Abstract
Background and purpose: Osteosarcoma is an aggressive malignant bone tumor in children and adolescents, which is more likely to recur and metastasize at the early stages. Cancer stem cells (CSC, CD133 is a biomarker of cancer stem cells), angiogenesis, and vasculogenic mimicry (VM) are closely related to tumor metastasis and recurrence. In this study, we investigated the associations among CD133, aldehyde dehydrogenase 1 (ALDH1), and VM in osteosarcoma, and their associations with clinical characteristics. Methods: Positive rates of CD133, ALDH1, and VM in 96 whole osteosarcoma tissue samples were detected by immunohistochemistry (IHC) and histochemistry staining. Patients’ clinical data were also collected. Results: Positive rates of CD133, ALDH1, and VM were significantly higher in osteosarcoma tissues compared with the control tissues. Positive rates of CD133, ALDH1, and VM were positively associated with lymph node metastasis, distant metastasis, Enneking stages, and patients’ overall survival (OS). A multivariate analysis indicated that the positive rates of CD133, ALDH1, and VM, as well as the Enneking stages were independent prognostic factors of osteosarcoma. Conclusion: The positive rates of CD133, ALDH1, and VM could represent potential biomarkers for metastasis and prognosis, which suggests these molecules might be promising therapeutic targets for osteosarcoma.
Keywords: Osteosarcoma, CD133, aldehyde dehydrogenase 1, vasculogenic mimicry, prognosis
Introduction
Osteosarcoma is a highly aggressive bone malignancy in children and adolescents, which accounts for approximately 60% of all malignant bone tumors [1,2]. Although advances in surgery, chemotherapy, and neoadjuvant chemotherapy have been achieved in modern medicine in the past decades, the 5-year survival rates of osteosarcoma have stagnated at 65% [2]. Lung metastasis and recurrence are the main reasons for poor prognosis [3]. This may be closely related to a small population of tumor cells called cancer stem cells (CSCs, also called tumor-initiating cells). CSCs have the capability of self-renewal, differentiation, and therapy-resistance in various human cancers and have a high tumorigenicity [2,4-6]. CD133, also called prominin-1, is a common biomarker of CSCs, which encodes a 120-kDa five transmembrane domain glycoprotein. CD133 was originally described as a CSCs biomarker in human hematopoietic stem and progenitor cells. Currently, the overexpression of CD133 was found and considered as a CSCs biomarker in various human cancers besides osteosarcoma [2,4,5,7,8]. CD133+ tumor cells are able to initiate tumor growth and to differentiate into other kinds of cells, such as osteoblasts and adipocytes [2,9]. Aldehyde dehydrogenase 1 (ALDH1) is another common biomarker of CSCs in a variety of human cancers [2,10-12]. ALDH1 is an important member of the ALDH family that is found in cytoplasms, mitochondria, and nuclei. ALDH1 promote cell proliferation, differentiation, and oxidative stress [13,14]. ALDH1 also enhances the detoxification and metabolism of lots of exogenous and endogenous aldehydes, and retinoic acid synthesis [15].
Recurrence and metastasis are also related to angiogenesis. Angiogenesis may form new blood vessels which appear in the lining of endothelial cells. Tumor cells can induce angiogenesis in order to acquire adequate nutrients and oxygen. Anti-angiogenesis therapy is a new strategy for treating many cancers. However, at this time, there is no known benefit from anti-angiogenesis therapy in the treatment of cancer [16-18]. Maniotis et al. found that some cancer cells were able to mimic endothelial cells and to form vascular channel-like structures which are known as vasculogenic mimicry (VM) [19]. VM can also convey nutrients and oxygen to promote tumor cell proliferation and metastasis [10,18,20].
Overall, studies of CD133, ALDH1, and VM in relation to metastasis and prognosis demonstrate that these biomarkers promote tumor progression. However, the relationships among CD133, ALDH1, and VM in osteosarcoma have not been widely researched. In our study, we examined the associations among CD133, ALDH1, and VM in osteosarcoma tissues and compared them with clinicopathologic changes and prognosis.
Materials and methods
Samples
We collected samples from 96 patients (median age: 19.2 years; range: 13-49 years) who were diagnosed with osteosarcoma at the First Affiliated Hospital of Bengbu Medical University, from January 2009 to December 2011, along with 96 samples of adjacent tissues. Patients who had received any anti-cancer therapies were excluded from our study. This study was approved by the Bengbu Medical University ethics committee. All samples were obtained with the patients’ written consent, and the study was conducted in accordance with the guidelines of the Declaration of Helsinki. All patients received surgical therapy and chemotherapy after being diagnosed. We collected the patients’ complete clinicopathological and follow-up data (at 4-month intervals by phone, mail, or applications). Overall survival (OS) time was counted from the patient’s surgery date to his/her death date or December 2016 (mean OS: 40.1 months; range: 13-69 months). The clinical stages were determined according to the Enneking staging system. Tumors were graded according to World Health Organization (WHO) standards. For specific characteristics, see Table 1.
Table 1.
Clinical and pathological characteristics of patients with osteosarcoma
| Patients characteristics | Frequency (n) | Percentage (%) |
|---|---|---|
| Age (years) | ||
| <19 | 53 | 55.2 |
| ≥19 | 43 | 44.8 |
| Gender | ||
| Male | 76 | 79.2 |
| Female | 20 | 20.8 |
| Site | ||
| Femur | 50 | 52.1 |
| Tibia or fibula | 33 | 34.4 |
| Other | 13 | 13.5 |
| Size (cm) | ||
| <8.0 | 42 | 43.8 |
| ≥8.0 | 54 | 56.3 |
| Type | ||
| Osteoblastic | 48 | 50.0 |
| Chondroblastic | 17 | 17.7 |
| Fibroblastic | 15 | 15.6 |
| Mixed | 16 | 16.7 |
| Grade | ||
| Low | 42 | 43.8 |
| High | 54 | 56.2 |
| Lung metastasis | ||
| No | 68 | 70.8 |
| Yes | 28 | 29.2 |
| Recurrence | ||
| No | 39 | 40.6 |
| Yes | 57 | 59.4 |
| Enneking stages | ||
| II | 66 | 68.8 |
| III | 30 | 31.3 |
Immunohistochemistry and histochemistry
Immunohistochemistry was conducted using the ElivisionTM Plus detection kit, following the manufacturer’s instructions (Lab Vision, USA). All osteosarcoma and corresponding adjacent tissues were fixed in 10% buffered formalin. All samples were embedded in paraffin, then cut into continuous 4 μm thick slices. All the slices were deparaffinized in xylene, then dehydrated in graded alcohol and washed using phosphate buffer saline (PBS, pH 7.2) for 10 min. Subsequently, all slices were incubated in 3% H2O2 to block endogenous peroxidase activity at room temperature for 10 min. For antigen repair, all slices were placed in a citrate buffer (pH 6.0) and heated to 95°C for 30 min. Then they went through several washes by PBS, and then all slices were quenched by goat serum for 30 min at room temperature. Lastly, all the slices were incubated with mouse monoclonal antibody against human CD133 (Abcam, USA), ALDH1 (Abcam, USA), and CD34 (Abcam, USA) at 37°C for 1 h. All slices underwent a periodic acid-Schiff (PAS)-CD34 dual staining to confirm endothelial cells in the glycosylated basement membrane of vessels or vasculogenic mimicry [5]. Subsequently, all slices were counterstained with hematoxylin, dehydrated, air dried, and mounted.
Evaluation of staining
The immunohistochemical staining results were semi-quantitatively assessed by two independent and experienced pathologists who were blind to all clinicopathological data. In order to avoid any intratumoral heterogeneity of biomarker expression, we analyzed ten representative fields at high-power-field (HPF) from different fields of the osteosarcoma slices. According to the intensity (no staining was 0; weak staining was 1; moderate staining was 2; strong staining was 3) and the extent score (positive cells <11% were 1; 11%-50% positive cells were 2; 51%-75% positive cells were 3; positive cells >75% were 4) system, we yielded final scores by multiplying the intensity score and the extent score that ranged from 0-12. The final scores >2 were defined as having a positive result. For slices that were positive for both CD133 and ALDH1, the average final score of each slice was calculated.
Statistical analysis
Relationships between positive rates of CD133, ALDH1, and VM and clinicopathological characteristics were compared using the Pearson chi-square test or Fisher’s exact test. Associations between positive rates of CD133, or ALDH1, or VM were compared using Spearman’s coefficient test. Univariate analysis between OS and the expression of CD133, ALDH1, or VM was compared using the Kaplan-Meier method with a log-rank test. The multivariate analysis for independent prognostic factors was compared using a COX regression model test. This study used SPSS 19.0 software for Windows (Chicago, IL). P<0.05 was considered significant.
Results
Associations between positive rates of CD133, ALDH1 and VM and clinicopathological characteristics
To evaluate the contributions of CD133, ALDH1, and VM to osteosarcoma, the results thereof were evaluated for both osteosarcoma and the corresponding adjacent tissues samples. The results were compared to patients’ clinicopathological characteristics. The positive rate of CD133 in osteosarcoma tissues (51.0%, 49/96) was significantly higher than that in the control tissues (9.4%, 9/96; P<0.05; Figure 1A and 1B). The positive rate of CD133 in osteosarcoma was significantly associated with the Enneking tumor stages, lung metastasis, grade, and recurrence, but not with patients’ age, gender, site, type, or size (Table 2).
Figure 1.

Immunostaining of CD133, or ALDH1, or VM in osteosarcoma or the control tissue. A: Negative staining of CD133 in control tissue (400× magnification); B: Positive staining of CD133 in the membranes and cytoplasms of tumor cells (400× magnification); C: Negative staining of ALDH1 in the control tissues (100× magnification); D: Positive staining of ALDH1 in the cytoplasm of tumor cells (100× magnification); E: Negative staining of VM in the control tissues (400× magnification); F: Positive staining of VM in the osteosarcoma tissues (100× magnification, the red arrow is VM structure, the black arrow is a microvessel).
Table 2.
The associations between expression of CD133, ALDH1, and VM and clinicopathological characteristics of osteosarcoma
| Variables | CD133 | P | ALDH1 | P | VM | P | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| - | + | - | + | - | + | ||||
| Age | 0.424 | 0.880 | 0.845 | ||||||
| <19 years | 24 | 29 | 23 | 30 | 31 | 22 | |||
| ≥19 years | 23 | 20 | 18 | 25 | 26 | 17 | |||
| Gender | 0.368 | 0.197 | 0.949 | ||||||
| Male | 39 | 37 | 35 | 41 | 45 | 31 | |||
| Female | 8 | 12 | 6 | 14 | 12 | 8 | |||
| Site | 0.355 | 0.539 | 0.738 | ||||||
| Femur | 21 | 29 | 22 | 28 | 29 | 21 | |||
| Tibia and Fibula | 19 | 14 | 12 | 21 | 19 | 14 | |||
| Other | 7 | 6 | 7 | 6 | 9 | 4 | |||
| Size (cm) | 0.857 | 0.035 | 0.034 | ||||||
| <8.0 | 21 | 21 | 23 | 19 | 30 | 12 | |||
| ≥8.0 | 26 | 28 | 18 | 36 | 27 | 27 | |||
| Type | 0.608 | 0.214 | 0.657 | ||||||
| Osteoblastic | 26 | 22 | 24 | 24 | 31 | 17 | |||
| Chondroblastic | 6 | 11 | 4 | 13 | 10 | 7 | |||
| Fibroblastic | 7 | 8 | 5 | 10 | 7 | 8 | |||
| Mixed | 8 | 8 | 8 | 8 | 9 | 7 | |||
| Grade | <0.001 | <0.001 | 0.011 | ||||||
| Low | 32 | 10 | 27 | 15 | 31 | 11 | |||
| High | 15 | 39 | 14 | 40 | 26 | 28 | |||
| Lung metastasis | 0.001 | <0.001 | <0.001 | ||||||
| No | 41 | 27 | 38 | 30 | 49 | 19 | |||
| Yes | 6 | 22 | 3 | 25 | 8 | 20 | |||
| Recurrence | <0.001 | <0.001 | <0.001 | ||||||
| No | 34 | 5 | 29 | 10 | 32 | 7 | |||
| Yes | 13 | 44 | 12 | 45 | 25 | 32 | |||
| Enneking stages | <0.001 | <0.001 | <0.001 | ||||||
| II | 42 | 24 | 38 | 28 | 50 | 16 | |||
| III | 5 | 25 | 3 | 27 | 7 | 23 | |||
Similar to CD133, the ALDH1+ expression was significantly higher in the osteosarcoma tissues (57.3%, 55/96) than in the control tissues (11.5%, 11/96; P<0.05; Figure 1C and 1D). The positive rate of ALDH1 expression in osteosarcoma was associated with lung metastasis, recurrence, size of tumors, grade, and Enneking staging, but not with the patients’ age, gender, site, or type (Table 2).
VM was some small vessel-like structures in osteosarcoma tissues which were PAS+ but CD34-. Lumens lined cells are tumor cells (Figure 1E and 1F). The VM patterns include tubular, linear, and network. Furthermore, there was no necrosis and hemorrhage near VM in tumors. VM was identified in osteosarcoma tissues 39 (40.6%) of 96 samples. There was a significant difference between the osteosarcoma group and the control group (0%, 0/96; P<0.001). Moreover, the positive staining of VM was found to be closely linked to lung metastasis, recurrence, size of tumors, grade, and Enneking staging. However, the positive rate of VM did not have a significant association with patients’ gender, age, tumor site, or tumor type (Table 2).
Associations among expressions of CD133 and ALDH1, and VM in osteosarcoma
A Spearman correlation coefficient analysis result showed that there were positive correlations between the expressions of CD133 and ALDH1 (r=0.587, P<0.001). Notably, the expressions of CD133 (r=0.471, P<0.001) and ALDH1 (r=0.457, P<0.001) are both positively associated with VM (Table 3).
Table 3.
Association between expression of CD133, ALDH1, and VM in osteosarcoma
| Variable | CD133 | r | P | VM | r | P | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| - | + | - | + | |||||
| CD133 | 0.471 | <0.001* | ||||||
| - | 39 | 8 | ||||||
| + | 18 | 31 | ||||||
| ALDH1 | 0.587 | <0.001 | 0.457 | <0.001* | ||||
| - | 34 | 7 | 35 | 6 | ||||
| + | 13 | 42 | 22 | 33 | ||||
positive association.
Univariate and multivariate analyzes
Follow-up data indicated that OS was significantly lower in osteosarcoma patients with CD133+ samples (27.4±10.9 months) compared with those with CD133- samples (53.4±14.4 months; log-rank =56.392, P<0.001; Figure 2A). Similarly, the OS of ALDH1+ patients (30.3±12.4 months) was significantly lower than those of ALDH1- patients (53.4±16.3 months; log-rank =45.990, P<0.001; Figure 2B). The OS of VM+ patients (26.3±11.3 months) was significantly shorter than those of VM- patients (49.6±15.9 months; log-rank =48.472, P<0.001; Figure 2C).
Figure 2.
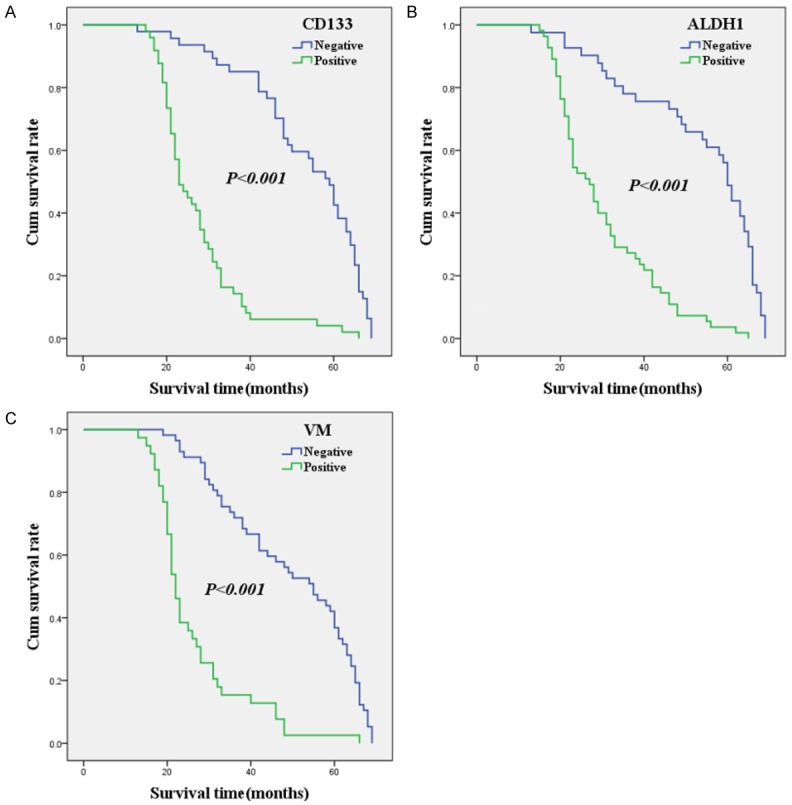
Kaplan-Meier analysis of the survival rate of patients with osteosarcoma. The y-axis represents the percentage of patients; the x-axis, their survival in months. (A) Overall survival of all patients in relation to CD133 (log-rank =56.392, P<0.001); (B) Overall survival of all patients in relation to ALDH1 expression (log-rank =45.990, P<0.001); (C) Overall survival of all patients in relation to VM (log-rank =48.472, P<0.001); In the (A-C) analyses, the green line represents patients with positive CD133, or ALDH1, or VM, and the blue line represents the negative CD133, or ALDH1, or VM patients.
Multivariate analysis data showed that the expression of CD133, ALDH1, and VM, the grade of differentiation, recurrence, as well as Enneking stages were independent prognostic factors for osteosarcoma (Table 4).
Table 4.
Results of multivariate analyses of overall survival (OS) time
| Covariate | B | SE | P | HR | 95% CI |
|---|---|---|---|---|---|
| CD133 | 0.690 | 0.288 | 0.016 | 1.994 | 1.135-3.504 |
| ALDH1 | 0.707 | 0.356 | 0.047 | 2.027 | 1.009-4.074 |
| VM | 1.077 | 0.315 | 0.001 | 2.935 | 1.584-5.438 |
| Grade | 1.012 | 0.279 | <0.001 | 2.751 | 1.591-4.757 |
| Recurrence | 1.388 | 0.331 | <0.001 | 4.009 | 2.094-7.674 |
| Enneking stages | 0.588 | 0.290 | 0.043 | 1.800 | 1.019-3.180 |
Discussion
Osteosarcoma is a highly heterogeneous tumor which can influence the repeatability of biomarker assessment. Therefore, the metastatic and prognostic values of biomarkers should be entirely evaluated to determine their validity. The leading causes of osteosarcoma treatment failure are lung metastasis and its recurrence [4]. This may be related to CSCs that have features correlated with normal stem cells [21]. It has been known that CSCs have the capability of self-renewal, differentiation, quiescence, and therapy-resistance (chemotherapy or radiotherapy), leading to tumor recurrence and metastasis [5,22,23]. CD133 is most commonly regarded as a biomarker for CSCs in many tumors [2,4,5,7,8]. In our study, we found that CD133 expression was positively related to lung metastasis, recurrence, and Enneking staging. Furthermore, a Kaplan-Meier overall survival (OS) analysis showed that CD133+ expression patients had a shorter OS time than did the CD133- patients. The above results suggest that CD133 should promote osteosarcoma progression and metastasis and should be considered as a useful biomarker for predicting progression and metastasis in this disease. Our findings are in accordance with the previous studies, enclosing osteosarcoma [5,24,25].
Similar to CD133, ALDH1 is also a biomarker of CSCs. ALDH1, an intracellular enzyme, not only detoxifies some intracellular cytotoxic drugs, but it also detoxifies the metabolism of various endogenous and exogenous aldehydes [13,26]. In this study, we found that ALDH1 expression is significantly associated with lung metastasis, recurrence, tumor size, and Enneking staging. In addition, Kaplan-Meier survival analysis data indicated that osteosarcoma patients with a positive ALDH1 expression had a significantly lower OS than ALDH negative patients did.
VM is a channel-like formation which is in the lining of cancer cells. The typical VM structure consists of three sections, just like cancer stem-like cells, with a plastic extracellular matrix and vascular-like structures. VM should participate in the development, progression, and metastasis of many tumors [5,10,17,18,27,28]. In this study, the positive rate of VM is positively associated with lung metastasis, recurrence, tumor size, and Enneking staging. A Kaplan-Meier survival analysis also showed that VM+ patients had a worse OS when compared with VM- patients. These findings demonstrated that VM should be considered as a valuable biomarker for predicting invasiveness and metastasis in osteosarcoma. Similar results were obtained in other studies which investigated the invasiveness and metastatic significance of VM in osteosarcoma [29,30].
The Enneking staging system cannot indicate thorough information about osteosarcoma’s biological behavior. So, it is urgent to find new and valuable molecular markers that will be useful in predicting the invasiveness, metastasis, and prognosis of osteosarcoma. In our study, multivariate analysis data demonstrated that CD133+, ALDH1+, VM+, lung metastasis, recurrence, and Enneking staging were independent prognostic factors for osteosarcoma patients. Our findings thus suggest that CD133, ALDH1, and VM should be thought of as trusted biomarkers for osteosarcoma, especially in predicting invasiveness, metastasis, and prognosis.
Moreover, CSCs should be involved in the initiation, development, and progression of osteosarcoma. Microvascular and lymphatic vessels constitute the survival environment of CSCs. Some studies have demonstrated that CSCs are capable of differentiation along tumor cells and the surrounding stromal cells (including endothelial cells) [31,32]. When the tumor size is more than a certain extent volume, tumor cells can induce angiogenesis to meet their rapid growth. Initially, CSCs are able to mimic endothelial cells to form a channel-like structure which could connect to the host’s microcirculation to convey nutrient and oxygen-which is vasculogenic mimicry. Along with tumor development, the lining CSCs of VM could differentiate endothelial cell to form new microvessels [31-34]. Overall, the above findings indicate that there is a complex relationship between CSCs and VM in osteosarcoma development, invasiveness, and metastasis.
Conclusions
We have demonstrated that CSCs are be involved in the evolution of osteosarcoma. The combined investigation shows that CD133, ALDH1, and VM should be considered as valuable and effective biomarkers for metastasis and prognosis in osteosarcoma patients.
Acknowledgements
This work was supported by the the Nature Science Key Program of College and University of Anhui Province (No. KJ2016A460) and General projects of support program for outstanding young talents in Colleges and Universities of Anhui Province (No. gxyq2017032), and the Nature Science Foundation of Bengbu Medical University (No. BYKF1771 and No. BYKF1708ZD).
Disclosure of conflict of interest
None.
References
- 1.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W, Gebhardt M, Goorin AM, Harris MB, Healey J, Huvos A, Link M, Montebello J, Nadel H, Nieder M, Sato J, Siegal G, Weiner M, Wells R, Wold L, Womer R, Grier H. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyltripeptide to cisplatin, doxorubicin and high-dose methotrexate. J. Clin. Oncol. 2005;23:2004–11. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Ni M, Xiong M, Zhang X, Cai G, Chen H, Zeng Q, Yu Z. Poly (lactic-co-glycolic acid) nanoparticles conjugated with CD133 aptamers for targeted salinomycin delivery to CD133+ osteosarcoma cancer stem cells. Int J Nanomedicine. 2015;10:2537–54. doi: 10.2147/IJN.S78498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2008;8:1257–69. doi: 10.1586/14737140.8.8.1257. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Wu S, Chen Y, Zhao J, Zhang K, Wang J, Chen S. microRNA-143 is associated with the survival of ALDH1+CD133+ osteosarcoma cells and the chemoresistance of osteosarcoma. Exp Biol Med (Maywood) 2015;240:867–75. doi: 10.1177/1535370214563893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu S, Yu L, Wang D, Zhou L, Cheng Z, Chai D, Ma L, Tao Y. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer. 2012;12:535. doi: 10.1186/1471-2407-12-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 7.Nair RM, Balla MM, Khan I, Kalathur RKR, Kondaiah P, Vumuganti GK. In vitro characterization of CD133Io cancer stem cells in retinoblastoma Y79 cell line. BMC Cancer. 2017;17:779. doi: 10.1186/s12885-017-3750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao Y, Ou Y, Yin H, Chen Y, Zhong S, Gao Y, Zhao Z, He B, Huang Q, Deng Q. Establishment and characterization of human osteosarcoma cells resistant to pyropheophorbide-α methyl ester-mediated photodynamic therapy. Int J Oncol. 2017;51:1427–38. doi: 10.3892/ijo.2017.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Zhong XY, Li ZY, Cai JF, Zou L, Li JM, Yang T, Liu W. CD133 expression in osteosarcoma and derivation of CD133+ cells. Mol Med Rep. 2013;7:577–84. doi: 10.3892/mmr.2012.1231. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Zhu B, Wu S, Zhou L, Song W, Gong X, Wang D. Evaluation of the correlation of vasculogenic mimicry, ALDH1, KiSS-1, and MACC1 in the prediction of metastasis and prognosis in ovarian carcinoma. Diagn Pathol. 2017;12:23. doi: 10.1186/s13000-017-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosse-Wilde A, Fouquier d’Hérouël A, McIntosh E, Ertaylan G, Skupin A, Kuestner RE, del Sol A, Walters KA, Huang S. Stemness of the bybrid epithelial/mesenchymal state in breast cancer and its association with poor prognosis. PLoS One. 2015;10:e0126522. doi: 10.1371/journal.pone.0126522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu S, Fourman MS, Mahjoub A, Mandell JB, Crasto JA, Greco NG, Weiss KR. Lung cells support osteosarcoma cell migration and survival. BMC Cancer. 2017;17:78. doi: 10.1186/s12885-017-3047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim IG, Lee JH, Kim SY, Kim JY, Cho EW. Fibulin-3 negatively regulates ALDH1 via c-MET suppression and increases c-radiation-induced sensitivity in some pancreatic cancer cell lines. Biochem Biophy Res Commun. 2014;454:369–75. doi: 10.1016/j.bbrc.2014.10.084. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Xu Q, Fu X, Luo W. ALDH1A1 overexpression is associated with the progression and prognosis in gastric cancer. BMC Cancer. 2014;14:705. doi: 10.1186/1471-2407-14-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huo W, Du M, Pan X, Zhu X, Li Z. Prognostic value of ALDH1 expression in lung cancer: a meta-analysis. Int J Clin Exp Med. 2015;8:2045–51. [PMC free article] [PubMed] [Google Scholar]
- 16.Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 17.Zhu B, Zhou L, Yu L, Wu S, Song W, Gong X, Wang D. Evaluation of the correlation of vasculogenic mimicry, ALDH1, KAI1 and microvessel density in the prediction of metastasis and prognosis in colorectal carcinoma. BMC Surg. 2017;17:47. doi: 10.1186/s12893-017-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Yu L, Zhu B, Wu S, Song W, Gong X, Wang D. Vasculogenic mimicry and expression of Twist1 and KAI1 correlate with metastasis and prognosis in lung squamous cell carcinoma. Int J Clin Exp Pathol. 2017;10:7542–50. [PMC free article] [PubMed] [Google Scholar]
- 19.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Guo H, Zhang D, Zhang W, Zhao X, Ren Z, Sun B. Microcirculation patterns in different stages of melanoma growth. Oncol Rep. 2006;15:15–20. [PubMed] [Google Scholar]
- 21.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 22.Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–23. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]
- 23.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–3. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu G, Zhou L, Song W, Wu S, Zhu B, Wang D. Expression of ORAOV1, CD133 and WWOX correlate with metastasis and prognosis in gastric adenocarcinoma. Int J Clin Exp Pathol. 2017;10:8916–24. [PMC free article] [PubMed] [Google Scholar]
- 25.Feng J, Lan R, Cai G, Lin J, Wang X, Lin J, Han D. Verification of TREX1 as a promising indicator of judging the prognosis of osteosarcoma. J Orthop Surg Res. 2016;11:150. doi: 10.1186/s13018-016-0487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou L, Yu L, Zhu B, Wu S, Song W, Gong X, Wang D. Metastasis-associated in colon cancer-1 and aldehyde dehydrogenase 1 are metastatic and prognostic biomarker for non-small cell lung cancer. BMC Cancer. 2016;16:876. doi: 10.1186/s12885-016-2903-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu A, Huang JJ, Jin XJ, Li JP, Tang YJ, Huang XF, Cui HJ, Xu WH, Sun GB. Curcumin suppresses invasiveness and vasculogenic mimicry of squamous cell carcinoma of the larynx through the inhibition of JAK-2/STAT-3 signaling pathway. Am J Cancer Res. 2015;5:278–88. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao N, Sun BC, Zhao XL, Wang Y, Meng J, Che N, Dong XY, Gu Q. Role of Bcl-2 and its associated miRNAs in vasculogenic mimicry of hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:15759–68. [PMC free article] [PubMed] [Google Scholar]
- 29.Yao N, Ren K, Wang Y, Jin Q, Lu X, Lu Y, Jiang C, Zhang D, Lu J, Wang C, Huo J, Chen Y, Zhang J. Paris polyphylla suppresses proliferation and vasculogenic mimicry of human osteosarcoma cells and inhibits tumor growth in vivo. Am J Chin Med. 2017;45:575–98. doi: 10.1142/S0192415X17500343. [DOI] [PubMed] [Google Scholar]
- 30.Ren K, Yao N, Wang G, Tian L, Ma J, Shi X, Zhang L, Zhang J, Zhou X, Zhou G, Wu S, Sun X. Vasculogenic mimicry: a new prognostic sign of human osteosarcoma. Hum Pathol. 2014;45:2120–9. doi: 10.1016/j.humpath.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Wang R, Chadalavad K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 32.Soda Y, Marumoto T, Friedmann-Morvinski D, Soda M, Liu F, Michiue H, Pastorino S, Yang M, Hoffman RM, Kesari S, Verman IM. Transdifferentiation of glioblastoma cells into vascular endothelial cell. Proc Natl Acad Sci U S A. 2011;108:4274–80. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.EI Hallani S, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL, Eichmann A, Delattre JY, Maniotis AJ, Sanson M. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133:973–82. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilbe W, Dirnhofer S, Oberwasserlechner F, Schmid T, Gunsilius E, Hilbe G, Wöll E, Kähler CM. CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung cancer. J Clin Pathol. 2004;57:965–9. doi: 10.1136/jcp.2004.016444. [DOI] [PMC free article] [PubMed] [Google Scholar]


