Abstract
Wnt7a is a known tumor suppressor gene in non-small cell lung cancer that regulates normal cellular proliferation and differentiation. The purpose of this study was to investigate the clinicopathologic significance of Wnt7a expression in colorectal adenocarcinoma. Wnt7a expression was immunohistochemically examined in 46 normal colorectal tissues, 47 tubular adenomas, 393 adenocarcinomas, and 93 lymph node metastases. Wnt7a was expressed in the cytoplasm. Loss of Wnt7a expression was more frequent in adenocarcinoma and lymph node metastasis compared to that in normal and tubular adenoma (P < 0.001). Wnt7a expression was inversely correlated with tumor size (P = 0.026), gross type (P = 0.008), differentiation (P = 0.009), vascular invasion (P = 0.038), tumor deposit (P = 0.007), tumor invasion (T category) (P = 0.003), lymph node metastasis (N category) (P < 0.001), and AJCC stage (P < 0.001). There was a significant correlation between loss of Wnt7a expression and overall survival and disease-free survival (P < 0.001 and P = 0.001, respectively) on univariable analysis. On multivariable analysis, loss of Wnt7a expression was an independent prognostic factor for both overall and disease-free survival (P = 0.002 and P = 0.047, respectively). Loss of Wnt7a expression may contribute to the carcinogenesis and tumor progression of colorectal adenocarcinoma and may be a new prognostic marker of colorectal adenocarcinoma.
Keywords: Wnt7a, carcinogenesis, prognosis, colorectum, adenocarcinoma
Introduction
Colorectal cancer is one of the most prevalent cancers worldwide, along with lung cancer and breast cancer and the second-leading cause of cancer-related death [1-3]. Although there have been marked advances in the understanding of colorectal cancer carcinogenesis and improvements in diagnostic and treatment modalities of colorectal cancer, the therapeutic problem is still unsolved [2]. Colorectal cancer patients without lymph node metastasis or distant metastasis can be treated by surgical resection alone. However, further treatment of colon cancer patients with distant metastasis and post-surgical recurrence still remains challenging [4,5]. Surgical treatment does not always prevent the recurrence of advanced colorectal cancer and about one-fourth of colorectal cancer patients present with liver or distant metastasis at initial diagnosis [1].
Currently, there is no targeted therapy available to improve the clinical outcome of colorectal cancer patients. A search for reliable molecular markers of prognosis in colorectal cancer patients would help patients with colorectal cancer [6,7]. Adjuvant chemotherapeutic approaches in colorectal cancer have proven beneficial, but do not prevent recurrence in all patients [8]. Therefore, there are numerous ongoing studies searching for alternative molecular markers to be used as adjuvant therapy. Cancer-associated monoclonal antibodies, targeting tumor-associated proteins and blocking essential processes of cancer, have been extensively investigated [9]. The identification of tumor-specific proteins that can be targeted is crucial and one of these targets is Wnt7a. Wnt7a, a 39 kDa secreted glycoprotein, is a member of the Wnt protein family which regulates normal cellular proliferation and differentiation, as well as tumorigenesis and progression [10]. Recent studies on human cancers have shown that Wnt7a has a critical role in malignant progression; however, there is conflicting evidence whether Wnt7a acts as a tumor promoter or tumor suppressor [11,12]. Wnt7a expression possesses controversial roles in different types of cancer [13].
In the present study, we investigated Wnt7a expression immunohistochemically in a series of 46 normal colorectal tissues, 47 tubular adenomas, 393 colorectal adenocarcinomas and 93 lymph node metastases and evaluated the association of Wnt7a expression with clinicopathological parameters, as well as the impact of Wnt7a expression on overall and disease-free survival in patients with colorectal adenocarcinoma.
Materials and methods
Patients and specimens
A consecutive series of 393 patients with colorectal adenocarcinoma was enrolled in this study. All patients underwent surgery at the Hanyang University Hospital (Seoul, South Korea) between January 2005 and December 2010. This study was approved by the Institutional Review Board of the Hanyang University Hospital (HYUH 2015-11-009-002). There were 242 male and 151 female patients. The patient age ranged from 28 years to 89 years (a mean age of 63.41 years and a median age of 64 years). Out of 393 cases, tumors were located in cecum (n = 21), ascending colon (n = 61), hepatic flexure (n = 3), transverse colon (n = 20), splenic flexure (n = 2), descending colon (n = 18), sigmoid colon (n = 126), and rectum (n = 142). The size of the tumor ranged from 0.5 to 13 cm (a mean size of 4.98 and a median size of 5.0). Tumors consisted of 366 non-mucinous adenocarcinomas and 27 mucinous adenocarcinomas. Mean follow-up interval was 4.55 years. 117 (29.8%) patients died and 276 (70.2%) patients remained alive. 53 cases were stage I, 119 cases were stage II, 194 cases were stage III, and 27 cases were stage IV according to the American Joint Committee on Cancer (AJCC) staging system. In addition, 46 samples of normal colorectal tissue, 47 samples of tubular adenoma, and 93 samples of lymph node metastasis were selected to evaluate the role of Wnt7a expression in colorectal carcinogenesis and tumor progression. All tissue samples were formalin-fixed and paraffin embedded. Pathologic reports, hematoxylin-eosin stained slides, and medical records were reviewed to confirm the final diagnosis and detail clinicopathologic parameters including age, gender, tumor location, tumor size, gross type, tumor subtype, differentiation, tumor budding, vascular invasion, perineural invasion, tumor deposit, perinodal tumor extension, T category, N category, M category, AJCC stage and patients’ survival.
Tissue microarray construction
A manual tissue microarrayer (Quick Ray Set, Unitama, Seoul, South Korea) was used for tissue microarray construction. As previously described [2], we selected an area rich in tumor cells without necrosis on light microscopy of H&E stained slides. We punched a tissue cylinder with a 2 mm diameter from a previously marked lesion of each donor paraffin block and transferred to the recipient block (Quick Ray Set, Unitama, Seoul, South Korea). Each tissue microarray was made up of 5 × 10 samples with two control samples.
Immunohistochemical staining
A polyclonal rabbit anti-Wnt7a antibody (ab183653, Abcam, Cambridge, UK) at 1:50 dilution was used. 4 μm sections were cut from tissue microarray block using Leica microtome and transferred to adhesive coated slides and deparaffinized. The staining was performed using the Bond Max automated immunostainer (Vision Biosystems, San Francisco, CA, USA). Before staining, the heat-induced epitope retrieval was performed in Bond epitope retrieval solution. Endogenous peroxidase activity was blocked using 0.3% hydrogen peroxide. The primary antibody was incubated for 30 minutes and the slides were incubated with post-primary reagent for 15 minutes at room temperature. The reactions were developed using a Bond polymer refine detection kit and followed by color development with 3,3’-diaminobenzidine tetrahydrochloride as a chromogen.
Interpretation of immunohistochemical staining
Wnt7a expression was evaluated semiquantitatively by two independent pathologists (Jang SM and Paik SS) who were blinded to the patients’ clinical outcome. We categorized the cytoplasmic Wnt7a expression in terms of both staining intensity and extent, as described previously [10,14]. Staining intensity was graded as negative (0), weak (1), moderate (2), and strong (3), and staining extent was graded as 0% (0), 1-25% (1), 26-50% (2), 51-75% (3), and 76-100% (4). The product of intensity grade and extent grade was used as the final staining score. Thus, the maximum combined score was 12 and the minimum score was 0. For the purpose of statistical analysis, a cut off value of 2 was adopted according to the receiver operating characteristic curve. The samples were finally classified as either negative (score 0-1) or positive (score 2-12) for Wnt7a expression.
Statistical analysis
Statistical analysis was performed using SPSS software, version 18.0 (Chicago, IL, USA). The expression pattern and median expression score of Wnt7a between normal colorectal mucosa, tubular adenoma, adenocarcinoma, and lymph node metastasis were evaluated by Chi-square test for linear trend and Kruskal-Wallis test. Chi-square test for linear trend, Chi-square test for independence, and Mann-Whitney U test were used to investigate the association between Wnt7a expression and clinicopathological features including age, gender, tumor location, tumor size, gross type, tumor subtype, differentiation, tumor budding, vascular invasion, perineural invasion, tumor deposit, perinodal tumor extension, T category, N category, M category, and AJCC stage. Spearman’s analysis was used to obtain a correlation coefficient. The Kaplan-Meier method with log-rank test was used to create the curves of overall survival and disease-free survival. Univariable analysis using the Cox proportional hazards regression model was performed to compare the survival rates of subgroups. Multivariable survival analysis using the Cox proportional hazards regression model was performed to determine independent prognostic factors. A difference of P < 0.05 was considered significant.
Results
Staining pattern of Wnt7a
Wnt7a expression was evaluated in 46 samples of normal colorectal tissue, 47 samples of tubular adenoma, 393 samples of adenocarcinoma, and 93 samples of lymph node metastasis. Wnt7a was expressed in the cytoplasm with various grades. Representative photographs of Wnt7a expression according to the staining intensity in colorectal adenocarcinoma are shown in Figure 1. Wnt7a expression was positive in all cases (100%) of normal colorectal tissue, 40 cases (85.1%) of tubular adenoma, 237 cases (60.3%) of adenocarcinoma, and 31 cases (33.3%) of lymph node metastasis. Loss of Wnt7a expression was more frequent in malignant tumors compared to that in benign lesions (P < 0.001) (Table 1). Median Wnt7a expression score was 8 (12-6) in normal colorectal tissue, 6 (9-3) in tubular adenoma, 3 (4-0) in adenocarcinoma, and 0 (3-0) in lymph node metastasis. Median Wnt7a expression score was significantly less in malignant tumors than in benign lesions (P < 0.001, Kruskal-Wallis test) (Figure 2).
Figure 1.
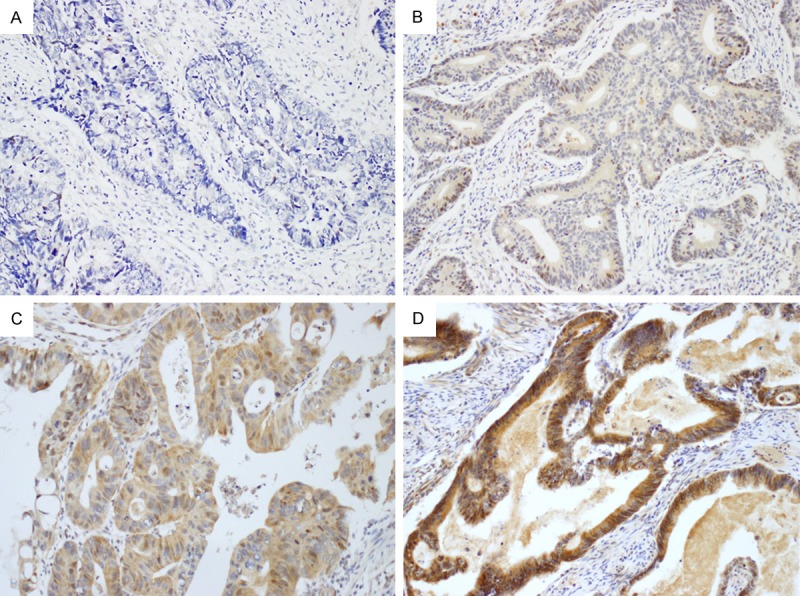
Representative photographs of Wnt7a immunostaining in colorectal adenocarcinoma (× 200). A: Negative; B: Weak; C: Moderate; D: Strong. Wnt7a is expressed in the cytoplasm of tumor cells.
Table 1.
Wnt7a expression in NL, TA, CA, and LNM (n = 579)
| Tissue samples | n | Wnt7a expression | |||
|---|---|---|---|---|---|
|
| |||||
| Negative (%) (n = 225) | Positive (%) n = 354) | p-value† | rs | ||
| NL | 46 | 0 (0.0) | 46 (100.0) | < 0.001 | -0.347 |
| TA | 47 | 7 (14.9) | 40 (85.1) | ||
| CA | 393 | 156 (39.7) | 237 (60.3) | ||
| LNM | 93 | 62 (66.7) | 31 (33.3) | ||
Chi-square test for linear trend.
NL: normal colorectal tissue; TA: tubular adenoma; CA: adenocarcinoma; LNM: lymph node metastasis; rs: Spearman’s rank correlation coefficient.
Figure 2.
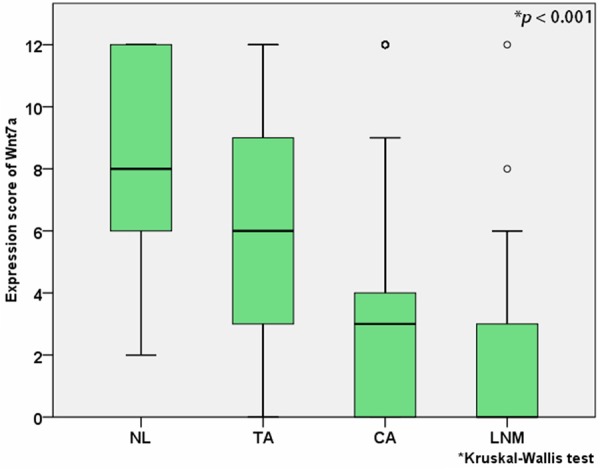
Median Wnt7a expression score in normal colorectal tissue (NL), tubular adenoma (TA), adenocarcinoma (CA), and lymph node metastasis (LNM).
Correlation between Wnt7a expression and various clinicopathological factors
A correlation was investigated between clinicopathological parameters and Wnt7a expression to assess the clinicopathological significance of Wnt7a expression in colorectal adenocarcinoma. Wnt7a expression was inversely correlated with tumor size (P = 0.026), gross type (P = 0.008), differentiation (P = 0.009), vascular invasion (P = 0.038), tumor deposit (P = 0.007), tumor invasion (T category) (P = 0.003), lymph node metastasis (N category) (P < 0.001), and AJCC stage (P < 0.001). However, there was no correlation with age, gender, tumor location, tumor subtype, tumor budding, perineural invasion, perinodal tumor extension, and M category (Table 2).
Table 2.
Correlation between Wnt7a expression and various factors in colorectal adenocarcinoma (n = 393)
| Factors | n | Expression of Wnt7a | |||
|---|---|---|---|---|---|
|
| |||||
| Negative (%) (n = 156) | Positive (%) (n = 237) | p value | rs | ||
| Age (years) | |||||
| Median (IQR) | 393 | 64 (71-58) | 65 (72-57) | 0.560† | 0.029 |
| Gender | |||||
| Male | 242 | 99 (40.9) | 143 (59.1) | 0.533‡ | 0.031 |
| Female | 151 | 57 (37.7) | 94 (62.3) | ||
| Tumor location | |||||
| Colon | 251 | 97 (38.6) | 154 (61.4) | 0.572‡ | -0.029 |
| Rectum | 142 | 59 (41.5) | 83 (58.5) | ||
| Tumor size | |||||
| Median (IQR) | 393 | 5.0 (6.5-4.0) | 4.5 (6.0-3.0) | 0.026† | -0.113 |
| Gross type | |||||
| Polypoid | 46 | 12 (26.1) | 34 (73.9) | 0.008* | -0.132 |
| Ulcerofungating | 154 | 56 (36.4) | 98 (63.6) | ||
| Ulceroinfiltrative | 193 | 88 (45.6) | 105 (54.4) | ||
| Tumor subtype | |||||
| Tubular | 366 | 141 (38.5) | 225 (61.5) | 0.081‡ | -0.088 |
| Mucinous | 27 | 15 (55.6) | 12 (44.4) | ||
| Differentiation | |||||
| Well | 26 | 6 (23.1) | 20 (76.9) | 0.009* | -0.129 |
| Moderately | 186 | 67 (36.0) | 119 (64.0) | ||
| Poorly | 181 | 83 (45.9) | 98 (54.1) | ||
| Tumor budding | |||||
| Low-grade | 238 | 91 (38.2) | 147 (61.8) | 0.464‡ | -0.037 |
| High-grade | 155 | 65 (41.9) | 90 (58.1) | ||
| Vascular invasion | |||||
| Absent | 315 | 117 (37.1) | 198 (62.9) | 0.038‡ | -0.105 |
| Present | 78 | 39 (50.0) | 39 (50.0) | ||
| Perineural invasion | |||||
| Absent | 198 | 70 (35.4) | 128 (64.6) | 0.076‡ | -0.089 |
| Present | 195 | 86 (44.1) | 109 (55.9) | ||
| Tumor deposits | |||||
| Absent | 318 | 116 (36.5) | 202 (63.5) | 0.007‡ | -0.135 |
| Present | 75 | 40 (53.3) | 35 (46.7) | ||
| PNTE¶ | |||||
| Absent | 119 | 57 (47.9) | 62 (52.1) | 0.894‡ | 0.009 |
| Present | 100 | 47 (47.0) | 53 (53.0) | ||
| T category | |||||
| T1 | 32 | 6 (18.8) | 26 (81.3) | 0.003* | -0.135 |
| T2 | 39 | 11 (28.2) | 28 (71.8) | ||
| T3 | 245 | 104 (42.4) | 141 (57.6) | ||
| T4 | 77 | 35 (45.5) | 42 (54.5) | ||
| N category | |||||
| N0 | 174 | 52 (29.9) | 122 (70.1) | < 0.001‡ | -0.179 |
| N1, N2 | 219 | 104 (47.5) | 115 (52.5) | ||
| M category | |||||
| M0 | 366 | 142 (38.8) | 224 (61.2) | 0.181‡ | -0.067 |
| M1 | 27 | 14 (51.9) | 13 (48.1) | ||
| AJCC stage | |||||
| I | 53 | 10 (18.9) | 43 (81.1) | < 0.001* | -0.208 |
| II | 119 | 40 (33.6) | 79 (66.4) | ||
| III | 194 | 92 (47.4) | 102 (52.6) | ||
| IV | 27 | 14 (51.9) | 13 (48.1) | ||
Mann-Whitney U test;
Chi-square test for independence;
Chi-square test for linear trend.
SD: standard deviation; IQR: interquartile range; PNTE: perinodal tumor extension; AJCC: American Joint Committee on Cancer; rs: Spearman’s rank correlation coefficient.
Cases without lymph node metastasis (N0 cases; n = 174) have been excluded.
Overall survival and disease-free survival analyses
In all 393 patients with colorectal adenocarcinoma, the impact of Wnt7a expression on overall and disease-free survival was evaluated. Patient age, tumor budding, vascular invasion, and AJCC stage show a significant effect on overall and disease-free survival in the univariable and multivariable analyses. There was a significant correlation between loss of Wnt7a expression and overall survival and disease-free survival (P < 0.001 and P = 0.001, respectively) on univariable analysis using Cox proportional hazards regression model. On multivariable survival analysis using Cox proportional hazards regression model, loss of Wnt7a expression was an independent prognostic factor for both overall and disease-free survival (P = 0.002 and P = 0.047, respectively) (Table 3). Kaplan-Meier survival curves with log-rank test revealed that loss of Wnt7a expression was significantly correlated with both overall survival and disease-free survival (P < 0.001 and P = 0.001, respectively) (Figure 3).
Table 3.
Effect of variables on overall and disease-free survival in 393 colorectal adenocarcinomas
| Variables | Univariable analysis† | Multivariable analysis† | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Overall survival | ||||
| Wnt7a expression (negative vs. positive) | 0.487 (0.338-0.701) | < 0.001 | 0.560 (0.385-0.813) | 0.002 |
| Patient age (< 64 yrs vs. ≥ 64 yrs) | 2.055 (1.395-3.028) | < 0.001 | 2.128 (1.443-3.138) | < 0.001 |
| Tumor budding (low vs. high) | 2.046 (1.421-2.945) | < 0.001 | 1.707 (1.171-2.488) | 0.005 |
| Vascular invasion (absent vs. present) | 3.070 (2.106-4.475) | < 0.001 | 2.524 (1.690-3.770) | < 0.001 |
| AJCC stage (I, IIvs. III, IV) | 2.079 (1.394-3.099) | < 0.001 | 1.306 (0.847-2.015) | 0.228 |
| Disease-free survival | ||||
| Wnt7a expression (negative vs. positive) | 0.599 (0.437-0.820) | 0.001 | 0.721 (0.522-0.995) | 0.047 |
| Patient age (< 64 yrs vs. ≥ 64 yrs) | 1.625 (1.175-2.247) | 0.003 | 1.762 (1.272-2.439) | 0.001 |
| Tumor budding (low vs. high) | 2.206 (1.609-3.026) | < 0.001 | 1.761 (1.270-2.441) | 0.001 |
| Vascular invasion (absent vs. present) | 3.286 (2.361-4,575) | < 0.001 | 2.509 (1.770-3.556) | < 0.001 |
| AJCC stage (I, IIvs. III, IV) | 2.445 (1.720-3.476) | < 0.001 | 1.661 (1.139-2.423) | 0.008 |
Cox proportional hazards regression model.
HR: hazard ratio; CI: confidence interval; AJCC: American Joint Committee on Cancer.
Figure 3.
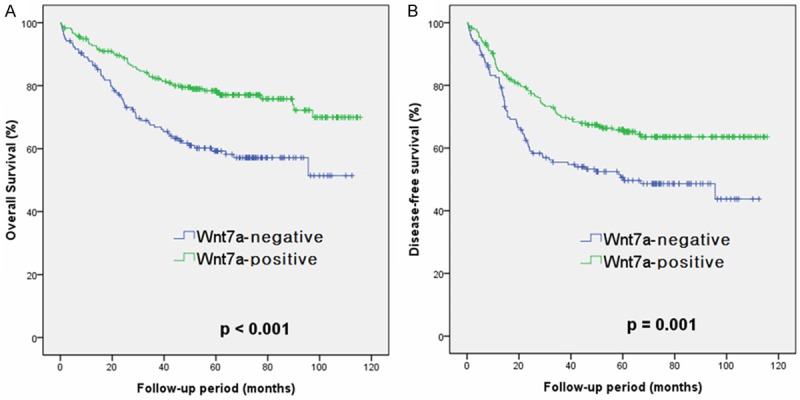
Cumulative survival curves according to Wnt7a expression in 393 patients with colorectal adenocarcinoma (Kaplan-Meier method with log-rank test). A: Overall; B: Disease-free.
Discussion
In the present study, we investigated Wnt7a expression in 46 samples of normal colorectal tissue, 47 samples of tubular adenoma, 393 samples of adenocarcinoma, and 93 samples of lymph node metastasis and evaluated the association between Wnt7a expression and clinicopathologic factors and patient survival in patients with colorectal adenocarcinoma. Loss of Wnt7a expression was more frequent in adenocarcinoma and lymph node metastasis compared to that in normal colorectal tissue and tubular adenoma. Median Wnt7a expression score was significantly higher in normal colorectal tissue and tubular adenoma than in adenocarcinoma and lymph node metastasis. Wnt7a expression was inversely correlated with tumor size, gross type, differentiation, vascular invasion, tumor deposit, tumor invasion, lymph node metastasis and AJCC stage. Kaplan-Meier survival curves revealed a significant effect of loss of Wnt7a expression on both overall survival and disease-free survival of patients with colorectal adenocarcinoma.
The Wnt family of genes encodes secreted, highly conserved glycoproteins that act through Frizzled receptor. Wnt proteins are involved in important cellular processes such as cell fate determination, differentiation, proliferation, mortality, tissue growth, apoptosis and carcinogenesis [14-18]. Wnt gene was initially discovered as a proto-oncogene in mammary tumors [19]. A Wnt protein as a ligand binds to a specific receptor located on the cell surface, activating the canonical or noncanonical pathway [14]. Wnt7a, a 39 kDa glycoprotein, is a member of the Wnt protein family, which is strongly implicated in female reproductive tract development [20]. Wnt7a is exclusively expressed in epithelial cells and acts via Frizzled receptor in the mesenchyme and epithelium [21]. Wnt7a increases cell proliferation via activation of the canonical Wnt/β-catenin pathway [22]. Wnt7a expression has controversial roles in different types of human cancers, including endometrial carcinoma, ovarian cancer, uterine cervical cancer, renal cell carcinoma, malignant pleural mesothelioma, and non-small cell lung carcinoma [10,13-15,23-26]. Recent studies on human cancers have demonstrated that Wnt7a has a critical role in malignant progression; however, there is conflicting evidence whether Wnt7a acts as a tumor promoter or tumor suppressor [10-12].
Winn et al. reported that antitumorigenic effect of Wnt7a/Fzd9 in non-small cell lung cancer cells was mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor γ. In non-small cell lung cancer cells, the restoration of Wnt7a/Fzd9 signaling promoted cell differentiation, inhibited cell proliferation, and reserved the transformed phenotype, suggesting that Wnt7a behaves as a tumor suppressor gene [26]. Ramos-Solano et al. described that reconstitution of Wnt7a expression in cervical cancer-derived cells negatively regulated not only its proliferation rate, but also its migration potential [13]. Peng et al. showed that lost or reduced Wnt7a expression was significantly associated with poor progression-free and overall survival in patients with endometrial carcinoma [14]. Kondratov et al. showed that the Wnt7a gene possessed tumor suppression function by colony-formation and cell proliferation assays in renal cell carcinoma cell line and proposed that inactivation of the Wnt7a gene may play an important role in the development of clear cell renal cell carcinoma [23]. Hirata et al. suggested that Wnt7a is possibly a novel tumor-suppressor gene in malignant pleural mesothelioma [24].
On the other hand, several reports have revealed that Wnt7a may play a role in promoting cancer progression. Merritt et al. described that Wnt7a expression was exclusively high in the malignant ovarian carcinomas and involved in increased migration and invasive capacity in an ovarian cancer cell line, acting as an oncogenic gene [27]. Liu et al. reported that Wnt7a was overexpressed in a large proportion of patients with endometrial carcinoma, and a positive Wnt7a expression was closely associated with tumor progression and unfavorable prognosis [10]. Zhang et al. showed that Wnt7a expression was higher in ovarian carcinomas compared with normal ovaries and benign tumors [28]. Yoshioka et al. suggested that re-expression of Wnt7a during malignant transformation of ovarian epithelial cells plays a critical role in ovarian cancer progression [15]. Wnt7a expression had been also explored in colorectal carcinomas by Wang et al. [29]. They have argued that high expression of Wnt7a was an unfavorable prognostic factor in their study with 212 cases. However, they used the additive scoring system for immunohistochemical semiquantitation of Wnt7a expression. Our study used the multiplying scoring system, which is more relevant and recommended in the scientific analysis because the negative results of additive scoring system can be incomplete [30].
In the present study, we found that Wnt7a was more frequently expressed in normal colorectal tissues and tubular adenomas compared to that in adenocarcinomas and lymph node metastases (P < 0.001). Median Wnt7a expression score was also significantly less in adenocarcinoms and lymph node metastases than in normal colorectal tissues and tubular adenomas (P < 0.001). These results suggest that loss of Wnt7a expression may be involved in the carcinogenesis of colorectal adenocarcinoma. The clinicopathological correlation analysis demonstrated that Wnt7a expression was inversely correlated with tumor size (P = 0.026), gross type (P = 0.008), differentiation (P = 0.009), vascular invasion (P = 0.038), tumor deposit (P = 0.007), tumor invasion (P = 0.003), lymph node metastasis (P < 0.001), and AJCC stage (P < 0.001). These results suggest that loss of Wnt7a expression may be associated with tumor progression and tumor aggressiveness of colorectal adenocarcinoma. In survival analyses, loss of Wnt7a expression revealed poorer overall survival and disease-free survival (P < 0.001 and P = 0.001, respectively) on univariable analysis. On multivariable survival analysis, loss of Wnt7a expression was an independent prognostic factor for both overall and disease-free survival (P = 0.002 and P = 0.047, respectively). Our results suggest that Wnt7a may be a tumor suppressor, not a cancer promoter, in colorectal adenocarcinoma.
In conclusion, our research shows a correlation between Wnt7a expression and clinicopathologic characteristics and the prognostic significance of Wnt7a expression in colorectal adenocarcinomas. Wnt7a expression was lost or reduced in a large proportion of colorectal adenocarcinomas and lymph node metastases and loss of Wnt7a expression was correlated with tumor progression and unfavorable prognosis in patients with colorectal adenocarcinoma. These results suggest that Wnt7a might play a role in tumor suppression in colorectal adenocarcinoma. The exact role of Wnt7a in colorectal adenocarcinoma and its potential as a novel therapeutic target for colorectal adenocarcinoma should be investigated in further studies.
Disclosure of conflict of interest
None.
References
- 1.Weng W, Feng F, Qin H, Ma Y. Molecular therapy of colorectal cancer: progress and future directions. Int J Cancer. 2015;136:493–502. doi: 10.1002/ijc.28722. [DOI] [PubMed] [Google Scholar]
- 2.Sim J, Yi K, Kim H, Ahn H, Chung Y, Rehman A, Jang SM, Lee KH, Jang K, Paik SS. Immunohistochemical expression of dual-specificity protein phosphatase 4 in patients with colorectal adenocarcinoma. Gastroenterol Res Pract. 2015;2015:283764. doi: 10.1155/2015/283764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binefa G, Rodríguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. 2014;20:6786–808. doi: 10.3748/wjg.v20.i22.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okuno K. Surgical treatment for digestive cancer. Current issues - colon cancer. Dig Surg. 2007;24:108–14. doi: 10.1159/000101897. [DOI] [PubMed] [Google Scholar]
- 5.Sawa M, Masuda M, Yamada T. Targeting the Wnt signaling pathway in colorectal cancer. Expert Opin Ther Targets. 2016;20:419–29. doi: 10.1517/14728222.2016.1098619. [DOI] [PubMed] [Google Scholar]
- 6.Jun YJ, Jang SM, Han H, Lee KH, Jang K, Paik SS. Clinicopathologic significance of GULT1 expression and its correlation with Apaf-1 in colorectal adenocarcinomas. World J Gastroenterol. 2011;17:1866–73. doi: 10.3748/wjg.v17.i14.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clinical Cancer Research. 2004;10:8465–71. doi: 10.1158/1078-0432.CCR-04-0653. [DOI] [PubMed] [Google Scholar]
- 8.André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 9.Blok EJ, Kuppen PJ, van Leeuwen JE, Sier CF. Cytoplasmic overexpression of HER2: a key factor in colorectal cancer. Clin Med Insights Oncol. 2013;7:41–51. doi: 10.4137/CMO.S10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Meng F, Xu Y, Yang S, Xiao M, Chen X, Lou G. Overexpression of Wnt7a is associated with tumor progression and unfavorable prognosis in endometrial cancer. Int J Gynecol Cancer. 2013;23:304–11. doi: 10.1097/IGC.0b013e31827c7708. [DOI] [PubMed] [Google Scholar]
- 11.Kirikoshi H, Katoh M. Expression of Wnt7A in human normal tissues and cancer, and regulation of Wnt7A and Wnt7B in human cancer. Int J Oncol. 2002;21:895–900. [PubMed] [Google Scholar]
- 12.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 13.Ramos-Solano M, Meza-Canales ID, Torres-Reyes LA, Alvarez-Zavala M, Alvarado-Ruíz L, Rincon-Orozco B, Garcia-Chagollan M, Ochoa-Hernández AB, Ortiz-Lazareno PC, Rösl F, Gariglio P, Jave-Suárez LF, Aguilar-Lemarroy A. Expression of WNT genes in cervical cancerderived cells: implication of WNT7A in cell proliferation and migration. Exp Cell Res. 2015;335:39–50. doi: 10.1016/j.yexcr.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Peng C, Zhang X, Wang Y, Li L, Wang Q, Zheng J. Expression and prognostic significance of Wnt7a in human endometrial carcinoma. Obstet Gynecol Int. 2012;2012:134962. doi: 10.1155/2012/134962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshioka S, King ML, Ran S, Okuda H, MacLean JA 2nd, McAsey ME, Sugino N, Brard L, Watabe K, Hayashi K. WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/β-catenin pathway. Mol Cancer Res. 2012;10:469–82. doi: 10.1158/1541-7786.MCR-11-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51. [PubMed] [Google Scholar]
- 17.Teo R, Möhrlen F, Plickert G, Müller WA, Frank U. An evolutionary conserved role of Wnt signaling in stem cell fate decision. Dev Biol. 2006;289:91–9. doi: 10.1016/j.ydbio.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Lee HY, Kléber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, Sommer L. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–3. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- 19.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 20.Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–10. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- 21.Tulac S, Nayak NR, Kao LC, Van Waes M, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E, Giudice LC. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab. 2003;88:3860–6. doi: 10.1210/jc.2003-030494. [DOI] [PubMed] [Google Scholar]
- 22.Lyu J, Joo CK. Wnt-7a up-regulates matrix metalloproteinase-12 expression and promotes cell proliferation in corneal epithelial cells during wound healing. J Biol Chem. 2005;280:21653–60. doi: 10.1074/jbc.M500374200. [DOI] [PubMed] [Google Scholar]
- 23.Kondratov AG, Kvasha SM, Stoliar LA, Romanenko AM, Zgonnyk YM, Gordiyuk VV, Kashuba EV, Rynditch AV, Zabarovsky ER, Kashuba VI. Alterations of the WNT7A gene in clear cell renal cell carcinomas. PLoS One. 2012;7:e47012. doi: 10.1371/journal.pone.0047012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata T, Zheng Q, Chen Z, Kinoshita H, Okamoto J, Kratz J, Li H, Lui N, Do H, Cheng T, Tseng HH, Koizumi K, Shimizu K, Zhou HM, Jablons D, He B. Wnt7A is a putative prognostic and chemosensitivity marker in human malignant pleural mesothelioma. Oncol Rep. 2015;33:2052–60. doi: 10.3892/or.2015.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohira T, Gemmill RM, Ferguson K, Kusy S, Roche J, Brambilla E, Zeng C, Baron A, Bemis L, Erickson P, Wilder E, Rustgi A, Kitajewski J, Gabrielson E, Bremnes R, Franklin W, Drabkin HA. WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci U S A. 2003;100:10429–34. doi: 10.1073/pnas.1734137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winn RA, Marek L, Han SY, Rodriguez K, Rodriguez N, Hammond M, Van Scoyk M, Acosta H, Mirus J, Barry N, Bren-Mattison Y, Van Raay TJ, Nemenoff RA, Heasley LE. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 2005;280:19625–34. doi: 10.1074/jbc.M409392200. [DOI] [PubMed] [Google Scholar]
- 27.Merritt MA, Parsons PG, Newton TR, Martyn AC, Webb PM, Green AC, Papadimos DJ, Boyle GM. Expression profiling identifies genes involved in neoplastic transformation of serous ovarian cancer. BMC Cancer. 2009;9:378. doi: 10.1186/1471-2407-9-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang XL, Peng CJ, Peng J, Jiang LY, Ning XM, Zheng JH. Prognostic role of Wnt7a expression in ovarian carcinoma patients. Neoplasma. 2010;57:545–51. doi: 10.4149/neo_2010_06_545. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Wei J, Zhang S, Li G, Zhang T, Yu X, Chen H, Liu M. Overexpression of Wnt7α protein predicts poor survival in patients with colorectal carcinoma. Tumour Biol. 2015;36:8781–7. doi: 10.1007/s13277-015-3633-6. [DOI] [PubMed] [Google Scholar]
- 30.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–8. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]


