tissues were determined by ELISA and qRT-PCR, respectively. (E) Expression of cleaved caspase-1 and caspase-11 at the protein level were determined by western blotting. (F) The expression of miR-424 at the mRNA level was determined by qRT-PCR. Data are presented as the mean ± S.E.M. n=6. *P<0.05 & **P<0.01 versus sham group.
IRI induces pyroptosis and up-regulates miR-424 expression in H9C2 cells
To investigate whether pyroptosis was induced in a rat heart cell line, H9C2 cells were subjected to H/R treatment. Figure 2A shows that compared with the control cells, H/R treatment markedly inhibited the cell proliferation (P<0.05). Consistently, in H9C2 cells, H/R treatment increased the MDA content (Figure 2B, P<0.05) but decreased the SOD activity (Figure 2C, P<0.05). FAC analysis (Figure 2D) showed that the percentage of apoptotic and necroptotic cells was remarkably increased after H/R treatment. Consistent with these findings, the expressions of IL-1β and IL-18 at the protein and mRNA levels were significantly increased in the H/R group (Figure 2E). The expressions of pyroptosis markers, cleaved caspase-1 and caspase-11 (Figure 2F), and miR-424 (Figure 2G), were significantly increased. These results suggested that pyroptosis and miR-424 expression were similarly induced in H9C2 cells after IRI (HRI).
Figure 2.
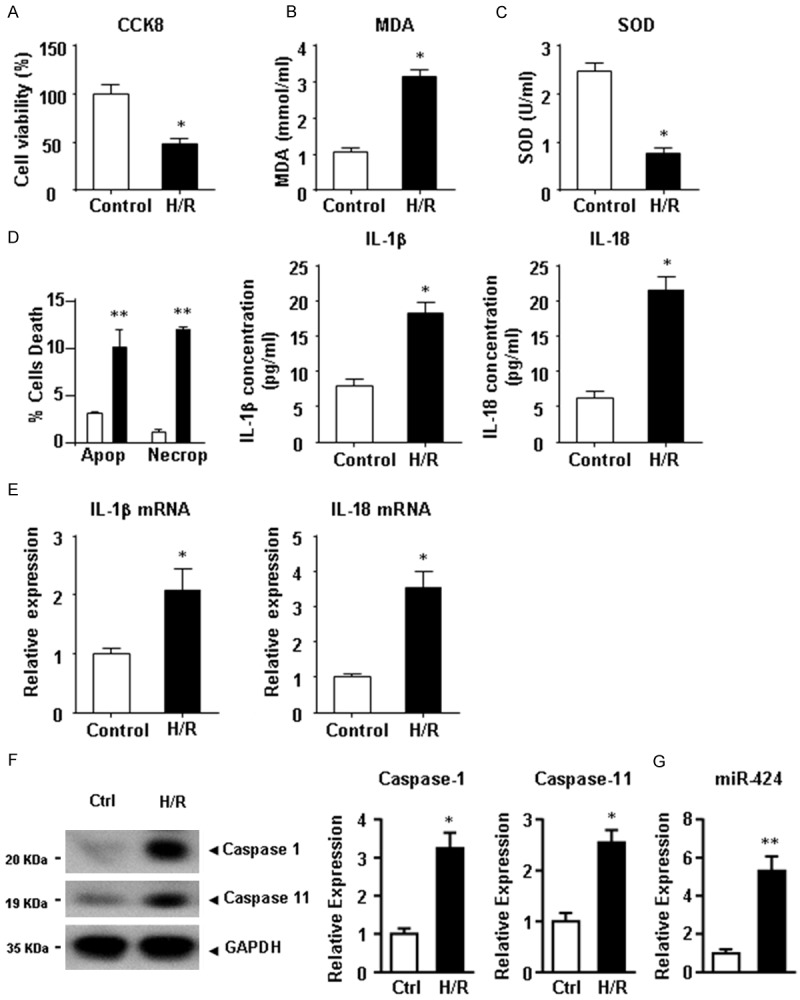
HRI induces cell pyroptosis and miR-424 up-regulation in H9C2 cells. After HRI, cell viability (A), MDA content (B) and SOD activity (C) of H9C2 cells were analyzed. (D) The proportions of apoptotic and necroptotic cells were analyzed by FACs. (D) The expressions of IL-1β and IL-18 at the protein level in culture medium and at the mRNA level (E) in H9C2 cells were determined by ELISA and qRT-PCR, respectively. (F) Expressions of cleaved caspase-1 and caspase-11 at the protein level were determined by western blotting. (G) The expression of miR-424 at the mRNA level was determined by qRT-PCR. Data are presented as the mean ± S.E.M. n=4. *P<0.05 & **P<0.01 versus control group.
CRISPLD2 is directly targeted by miR-424 and down-regulated in I/R hearts and H/R-challenged H9C2 cells
We further investigated the possible targets of miR-424 in I/R cardiac tissues. We fund that CRISPLD2 was significantly down-regulated in IRI heart tissues and H/R-challenged H9C2 cells as determined by western blotting (Figure 3A). We next performed a series of functional studies to determine the link between miR-424 and CRISPLD2. Computational analysis predicted a conserved binding site for miR-424 in the 3’-UTR of the CRISPLD2 gene (Figure 3B). To verify whether miR-424 directly targeted CRISPLD2, luciferase report constructs carrying the wild-type or mutant CRISPLD2 3’-UTR were prepared (Figure 3C). Co-transfection of miR-424 and the wild-type luciferase reporter vector into H9C2 cells caused a sharp decrease in luciferase activity compared with the transfection of luciferase vector alone (Figure 3D). Interesting, an antisense inhibitor oligonucleotide (miR-424 inhibitor) restored such miR-424 mimic-induced depression of luciferase activity (Figure 3D). However, miR-424 failed to affect the luciferase activity elicited by the construct containing the CRISPLD2 3’-UTR with the mutant miR-424 binding site (Figure 3D). Figure 3E and 3F exhibit that transfection of miR-424 into H9C2 cells remarkably reduced the CRISPLD2 expression at the mRNA and protein levels. Conversely, CRISPLD2 was significantly up-regulated when miR-424 inhibitor was transfected into H9C2 cells upon H/R treatment, indicating that CRISPLD2 was a direct target of miR-424. Over-expression of miR-424 was consistently observed in H/R-challenged HK2 cells compared with the control group (Figure 3E). We therefore suggested that miR-424 was involved in IRI by directly targeting CRISPLD2.
Figure 3.
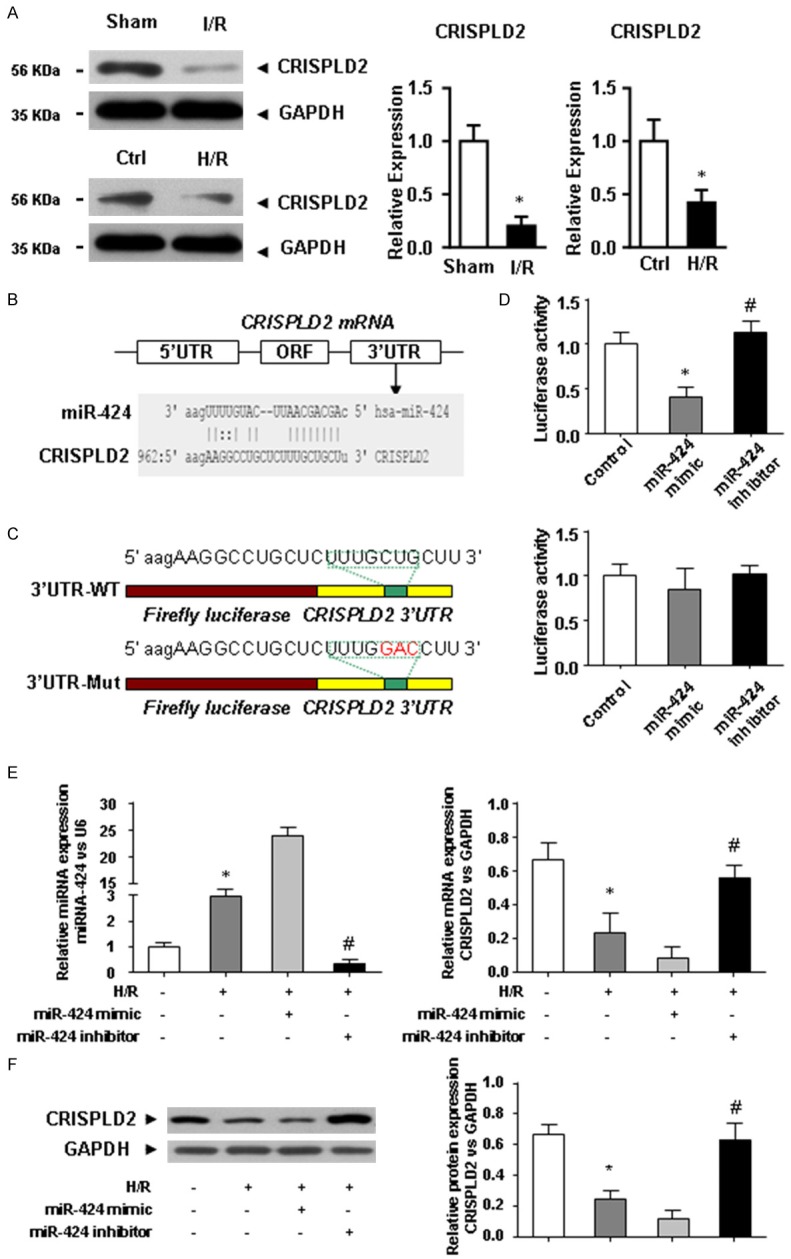
miR-424 directly targets CRISPLD2 in IRI hearts and H/R-challenged H9C2 cells. A. The expression of FoxO3a at the protein level in IRI heart tissues and H9C2 cells subjected to HRI was determined using western blotting. B. Alignment of the miR-424 sequence with that of the 3’-UTR of CRISPLD2. C. CRISPLD2 3’-UTR construct mutated at the predicted miR-424 binding site. D. Luciferase reporter activities of chimeric vectors carrying the luciferase gene and a fragment of the CRISPLD2 3’-UTR containing the miR-424 binding sites in the control, miR-424 mimic, and miR-424 inhibitor groups. E. The expression of miR-424 and CRISPLD2 at the protein level in the control, H/R, H/R + miR-424 mimic, and H/R + miR-424 inhibitor groups. F. The expression of CRISPLD2 at the protein level in the control, H/R, H/R + miR-424 mimic, and H/R + miR-424 inhibitor groups. Data are presented as the mean ± S.E.M. n=4; *P<0.05 versus control group; #P<0.05 versus H/R group.
miR-424 regulates H9C2 cell pyroptosis via targeting CRISPLD2
We explored the possible mechanisms underlying miR-424-induced pyroptosis of H9C2 cells. Figure 4A illustrates that transient expression of miR-424 inhibitor dramatically preserved the CRISPLD2 expression at the protein and mRNA levels in H/R-challenged H9C2 cells, while its expression was down-regulated by CRISPLD2 specific siRNA transfection (Figure 4A). Moreover, miR-424 inhibitor promoted the cell viability (Figure 4B) and SOD activity (Figure 4D), and decreased the MDA content (Figure 4C) in H/R-challenged H9C2 cells. However, neither miR-424 inhibitor nor CRISPLD2 specific siRNA affected H/R-induced apoptosis and necroptosis (Figure 4E). On the other hand, H/R induced the expressions of pro-inflammatory factors IL-1β and IL-18 at the protein (Figure 4F) and mRNA (Figure 4G) levels, while such effect was abolished upon transfection with miR-424 inhibitor. The protein levels of cleaved caspase-1 and caspase-11 were similar (Figure 4H). These data indicated that knockdown of miR-424 attenuated H/R-induced pyroptosis and inflammation in H9CE cells through targeting CRISPLD2.
Figure 4.
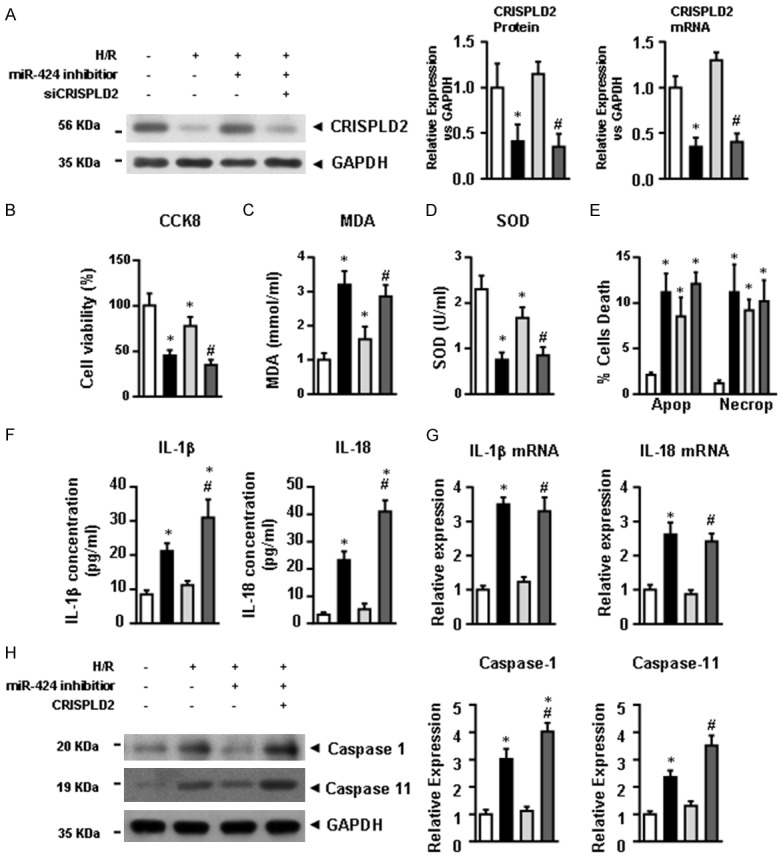
miR-424 regulates H9C2 cell pyroptosis by targeting CRISPLD2. (A) The expression of CRISPLD2 at the protein and mRNA levels in control, H/R, H/R + miR-424 inhibitor, and H/R + miR-424 inhibitor + CRISPLD2 groups. After HRI, cell viability (B), MDA content (C) and SOD activity (D) of H9C2 cells were analyzed. (E) The proportions of apoptotic and necroptotic cells were analyzed by FACs. (F) The expressions of IL-1β and IL-18 at the protein level in culture medium and at the mRNA level (G) in H9C2 cells were determined by ELISA and qRT-PCR, respectively. (H) Expression of cleaved caspase-1 and caspase-11 at the protein level were determined by western blotting. Data are presented as the mean ± S.E.M. n=4; *P<0.05 versus control group; #P<0.05 versus H/R group.
miR-424 regulates pyroptosis in IRI heart tissues via targeting CRISPLD2
To further exploit the pro-pyroptotic roles of miR-424 and CRISPLD2 in IRI heart tissues, we first evaluated the effects of miR-424 inhibitor on the expression of CRISPLD2 in IRI heart tissues. Similar with the in vitro analysis, caudal vein injection of miR-424 inhibitor dramatically preserved the expression of CRISPLD2 at the protein and mRNA levels in IRI heart tissues, which was next down-regulated by pre-transfection of recombinant adeno associated virus type 9 encoding a CRISPLD2 specific siRNA (AAV9-siCRISPLD2) (Figure 5A). Echocardiography evaluation revealed that miR-424 inhibitor significantly attenuated the impaired cardiac function, indicated by the parameters of EF%, FS%, LVEDD, LVEDS, LVEDV and LVESV (Figure 5B). As expected, down-regulation of CRISPLD2 was abolished by the cardiac protective effect of miR-424 inhibitor. Moreover, the expressions of pro-inflammatory factors IL-1β and IL-18 detected by ELISA and qPCR were aggravated after down-regulation of CRISPLD2 compared with IRI hearts (Figure 5D). Similarly, the levels of cleaved caspase-1 and caspase-11 were aggravated after CRISPLD2 down-regulation. These results suggested that miR-424 promoted the pyroptosis of cardiomyocytes in IRI hearts by down-regulating CRISPLD2.
Figure 5.
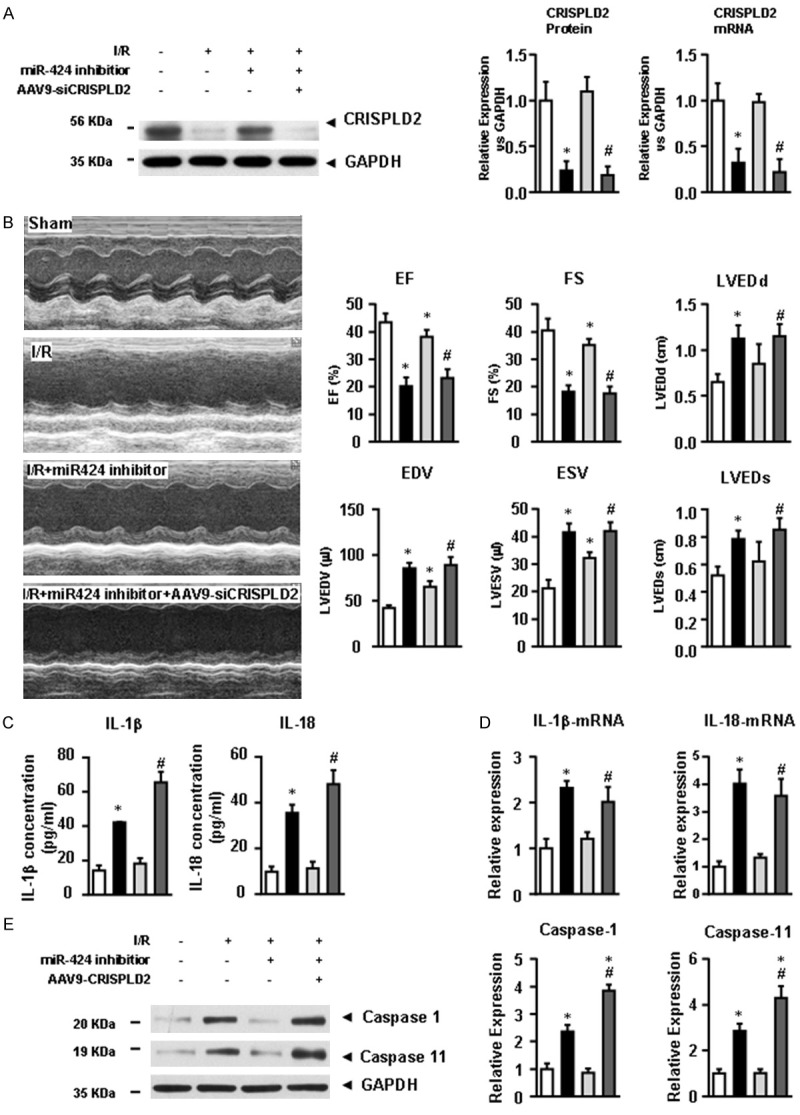
miR-424 regulates pyroptosis in IRI heart tissues via targeting CRISPLD2. (A) The expression of CRISPLD2 at the protein and mRNA levels in the control, I/R, I/R + miR-424 inhibitor, and I/R + miR-424 inhibitor + AAV9-siCRISPLD2 groups. (B) The evaluation of cardiac function was detected by echocardiography. The parameters of cardiac function, including EF%, FS%, LVEDD, LVESD, LVEDV and LVESV, were analyzed. (C) Expression of IL-1β and IL-18 at the serum and mRNA levels (D) in heart tissues were determined by ELISA and qRT-PCR, respectively. (E) Expressions of cleaved caspase-1 and caspase-11 at the protein level were determined by western blotting. Data are presented as the mean ± S.E.M. n=5; *P<0.05 versus sham group; #P<0.05 versus I/R group.
Discussion
IRI-induced death of cardiomyocytes is the main cause of the development and progression of ischemic heart disease [7]. Previous studies have shown that apoptosis and necrosis are the major pathways that lead to cardiomyocyte death after IRI. Pyroptosis is a unique type of programmed cell death distinct from apoptosis and necrosis [23-26]. In the present study, we unraveled a novel role of miR-424 in IRI. We showed that miR-424 was up-regulated in IRI heart tissues and H/R-injured H9C2 cells, leading to directly repressed expression of CRISPLD2. Moreover, such up-regulation of miR-424 subsequently increased the expressions of inflammatory molecules and promoted pyroptosis in vitro and in vivo. Knockdown of miR-424 by its antisense inhibitor markedly attenuated the effects of miR-424 (Figure 6).
Figure 6.
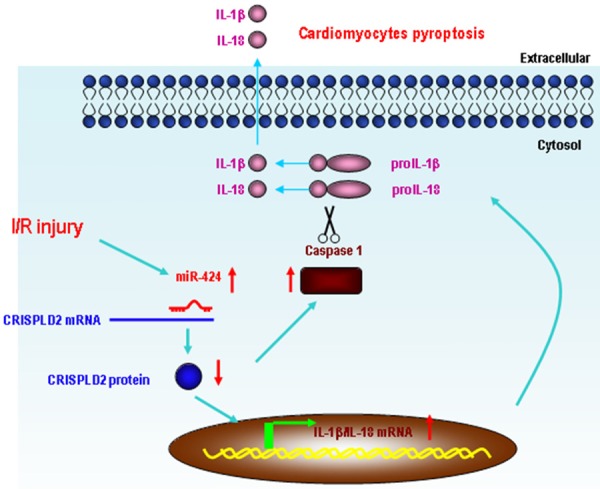
Schematic diagram of the proposed miR-424-induced pyroptosis signaling pathways. miR-424 was significantly up-regulated under IRI conditions and played a regulatory role in pyroptosis by down-regulating CRISPLD2, leading to up-regulation of caspase-1 and the pro-inflammatory cytokines, IL-1β and IL-18.
miRNAs have emerged as important mediators of translational control and as regulators of a wide range of biological processes [17-21]. Up-regulation of miRNAs is a common observation that occurs in various diseases, and aberrantly expressed miRNAs often participate in the pathogenesis of specific diseases, including ischemic heart diseases. The most reported protective microRNAs in myocardial I/R include miRNA-126, miRNA-133, miRNA-144, miRNA-145, miRNA-199, miRNA-210, miRNA-214, miRNA-494, miRNA-451 and miRNA-499 [38-40]; while microRNAs with opposite effects in myocardial I/R include miRNA-1, miRNA-15, miRNA-92a, miRNA-320 and miR-424 [39,41-43]. Moreover, some miRNAs show a dual role in the pathomechanism of myocardial IRI, such as miRNA-21, miRNA-24 and miRNA-29. It has been previously reported that the miR-424 is a key up-regulator of hypoxia inducible factor-1α (HIF-1α), the primary hypoxia-driven signaling pathway of the ischemic heart disease. Therefore, it is conceivable that an increase in miR-424 underlies HIF-1α up-regulation, which is a phenomenon observed in most forms of ischemic heart disease [44]. A previous study has revealed that hypoxia induces PAECs to up-regulate and secrete miR-424, which are at least partially transported in EXOs and can be taken up by cardiomyocytes, resulting in down-regulation of SMURF1 and contributing to RVH and heart failure at last [43]. However, there is no direct evidence indicating a role of miR-424 in cardiomyocyte pyroptosis in the setting of IRI. In the present study, we identified that miR-424 promoted pyroptosis in H9C2 cells (a rat heart cell line) subjected to HRi and in heart tissues from IRI mice.
CRISPLD2 is a GC- and developmentally regulated gene encoding a secreted mesenchymal protein in the lung and other organs [36,37,45-47]. Previous studies have reported that CRISPLD2 serves as an endogenous anti-inflammatory gene in lung fibroblasts, and it can also curtail proinflammatory signaling by lung epithelial cells through mesenchymal-epithelial interactions [45]. In this study, we validated that CRISPLD2 was a direct target of miR-424. More importantly, knockdown of miR-424 attenuated the inflammatory cell death of H9C2 cells, which was aggravated by CRISPLD2 down-regulation. These results suggested that CRISPLD2 played a critical anti-pyroptotic role in IRI cardiomyocytes, and over-expression of CRISPLD2 could be a strategy for the prevention of cardiac pyroptosis.
Pyroptosis is a recently identified type of programmed cell death. Caspase-1-dependent pyroptosis has been first reported in mouse macrophages infected with the Gram-negative bacteria Shigella flexneri [23-26,48]. As a protease, caspase-1 has a basic function in processing the inactive precursors of IL-1β into mature inflammatory cytokines, thus it is called IL-1β-converting enzyme [49,50]. Activation of caspase-1 induces pore formation on the cell membrane, leading to the generation and release of abundant inflammatory factors that subsequently contribute to pyroptosis. Our in vivo study showed that pyroptosis was characterized by increased expression of caspase-1, IL-1β and IL-18 after IRI. The altered levels of caspase-1, IL-1β and IL-18 in heart tissues were correlated with cardiac functional changes. Our in vitro study revealed that HRI caused up-regulation of caspase-1, IL-1β and IL-18 in H9C2 cells, which was accompanied by decreased cell viability and SOD activity as well as increased MDA content. Collectively, pyroptosis and inflammatory responses were closely associated with the development and progression of IRI in cardiomyocytes.
In conclusion, our results provided the first evidence that IRI-triggered cardiac pyroptosis was promoted with miR-424 by direct targeting CRISPLD2. These findings illustrated a previously unknown pathway miR-424-CRISPLD2 that regulates cardiac pyroptosis. However, it was undeniable that the effects of miR-424 on ischemic heart disease might be mediated by a variety of mechanisms in addition to the miR-424-CRISPLD2 pathway, and further investigation is required to uncover any other potential mechanisms. The salient findings from the present study indicated an essential role of miR-424 in IRI, greatly contributing to our understanding of the role of pyroptosis in ischemic heart disease.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No: 81571942).
Disclosure of conflict of interest
None.
References
- 1.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu G, Zhang H, Hao F, Hao J, Pan L, Zhao Q, Wo J. Clusterin reduces cold ischemia-reperfusion injury in heart transplantation through regulation of NF-kB signaling and Bax/Bcl-xL expression. Cell Physiol Biochem. 2018;45:1003–1012. doi: 10.1159/000487295. [DOI] [PubMed] [Google Scholar]
- 3.Maimaitiaili A, Li J, Aibibula A, Abudureheman M. Inhibition of nuclear factor kappa B pathway protects myocardial ischemia/reperfusion injury in rats under treatment with abnormal savda munziq. Am J Transl Res. 2018;10:77–85. [PMC free article] [PubMed] [Google Scholar]
- 4.Du Y, Liu P, Xu T, Pan D, Zhu H, Zhai N, Zhang Y, Li D. Luteolin modulates SERCA2a leading to attenuation of myocardial ischemia/reperfusion injury via sumoylation at lysine 585 in mice. Cell Physiol Biochem. 2018;45:883–898. doi: 10.1159/000487283. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Quan N, Sun W, Chen X, Cates C, Rousselle T, Zhou X, Zhao X, Li J. Cardiomyocyte specific deletion of Sirt1 gene sensitizes myocardium to ischemia and reperfusion injury. Cardiovasc Res. 2018;114:805–821. doi: 10.1093/cvr/cvy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu SY, Dong B, Tang L, Zhou SH. LncRNA MALAT1 sponges miR-133 to promote NLRP3 inflammasome expression in ischemia-reperfusion injured heart. Int J Cardiol. 2018;254:50. doi: 10.1016/j.ijcard.2017.10.071. [DOI] [PubMed] [Google Scholar]
- 7.Levraut J, Iwase H, Shao ZH, Vanden Hoek TL, Schumacker PT. Cell death during ischemia: relationship to mitochondrial depolarization and ROS generation. Am J Physiol Heart Circ Physiol. 2003;284:H549–558. doi: 10.1152/ajpheart.00708.2002. [DOI] [PubMed] [Google Scholar]
- 8.Chin KY, Silva LS, Darby IA, Ng DCH, Woodman OL. Protection against reperfusion injury by 3’,4’-dihydroxyflavonol in rat isolated hearts involves inhibition of phospholamban and JNK2. Int J Cardiol. 2018;254:265–271. doi: 10.1016/j.ijcard.2017.11.101. [DOI] [PubMed] [Google Scholar]
- 9.Lui JC. Regulation of body growth by microRNAs. Mol Cell Endocrinol. 2017;456:2–8. doi: 10.1016/j.mce.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alipoor SD, Adcock IM, Garssen J, Mortaz E, Varahram M, Mirsaeidi M, Velayati A. The roles of miRNAs as potential biomarkers in lung diseases. Eur J Pharmacol. 2016;791:395–404. doi: 10.1016/j.ejphar.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ojha CR, Rodriguez M, Dever SM, Mukhopadhyay R, El-Hage N. Mammalian microRNA: an important modulator of host-pathogen interactions in human viral infections. J Biomed Sci. 2016;23:74. doi: 10.1186/s12929-016-0292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lima TI, Araujo HN, Menezes ES, Sponton CH, Araujo MB, Bomfim LH, Queiroz AL, Passos MA, TA ES, Hirabara SM, Martins AR, Sampaio HC, Rodrigues A, Curi R, Carneiro EM, Boschero AC, Silveira LR. Role of microRNAs on the regulation of mitochondrial biogenesis and insulin signaling in skeletal muscle. J Cell Physiol. 2017;232:958–966. doi: 10.1002/jcp.25645. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Wu YY, Jiang JN, Liu XS, Ji FJ, Fang XD. MiRNA-3978 regulates peritoneal gastric cancer metastasis by targeting legumain. Oncotarget. 2016;7:83223–83230. doi: 10.18632/oncotarget.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Hong Q, Wang Z, Yu Y, Zou X, Xu L. MiR-21 inhibits autophagy by targeting Rab11a in renal ischemia/reperfusion. Exp Cell Res. 2015;338:64–69. doi: 10.1016/j.yexcr.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Ma N, Bai J, Zhang W, Luo H, Zhang X, Liu D, Qiao C. Trimetazidine protects against cardiac ischemia/reperfusion injury via effects on cardiac miRNA21 expression, Akt and the Bcl2/Bax pathway. Mol Med Rep. 2016;14:4216–4222. doi: 10.3892/mmr.2016.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei Q, Liu Y, Liu P, Hao J, Liang M, Mi QS, Chen JK, Dong Z. MicroRNA-489 induction by hypoxia-inducible factor-1 protects against ischemic kidney injury. J Am Soc Nephrol. 2016;27:2784–2796. doi: 10.1681/ASN.2015080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guclu A, Kocak C, Kocak FE, Akcilar R, Dodurga Y, Akcilar A, Secme M. Micro RNA-320 as a novel potential biomarker in renal ischemia reperfusion. Ren Fail. 2016;38:1468–1475. doi: 10.1080/0886022X.2016.1227915. [DOI] [PubMed] [Google Scholar]
- 18.Hao J, Wei Q, Mei S, Li L, Su Y, Mei C, Dong Z. Induction of microRNA-17-5p by p53 protects against renal ischemia-reperfusion injury by targeting death receptor 6. Kidney Int. 2017;91:106–118. doi: 10.1016/j.kint.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amrouche L, Desbuissons G, Rabant M, Sauvaget V, Nguyen C, Benon A, Barre P, Rabate C, Lebreton X, Gallazzini M, Legendre C, Terzi F, Anglicheau D. MicroRNA-146a in human and experimental ischemic AKI: CXCL8-dependent mechanism of action. J Am Soc Nephrol. 2017;28:479–493. doi: 10.1681/ASN.2016010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatt K, Wei Q, Pabla N, Dong G, Mi QS, Liang M, Mei C, Dong Z. MicroRNA-687 induced by hypoxia-inducible factor-1 targets phosphatase and tensin homolog in renal ischemia-reperfusion injury. J Am Soc Nephrol. 2015;26:1588–1596. doi: 10.1681/ASN.2014050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Choi E, Cha MJ, Hwang KC. Looking for pyroptosis-modulating miRNAs as a therapeutic target for improving myocardium survival. Mediators Inflamm. 2015;2015:254871. doi: 10.1155/2015/254871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nie H, Xue X, Li J, Liu X, Lv S, Guan G, Liu H, Liu G, Liu S, Chen Z. Nitro-oleic acid attenuates OGD/R-triggered apoptosis in renal tubular cells via inhibition of Bax mitochondrial translocation in a PPAR-gamma-dependent manner. Cell Physiol Biochem. 2015;35:1201–1218. doi: 10.1159/000373944. [DOI] [PubMed] [Google Scholar]
- 23.Dong T, Liao D, Liu X, Lei X. Using small molecules to dissect non-apoptotic programmed cell death: necroptosis, ferroptosis, and pyroptosis. Chembiochem. 2015;16:2557–2561. doi: 10.1002/cbic.201500422. [DOI] [PubMed] [Google Scholar]
- 24.Croker BA, Silke J, Gerlic M. Fight or flight: regulation of emergency hematopoiesis by pyroptosis and necroptosis. Curr Opin Hematol. 2015;22:293–301. doi: 10.1097/MOH.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Jiang G, Zhang P, Fan J. Programmed cell death and its role in inflammation. Mil Med Res. 2015;2:12. doi: 10.1186/s40779-015-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoenlaub L, Cherla R, Zhang Y, Zhang G. Coxiella burnetii avirulent Nine Mile phase II induces caspase-1 dependent pyroptosis in murine peritoneal B1a B cells. Infect Immun. 2016;84:3638–3654. doi: 10.1128/IAI.00694-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorgensen I, Lopez JP, Laufer SA, Miao EA. IL-1beta, IL-18, and eicosanoids promote neutrophil recruitment to pore-induced intracellular traps following pyroptosis. Eur J Immunol. 2016;46:2761–2766. doi: 10.1002/eji.201646647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Napier BA, Brubaker SW, Sweeney TE, Monette P, Rothmeier GH, Gertsvolf NA, Puschnik A, Carette JE, Khatri P, Monack DM. Complement pathway amplifies caspase-11-dependent cell death and endotoxin-induced sepsis severity. J Exp Med. 2016;213:2365–2382. doi: 10.1084/jem.20160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pillon NJ, Chan KL, Zhang S, Mejdani M, Jacobson MR, Ducos A, Bilan PJ, Niu W, Klip A. Saturated fatty acids activate caspase-4/-5 in human monocytes, triggering IL1beta and IL18 release. Am J Physiol Endocrinol Metab. 2016;311:E825–E835. doi: 10.1152/ajpendo.00296.2016. [DOI] [PubMed] [Google Scholar]
- 30.Eichholz K, Bru T, Tran TT, Fernandes P, Welles H, Mennechet FJ, Manel N, Alves P, Perreau M, Kremer EJ. Immune-complexed adenovirus induce AIM2-mediated pyroptosis in human dendritic cells. PLoS Pathog. 2016;12:e1005871. doi: 10.1371/journal.ppat.1005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Zhao Y, Zhang P, Li Y, Yang Y, Yang Y, Zhu J, Song X, Jiang G, Fan J. Hemorrhagic shock primes for lung vascular endothelial cell pyroptosis: role in pulmonary inflammation following LPS. Cell Death Dis. 2016;7:e2363. doi: 10.1038/cddis.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vande Walle L, Lamkanfi M. Pyroptosis. Curr Biol. 2016;26:R568–572. doi: 10.1016/j.cub.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Hutton HL, Ooi JD, Holdsworth SR, Kitching AR. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology (Carlton) 2016;21:736–744. doi: 10.1111/nep.12785. [DOI] [PubMed] [Google Scholar]
- 34.Lin CF, Kuo YT, Chen TY, Chien CT. Quercetin-rich guava (Psidium guajava) juice in combination with trehalose reduces autophagy, apoptosis and pyroptosis formation in the kidney and pancreas of type II diabetic rats. Molecules. 2016;21:334. doi: 10.3390/molecules21030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang CC, Yao CA, Yang JC, Chien CT. Sialic acid rescues repurified lipopolysaccharide-induced acute renal failure via inhibiting TLR4/PKC/gp91-mediated endoplasmic reticulum stress, apoptosis, autophagy, and pyroptosis signaling. Toxicol Sci. 2014;141:155–165. doi: 10.1093/toxsci/kfu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Kho AT, Wu Q, Halayko AJ, Limbert Rempel K, Chase RP, Sweezey NB, Weiss ST, Kaplan F. CRISPLD2 (LGL1) inhibits proinflammatory mediators in human fetal, adult, and COPD lung fibroblasts and epithelial cells. Physiol Rep. 2016:4. doi: 10.14814/phy2.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoo JY, Shin H, Kim TH, Choi WS, Ferguson SD, Fazleabas AT, Young SL, Lessey BA, Ha UH, Jeong JW. CRISPLD2 is a target of progesterone receptor and its expression is decreased in women with endometriosis. PLoS One. 2014;9:e100481. doi: 10.1371/journal.pone.0100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diao H, Liu B, Shi Y, Song C, Guo Z, Liu N, Song X, Lu Y, Lin X, Li Z. MicroRNA-210 alleviates oxidative stress-associated cardiomyocyte apoptosis by regulating BNIP3. Biosci Biotechnol Biochem. 2017;81:1712–1720. doi: 10.1080/09168451.2017.1343118. [DOI] [PubMed] [Google Scholar]
- 39.Fan ZX, Yang J. The role of microRNAs in regulating myocardial ischemia reperfusion injury. Saudi Med J. 2015;36:787–793. doi: 10.15537/smj.2015.7.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu PY, Tian Y, Xu SY. Mediated protective effect of electroacupuncture pretreatment by miR-214 on myocardial ischemia/reperfusion injury. J Geriatr Cardiol. 2014;11:303–310. doi: 10.11909/j.issn.1671-5411.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bian B, Yu XF, Wang GQ, Teng TM. Role of miRNA-1 in regulating connexin 43 in ischemia-reperfusion heart injury: a rat model. Cardiovasc Pathol. 2017;27:37–42. doi: 10.1016/j.carpath.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Liu P, Zhao H, Wang R, Wang P, Tao Z, Gao L, Yan F, Liu X, Yu S, Ji X, Luo Y. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke. 2015;46:513–519. doi: 10.1161/STROKEAHA.114.007482. [DOI] [PubMed] [Google Scholar]
- 43.Baptista R, Marques C, Catarino S, Enguita FJ, Costa MC, Matafome P, Zuzarte M, Castro G, Reis A, Monteiro P, Pego M, Pereira P, Girao H. MicroRNA-424(322) as a new marker of disease progression in pulmonary arterial hypertension and its role in right ventricular hypertrophy by targeting SMURF1. Cardiovasc Res. 2018;114:53–64. doi: 10.1093/cvr/cvx187. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y, Ramakrishnan S. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J Clin Invest. 2010;120:4141–4154. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Liu Z, Liu S. HMGB1 induced inflammatory effect is blocked by CRISPLD2 via MiR155 in hepatic fibrogenesis. Mol Immunol. 2016;69:1–6. doi: 10.1016/j.molimm.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Wang ZQ, Xing WM, Fan HH, Wang KS, Zhang HK, Wang QW, Qi J, Yang HM, Yang J, Ren YN, Cui SJ, Zhang X, Liu F, Lin DH, Wang WH, Hoffmann MK, Han ZG. The novel lipopolysaccharide-binding protein CRISPLD2 is a critical serum protein to regulate endotoxin function. J Immunol. 2009;183:6646–6656. doi: 10.4049/jimmunol.0802348. [DOI] [PubMed] [Google Scholar]
- 47.Chiquet BT, Lidral AC, Stal S, Mulliken JB, Moreno LM, Arcos-Burgos M, Valencia-Ramirez C, Blanton SH, Hecht JT. CRISPLD2: a novel NSCLP candidate gene. Hum Mol Genet. 2007;16:2241–2248. doi: 10.1093/hmg/ddm176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorenz G, Darisipudi MN, Anders HJ. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant. 2014;29:41–48. doi: 10.1093/ndt/gft332. [DOI] [PubMed] [Google Scholar]
- 49.Bergsbaken T, Cookson BT. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]


