Abstract
Breast cancer-associated leptomeningeal metastasis (LM) is a particularly aggressive syndrome with an abysmal prognosis. Here we applied the SE-i•FISH platform for surveillance and function analysis of cerebrospinal fluid-derived circulating tumor cells (CSFTCs) from five breast cancer patients with LM. We observed a negative correlation of CK18 expression on CSFTCs with clinical symptom remission, and confirmed that at least six intrathecal chemotherapies were necessary. We also present the case of a breast cancer patient with bladder and leptomeningeal metastasis. The bladder is an extremely unusual site for metastasis from primary breast cancer, with only 14 cases sporadically reported worldwide. Our platform could help monitor CSFTC numbers and treatment responses for patients with LM.
Keywords: Breast cancer, bladder metastasis, leptomeningeal metastasis, cerebrospinal fluid-derived tumor cell, precision medicine
Introduction
Breast cancer commonly metastasizes to numerous organs including lymph nodes, lung, bone, liver, and brain. Leptomeningeal metastasis (LM) of breast cancer, the diffuse infiltration of the meninges by metastatic breast cancer cells, is a rare metastatic site [1]. Due to the lack of prospective clinical studies and advanced detection methods for LM, current treatment recommendations only rely on clinical experience or studies with a low level of evidence [2]. Therefore, there is an urgent need to develop a feasible and reliable method to help evaluate treatment responses and identify clinical regimens. In this study, we applied the established SE-i•FISH platform for surveillance and function analysis of cerebrospinal fluid-derived tumor cells (CSFTCs) [3]. We examined the status of CK18 expression on CSFTCs by SE-i•FISH platform, and observed the correlation of CK18 expression status with clinical remission and CSFTC numbers. To optimize treatment regimens, we retrospectively analyzed the correlation of CSFTC number with treatment times. To the best of our knowledge, at least 6 continuous intrathecal regimens are recommended for breast cancer leptomeningeal metastasis in the English-language literature for the first time.
Materials and methods
SE-i•FISH platform
Briefly, CSF was collected and centrifuged. Supernatant was incubated with anti-WBC (CD45) and endothelial cell immunomagnetic beads as described previously [3]. Images of cells were visualized with a fluorescence microscope. Immunofluorescence intensity of CK18 corresponding to the known CK18 expression status served as the reference standard for quantification of CK18 status on CSFTCs. Enumeration was performed by two experienced researchers in a blinded fashion.
Results
Correlation of CK18 expression on CSFTCs with clinical remission
The up-regulation of CK18 protein correlates with cancer progression [4], cell migration [5], metastasis, and recurrence [6-8] in breast cancer. However, the clinical significance of CK18 in CSFTCs has not been reported. We applied the SE-i•FISH platform (as described previously [3]) to examine the status of CK18 expression on CSFTCs. Strikingly, immunofluorescence intensity of CK18 before intrathecal chemotherapy was strongly positive (+++), whereas with the intrathecal treatment, the number of CSFTCs and immunofluorescence intensity of CK18 gradually reduced (Figure 1A). Together, our data validated the negative correlation of CK18 expression status on CSFTCs with clinical remission and CSFTC numbers.
Figure 1.

The dynamic change of CSFTCs along with intracranial treatment. A. Representative images of immunofluorescence, anti-CK18 (green), DAPI (blue) and CD45 (red). B. The horizontal axis represents times of intracranial chemotherapy. The Y-axis represents the number of CSFTCs. The CSFTC number is recorded in the table (below).
Six intrathecal chemotherapies are recommended
To explore and optimize treatment regimens, we retrospectively examined whether the number of CSFTCs correlated with the treatment times. Since we had counted CSFTCs in five LM patients as described previously [3], the dynamic changes of CSFTC numbers could be used to analyze the optimal regimens (Figure 1B). The number was blinded to clinical history, and patient treatment selection was not informed. Interestingly, when the patients were treated after the sixth intrathecal chemotherapy, the number of tumor cells was found to be less than 15 per ml of cerebrospinal fluid, indicated of a low level and a clinically stable course. However, the CSFTC number of case NM01 and case NM07 tended to rebound at the fifth and fourth intrathecal chemotherapy, respectively, suggesting that fewer than six times of treatment were not enough to control this disease (Figure 1B). These data indicated that the LM patients with breast cancer needed at least six intrathecal chemotherapies.
A breast cancer patient with bladder metastasis and leptomeningeal metastasis
We describe a case of a 44-year-old female with a left breast cancer (Case NM04), and the pathology result reported triple negative breast cancer (Figure 2). After one year, the patient suffered from left flank pain, hematuresis and irritative symptoms. Abdominal computed tomography (CT) revealed diffuse thickening of the urinary bladder wall (Figure 2). Subsequently, the transuretheral biopsy of bladder tumor was performed and the H&E staining showed a sub-mucosal nest of carcinoma cells reported likely source from breast cancer (Figure 2). IHC analysis was consistent with primary breast cancer, ER, PR and HER2 negative. Several cycles of adjuvant chemotherapy were given to the patient and the treatment was suspended after six months as she presented with extreme headache and vomiting, indicative intracranial hypertension and probableleptomeningeal metastasis. The brain CT revealed leptomeningeal metastases (Figure 2). The patient received regularly intrathecal chemotherapy twice a week (Methotrexate/Cytarabine). Unfortunately, the disease progressed and the patient died after 6 months.
Figure 2.
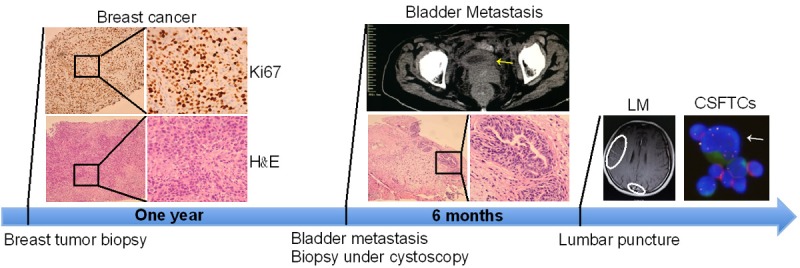
Treatment timelines. A 44-year-old female was diagnosed with breast cancer. After one year, the bladder metastasis was diagnosed by transurethral biopsy. Subsequently, leptomeningeal metastasis appeared. Representative images of hematoxylin and eosin (H&E) staining and computed tomography (CT) in the top panel.
Clinical characteristics of bladder metastasis from breast cancer
Through analyzing the cases in the Table 1, the bladder metastasis median age was 68 years (range 44-91). According to molecular classification of breast cancer, primary breast cancers were 80% (12/15) estrogen receptor positive and 29% (2/7) HER2 positive. 73% (11/15) estrogen receptor positive and 29% (2/7) HER2 positive were observed in bladder metastases. Interestingly, there was discordance between primary tumors and matching bladder metastases: IHC analysis of bladder metastases from Case 2 and Case 9 revealed loss of estrogen receptor (ER) compared with matching primary tumor, whereas the bladder metastasis of Case 8 represented positive estrogen receptor in ER negative breast cancer (Table 1). In Case 3, the primary tumor showed HER2 positive, but the bladder metastasis lost HER2 expression (Table 1).
Table 1.
Reported cases of breast cancer metastasis to the bladder
| NO. | Primary Cancer Age | Primary breast Cancer | Bladder Mets Age | Bladder Mets | Bladder mets to death | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| ER | PR | HER2 | ER | PR | HER2 | |||||
| 1 | 44 | - | - | Neg. | 46 | - | - | Neg. | 1 year | Our case |
| 2 | 50 | + | + | Neg. | 59 | - | - | Neg. | NR | Ahmed A et al. 2016 [15] |
| 3 | 86 | + | - | Pos. | 91 | + | - | Neg. | NR | Carsten Nieder et al. 2014 [16] |
| 4 | 45 | 70% | 80% | Neg. | 53 | + | + | Neg. | NR | Luigi Cormio et al. 2014 [17] |
| 5 | 53 | + | + | Neg. | 83 | + | + | Equivocal | >6 years | MH Wong et al. 2013 [10] |
| 6 | 64 | + | + | Neg. | 69 | + | - | Neg. | 1 year | RA Ghaida et al. 2013 [18] |
| 7 | 46 | + | + | NR | 56 | + | + | Pos. | NR | FA HERRERA et al. 2010 [19] |
| 8 | 65 | - | - | Pos. | 68 | + | + | Pos. | >2 years | WeiChing Lin et al. 2007 [20] |
| 9 | 67 | + | + | NR | 72 | - | - | NR | NR | RalphM Zagha et al. 2007 [21] |
| 10 | 57 | + | + | NR | NR | + | + | NR | >2 years | James Forster et al. 2006 [22] |
| 11 | NR | + | - | NR | 74 | + | - | NR | NR | Nathan L et al. 2005 [11] |
| 12 | NR | + | + | NR | 44 | + | - | NR | NR | |
| 13 | 46 | + | + | NR | 56 | + | + | NR | 2 months | MB Fisher, et al. 2005 [13] |
| 14 | 57 | 90% | 90% | NR | 59 | + | - | NR | >3 months | P Ramalingam et al. 2003 [12] |
| 15 | 61 | - | - | NR | 68 | - | - | NR | NR | Feldman PA et al. 2002 [9] |
Note: NR, not reported.
Discussion
Bladder metastasis generally tends to occur as a diffuse thickening of mucosa rather than a solitary nodule [9-11]. We can observe unusual monomorphic patterns with no accompanying urothelial tumor features and the infiltration of the luminal surfaces [12]. Bladder metastasis is not curable as the deposit is muscular rather than mucosal [13,14]. Leptomeningeal metastasis (LM) usually occurs at a late stage of advanced malignancy with limited treatment options. With progress in effective drugs and advanced diagnostic technology, the incidence of breast cancer-associated leptomeningeal metastases is currently on the rise [1,3]. The leptomeninges may act as a sanctuary site for cancer recurrence. In this study, we observed the negative correlation of CK18 expression status on CSFTCs with clinical symptoms and treatment responses. After intrathecal treatments, the number of CSFTCs and immunofluorescence intensity of CK18 were gradually reduced (Figure 1A). Cancer progression, metastasis, and recurrence in breast cancer were correlated with the expression of CK18 protein [4-7]. Further studies using cancer cell lines and animal models will be explored to gain more mechanistic insights into the implications of CK18 expression in disease progression. Though analyzing the dynamic changes of CSFTC numbers (Figure 1B), we optimized treatment regimens and drew a conclusion that at least six intrathecal chemotherapies were necessary, which was a reliable platform for individualized LM patients (Figure 3). In contrast, the regular CSF cytology in clinical practice only detected the existence of cancer cells but did not sensitively monitor the change of CSFTC numbers. However, the major limitation of this current study is due to the limited case number. The small sample size reduces the statistical power.
Figure 3.

A reliable platform for individualized LM patients. As discussed in the text, CSF samples were obtained from LM patients. The SE-i•FISH method can be applied to detect (A) and enumerate (B) cerebrospinal fluid-derived tumor cells. (C) The isolation and ex vivo culture of CSFTCs can be performed by this platform. The drug sensitivity test on cultured CSFTCs based on genomic analysis data (D) helped predict potential effective treatment regimens for individual LM patients (E).
Acknowledgements
We are indebted to the clinical teams and to Dr. Xuelu Li for their guidance in the core technique. This work was supported by the Provincial Natural Science Foundation of Liaoning (LQ2017026 and 20170540238 to Dandan Zhu).
Disclosure of conflict of interest
None.
References
- 1.Corbin ZA, Nagpal S. Leptomeningeal metastases. JAMA Oncol. 2016;2:839. doi: 10.1001/jamaoncol.2015.3502. [DOI] [PubMed] [Google Scholar]
- 2.Mack F, Baumert BG, Schäfer N, Hattingen E, Scheffler B, Herrlinger U, Glas M. Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat Rev. 2016;43:83–91. doi: 10.1016/j.ctrv.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Li XL, Zhang Y, Ding JL, Wang M, Li N, Yang H, Wang K, Wang D, Lin PP, Li M, Zhao Z, Liu P. Clinical significance of detecting CSF-derived tumor cells in breast cancer patients with leptomeningeal metastasis. Oncotarget. 2018;9:2705–2714. doi: 10.18632/oncotarget.23597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weng YR, Cui Y, Fang JY. Biological functions of cytokeratin 18 in cancer. Mol Cancer Res. 2012;10:485–493. doi: 10.1158/1541-7786.MCR-11-0222. [DOI] [PubMed] [Google Scholar]
- 5.Woelfle U, Sauter G, Santjer S, Brakenhoff R, Pantel K. Down-regulated expression of cytokeratin 18 promotes progression of human breast cancer. Clin Cancer Res. 2004;10:2670–2674. doi: 10.1158/1078-0432.ccr-03-0114. [DOI] [PubMed] [Google Scholar]
- 6.Fortier AM, Asselin E, Cadrin M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J Biol Chem. 2013;288:11555–11571. doi: 10.1074/jbc.M112.428920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge F, Zhang H, Wang DD, Li L, Lin PP. Enhanced detection and comprehensive in situ phenotypic characterization of circulating and disseminated heteroploid epithelial and glioma tumor cells. Oncotarget. 2015;6:27049–27064. doi: 10.18632/oncotarget.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Sun S, Li N, Gao J, Yu J, Zhao J, Li M, Zhao Z. High expression of CCR7 predicts lymph node metastasis and good prognosis in triple negative breast cancer. Cell Physiol Biochem. 2017;43:531–539. doi: 10.1159/000480526. [DOI] [PubMed] [Google Scholar]
- 9.Feldman PA, Madeb R, Naroditsky I, Halachmi S, Nativ O. Metastatic breast cancer to the bladder: a diagnostic challenge and review of the literature. Urology. 2002;59:138. doi: 10.1016/s0090-4295(01)01489-3. [DOI] [PubMed] [Google Scholar]
- 10.Wong MH, Yiu MK, Ho KL. Metastatic carcinoma of breast in the urinary bladder. Hong Kong Med J. 2013;19:455–7. doi: 10.12809/hkmj133768. [DOI] [PubMed] [Google Scholar]
- 11.Lawrentschuk N, Chan Y, Bolton DM. Metastatic breast cancer to the bladder. Breast J. 2005;11:143. doi: 10.1111/j.1075-122X.2005.21427.x. [DOI] [PubMed] [Google Scholar]
- 12.Ramalingam P, Middleton LP, Tamboli P, Troncoso P, Silva EG, Ayala AG. Invasive micropapillary carcinoma of the breast metastatic to the urinary bladder and endometrium: diagnostic pitfalls and review of the literature of tumors with micropapillary features. Ann Diagn Pathol. 2003;7:112–119. doi: 10.1053/adpa.2003.50015. [DOI] [PubMed] [Google Scholar]
- 13.Fisher MB, Weise AJ, Powell IJ. Breast carcinoma metastatic to the bladder and renal pelvis requiring fulguration. Clin Breast Cancer. 2005;6:173–174. doi: 10.3816/CBC.2005.n.021. [DOI] [PubMed] [Google Scholar]
- 14.Iguchi C, Nio Y, Itakura M. Heterogeneic expression of estrogen receptor between the primary tumor and the corresponding involved lymph nodes in patients with node-positive breast cancer and its implications in patient outcome. J Surg Oncol. 2003;83:85–93. doi: 10.1002/jso.10243. [DOI] [PubMed] [Google Scholar]
- 15.Al Ibraheemi AA. Case report of metastatic invasive breast lobular carcinoma to the urinary bladder. Int J Hematol Oncol Stem Cell Res. 2016;10:51–55. [PMC free article] [PubMed] [Google Scholar]
- 16.Nieder C, Pawinski A. A case of recurrent breast cancer with solitary metastasis to the urinary bladder. Case Rep Oncol Med. 2014;2014:931546. doi: 10.1155/2014/931546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cormio L, Sanguedolce F, Di Fino G, Massenio P, Liuzzi G, Ruocco N, Bufo P, Carrieri G. Asymptomatic bladder metastasis from breast cancer. Case Rep Urol. 2014;2014:672591. doi: 10.1155/2014/672591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghaida RA, Ayoub H, Nasr R, Issa G, Bulbul M. Bladder metastasis from primary breast cancer: a case report and literature review. Cent Eur J Urol. 2013;66:177–184. doi: 10.5173/ceju.2013.02.art17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera FA Jr, Hassanein AH, Cosman BC, Bouvet M. Breast carcinoma metastatic to the gallbladder and urinary bladder. Eur Rev Med Pharmacol Sci. 2010;14:883–886. [PubMed] [Google Scholar]
- 20.Lin WC, Chen JH. Urinary bladder metastasis from breast cancer with heterogeneic expression of estrogen and progesterone receptors. J Clin Oncol. 2007;27:4308–4310. doi: 10.1200/JCO.2007.12.9379. [DOI] [PubMed] [Google Scholar]
- 21.Zagha RM, Hamawy KJ. Solitary breast cancer metastasis to the bladder: an unusual occurrence. Urol Oncol. 2007;25:236–239. doi: 10.1016/j.urolonc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Forster J, Agrawal V, Anathhanam AJ, Spencer N, Biyani CS. Breast carcinoma metastasizing to the urinary bladder presenting as bilateral hydronephrosis treated with ureteral stenting and chemotherapy. Urol Oncol. 2006;24:33–35. doi: 10.1016/j.urolonc.2005.07.012. [DOI] [PubMed] [Google Scholar]


