Abstract
The receptor tyrosine kinase-like orphan receptor 1 (ROR1) is a type I surface transmembrane protein that contributes to progression of tumor-cell growth and metastasis. We and others have shown that the roles of ROR1 include inhibiting apoptosis, potentiating EGFR signaling, and inducing proliferation in lung cancer, but the roles and mechanisms of ROR1 in lung adenocarcinoma metastasis have not been elucidated. Here we chose four lung adenocarcinoma cell lines, PC9 (erlotinib-sensitive), PC9erlo (acquired erlotinib-resistant), NCI-H358 (partial erlotinib-resistant), and NCI-H1975 (erlotinib-resistant) as cell models to simulate the clinical situation. We found that ROR1 prompted epithelial to mesenchymal transition (EMT) by increasing the expression level of a key epithelial gene, E-cadherin, while decreasing the expression level of the key mesenchymal gene vimentin. Silencing ROR1 by siRNA significantly reduced the migration and invasion of lung adenocarcinoma cells in vitro and also significantly inhibited the phosphorylation of Akt (Ser473), mTOR (Ser2448), Raptor (Ser792) and p70S6K (Thr389) in all four cell lines. This strongly supports our proposal that ROR1 may play a central role in tumor progression and metastasis in lung adenocarcinoma through mTOR signaling, regardless of its EGFR-TKI sensitivity status.
Keywords: ROR1, EMT, lung adenocarcinoma, invasion, migration, AKT/mTOR signaling pathway
Introduction
Lung cancer is currently the most common cause of global cancer morbidity and mortality [1]. There are two major histologic types: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Non-small cell lung cancer accounts for approximately 80% of all diagnosed lung cancers. The most common subtype of NSCLC lung adenocarcinoma has a poor 5-year survival rate because of tumor invasion and metastasis [2].
Emerging evidence suggests that the acquisition of invasiveness in cancer is accompanied by the loss of epithelial features and the gain of a mesenchymal phenotype, a process known as epithelial-to-mesenchymal transition (EMT) [3]. Induction of a partial or full EMT has been associated with enhanced tumor-initiation, resistance against multiple therapies, immune-evasion, altered metabolism, and genomic instability [4-8]. Cells undergoing EMT display decreased expression levels of epithelial genes (such as E-cadherin, ZO-1 and occludin) and increased expression levels of mesenchymal genes (such as N-cadherin, vimentin and fibronectin) [9]. In most cases, loss of E-cadherin is a hallmark of EMT [10]. There is evidence accumulating from genetic and cancer biology studies that the PI3K/AKT pathway is a central mechanism controlling EMT features [11-18].The signaling pathway ultimately leads to activation of EMT transcription factors (EMT-TFs) [19,20]. Several transcription factors have been identified as master regulators of EMT, including SNAIL factors (SNAI1, also known as Snail and SNAI2, also known as Slug), bHLH factors (E12 and E47, TWIST1 and TWIST2) and ZEB factors (ZEB1 and ZEB2) [21-23].
The receptor tyrosine kinase-like orphan receptor 1 (ROR1) is a type I surface transmembrane protein that contributes to the development and migration of fetal organs during embryonic development [24,25]. We and others found that ROR1 was present and active in numerous blood and some solid malignancies, as well as in lung cancer cell lines and tissues. ROR1 has been shown to inhibit apoptosis, potentiate EGFR signaling, and induce proliferation, and is thus critically involved in progression of tumor cells [26-33]. In 2013, Kipps first reported the role of ROR1 in breast cancer metastasis [34], then several additional studies also demonstrated that targeting ROR1 inhibits invasion and adhesion in ovarian, breast cancer, and chronic lymphocytic leukemia metastasis [35-39], but the role of ROR1 in lung adenocarcinoma metastasis is still unclear.
In our previous study, we demonstrated that over 60% of human lung adenocarcinomas expressed ROR1 and silencing ROR1 with siRNA can induce tumor cell death and apoptosis via the PI3K/AKT/mTOR pathway [33]. Here, we investigated the mechanistic links that could explain the extraordinary potency of ROR1 in driving lung adenocarcinoma metastasis, and we show a direct effect of ROR1 on the EMT process. Thus, our findings provide new insights into the mechanism of ROR1-induced EMT in lung adenocarcinoma.
Material and methods
Cell lines
The NSCLC cell line NCI-H358 was purchased from Typical Culture Preservation Commission Cell Bank, Chinese Academy of Sciences. We received a kind gift of PC9 from Dr. Jun Zhang of Shanghai Pulmonary Hospital. The NSCLC cell line NCI-H1975 was kindly provided by Stem Cell Bank, Chinese Academy of Sciences. The three cell lines were cultured at 37°C in a 5% CO2/95% humidified air incubator (Panasonic, Ehime, Japan) in RPMI-1640 (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, Transgen, Beijing, China) and 100 μg/ml penicillin/streptomycin (HyClone, Logan, UT, USA).
Establishment of an acquired erlotinib-resistant cell line PC9erlo
The acquired erlotinib-resistant cell line was established by our own laboratory. The PC9 cell lines were seeded in a 10 cm2 dish and erlotinib (Cayman, MI, USA) was added to a final concentration of 10 μM. The medium was discarded and the drug was washed with PBS 48 hours later. In order to obtain and maintain erlotinib resistance ability, these cells were collected and gradually exposed to increasing concentrations of erlotinib (0.1 μM for 2 months, 0.5 μM for 2 months, 1.25 μM for 2 months, 2.5 μM till now). The IC50 value of erlotinib in PC9erlo was 2.62±0.82 μM, which was tested 2 months after exposing cells to 2.5 μM erlotinib.
Silencing of human ROR1
The pre-designed siRNA sequence to target endogenous ROR1 mRNA was obtained from Ambion (Thermo Scientific, Grand Island, NY, USA): ROR1 siRNA(siROR1) 5’-GUACUGCGAUGAAACUUCATT-3’; antisense 5’-UGAAGUUUCAUCGCAGUACGG-3’. The siRNA duplex (siNC, Invitrogen, Thermo Scientific, Grand Island, NY, USA) was used as a negative control. Cells were seeded in 6-well plates at a density of 5×105 cells/well and incubated in a CO2 incubator overnight. Cells were transfected with 20 nM siROR1 or siControl, serum-starved for 6 h, and then replaced into complete medium. All siRNA transfections were performed in Opti-MEM reduced serum medium (Gibco, Life Technologies, Grand Island, NY, USA) using lipofectamine RNAiMAX (Invitrogen, Thermo Scientific, Grand Island, NY, USA) according to the manufacturer’s instruction.
Flow cytometry assay
Cells (5×105) were collected and washed twice with ice-cold flow cytometry buffer (PBS with 1% FBS). Five μg/ml of chimeric rabbit/human anti-ROR1 monoclonal antibody R12 with HA tag that was developed by Christoph Rader [40], or human IgG (Jackson ImmunoResearch, West Grove, PA, USA) was added to the cells and incubated on ice for 30 min. After washing twice with flow cytometry buffer, PE-conjugated anti-HA monoclonal Ab (mAb) (Miltenyi Biotech, San Diego, CA, USA) was added and incubated on ice for 30 min. Finally, cells were washed and suspended in 500 μl of flow cytometry buffer. A BD Accuri C6 flow cytometer (BD Bioscience, USA) was used to analyze ROR1 expressing cells and the data were analyzed using the FlowJo 7.6.2 software program.
Wound healing assay
Cell migration was determined by a wound healing assay. Cells were seeded in 6-well plates at a concentration of 5×105 cells/well and incubated overnight. The next day, cells were transfected with siRNA when cell confluency was >90%. After 48 hours, the wounds were drawn perpendicularly with a 200-μl pipette tip. Cell debris was washed twice with PBS, and serum-free 1640 medium was added for further incubation. The wound area was observed with a microscope at 0 h and 48 h and images obtained were analyzed using Image-Pro Plus 6.0 software.
Transwell invasion assay
Transwell invasion assays were performed using 24-well invasion chambers with an 8 micron pore size PET membrane (Corning, USA) that was pretreated with 200 μg/ml Matrigel Matrix (Corning, USA). Forty-eight hours after transfection with 20 nM siROR1, cells were trypsinized, resuspended, and seeded into the upper Matrigel chamber in 200 μl of serum-free 1640 medium at a concentration of 1×105 cells/well, and in the lower chamber 700 μl of RPMI-1640 with 10% fetal calf serum acting as a chemoattractant. After 24 hour-incubation, non-invasive cells on the surface of the upper membrane were erased with a cotton swab. Invasive cells on the lower surface of the membrane were fixed and stained with crystal violet for 5 min. Cells in 5-random fields were counted using an inverted microscope.
Western blot assay
Protein extracts from lung adenocarcinoma cell lines were prepared using RIPA lysis buffer (Beyotime, Shanghai, China) which were supplemented with protease inhibitor cocktail (Millipore, Bedford, MA, USA) and phosphatase inhibitor (Roche, Basel, Switzerland). The protein concentration was confirmed in accordance with the BCA Protein Assay Kit (Beyotime, Shanghai, China) with bovine serum albumin utilized as a standard. After the desired amount of protein was electrophoresed, and transferred to a PVDF membrane (Millipore, Bedford, MA, USA), immunoblotting was performed using antibodies detecting mTOR, phospho-mTOR, AKT, phospho-AKT, Raptor, phospho-Raptor, phospho-p70S6K, Snail, Slug (Cell Signaling Technology, Danvers, MA, USA), E-cadherin, p70S6K (BD Bioscience, Mountain View, CA, USA), vimentin (Santa Cruz Biotechnology, Dallas, TX, USA) with β-actin (TransGen, Beijing, China) used as loading control. HRP conjugated anti-mouse IgG or anti-rabbit IgG (TransGen, Beijing, China) were used as a secondary antibody. An enhanced Pierce ECL Western Blotting Substrate (Thermo Scientific, Rockford, IL, USA) was used to detect chemiluminescence. The images obtained were analyzed using Image J software.
Immunofluorescence
Cells were grown on 96-well plates, fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 1% Triton X100 for 30 minutes, and blocked with PBS containing 5% fetal goat serum for 1 hour. Cells were incubated with primary antibody in PBS for 1 hour, washed three times with PBS, incubated with Alexa Fluor 488 secondary antibody (Thermo Scientific, Rockford, IL, USA) in PBS for 30 minutes, and then analyzed using a inverted fluorescence microscope (Nikon, Tokyo, Japan).
Statistical analysis
All of the data were analyzed using GraphPad Prism 5 software (San Diego, CA, USA). The data were expressed as the mean ± standard deviation.Comparisons of multiple groups were achieved by one-way analysis of variance following by Turkey’s multiple comparison procedures. P<0.05 were considered to be statistically significant differences. All of the assays were performed at least three times independently.
Results
ROR1 expression in human lung adenocarcinoma cells is significantly inhibited via siROR1
PC9, PC9erlo, NCI-H358, and NCI-H1975 cell lines were transfected with pre-designed siROR1, and ROR1 expression was examined by flow cytometry at 72 h after transfection. The flow cytometry results shown in Figure 1 indicate the siROR1 silenced ROR1 expression by 50~90% compared to cells transfected with the control, non-silencing siRNA (siNC) (ROR1 expression considered to be 100%) (Figure 1).
Figure 1.
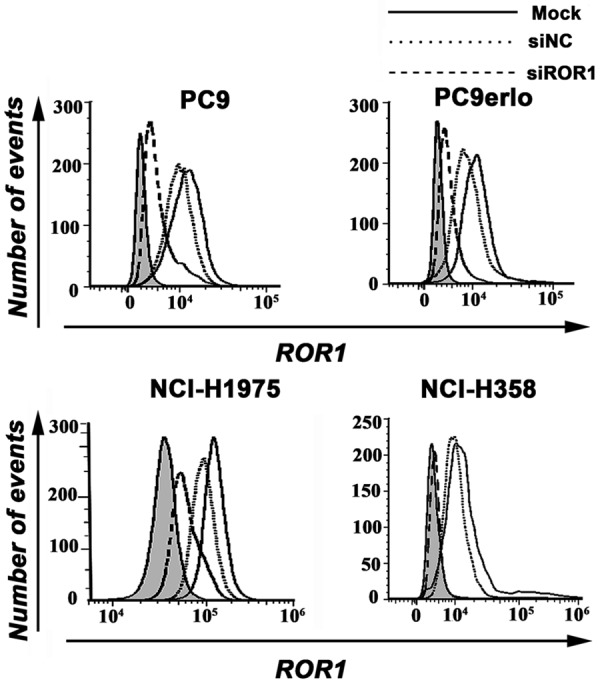
Silencing ROR1 expression by siRNA. PC9, PC9erlo, NCI-H358, and NCI-H1975 were treated with ROR1 siRNA (siROR1) or control siRNA (siNC) for 72 h and examined for ROR1 protein expression with chimeric rabbit/human anti-ROR1 monoclonal antibody R12 by flow cytometry. Mock, wild-type cells; siNC, negative control small interfering RNA-transfected cells; siROR1, ROR1 siRNA-transfected cells.
Silencing ROR1 inhibits migration in human lung adenocarcinoma cells
To investigate whether silencing ROR1 can inhibit the migration of lung cancer cells, a wound healing assay was performed. PC9erlo, an acquired erlotinib-resistant cell line established in our lab and its parental cell line PC9 which is erlotinib-sensitive, were used in the assay. Compared with cells transfected with the control siRNA (siNC), PC9 cells and PC9erlo cells transfected with siROR1 migrated into the wound area more slowly (Figure 2). These results suggested that silencing of ROR1 could suppress the migration of lung adenocarcinoma cells, even those with acquired erlotinib-resistance.
Figure 2.
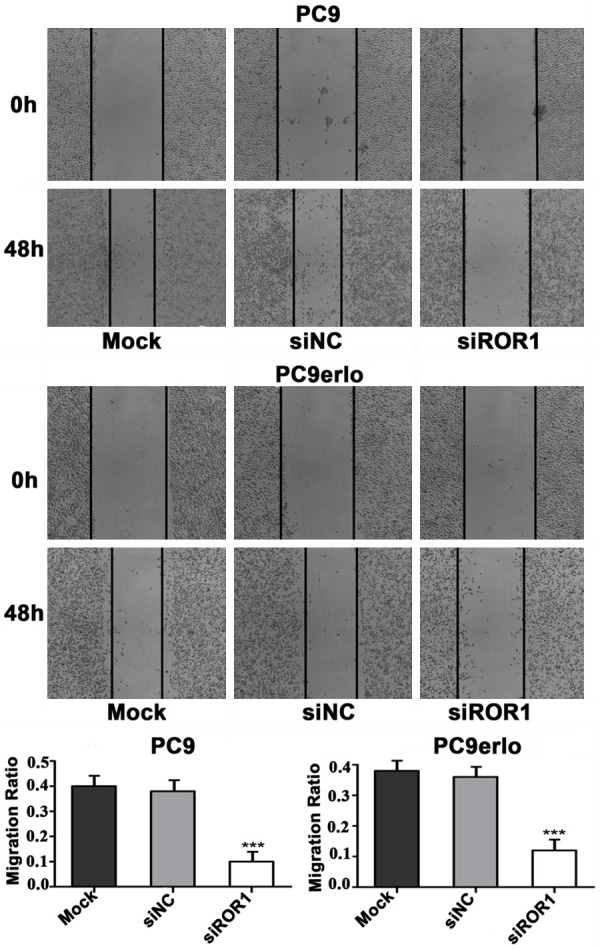
Knockdown of ROR1 inhibits lung adenocarcinoma cell migration. The cells were treated with siROR1 for 48 h, then scraped with a pipette tip. The migration of the cells was observed using a microscope (magnification, 100×) prior to and following injury. The migration of the PC9 and PC9erlo cells were quantified by measuring the wound closure areas pre- and post-injury. The experiments were repeated three times. Mock, wild-type cells; siNC, negative control small interfering RNA-transfected cells; siROR1, ROR1 siRNA-transfected cells.
Silencing ROR1 inhibits invasion in human lung adenocarcinoma cells
We used a 24-well invasive chamber for invasion assay. One erlotinib-sensitive cell line PC9 and two erlotinib-resistant cell lines PC9erlo and NCI-H1975 were involved in the assay. Our data clearly demonstrated that in all cell lines the number of invading cells were significantly decreased after silencing with siROR1 compared with silencing with siNC (Figure 3). These results strongly suggest that ROR1 silencing effectively inhibited the invasive abilities of lung adenocarcinoma cells, even those with acquired erlotinib-resistance.
Figure 3.
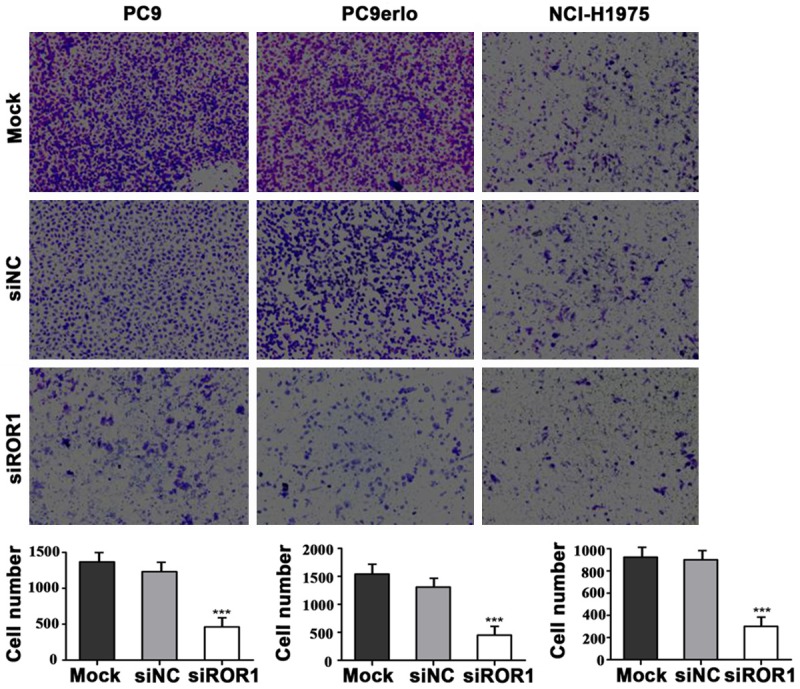
Knockdown of ROR1 inhibits lung adenocarcinoma cell invasion. Transwell invasion assay was performed in PC9, PC9erlo and NCI-H1975 cells. The three cell lines were transfected with siROR1 for 48 h and then plated into 24-well chambers. Invasive cells were stained with crystal violet 24 h later and observed using a microscope (magnification, 100×). Three independent experiments were performed for quantification. Mock, wild-type cells; siNC, negative control small interfering RNA-transfected cells; siROR1, ROR1 siRNA-transfected cells.
ROR1 promoted the EMT process in lung adenocarcinoma cells
EMT is a critical process involved in cancer invasion and metastasis. To further investigate the potential molecular mechanism of ROR1-induced migration and invasion, western blot and immunofluorescent assays were used to examine the EMT-related molecules in lung adenocarcinoma cells. PC9 (erlotinib-sensitive), PC9erlo (acquired erlotinib-resistant), NCI-H358 (partial erlotinib-resistant), and NCI-H1975 (erlotinib-resistant) were used as cell models to simulate the clinical situation. Silencing ROR1 with siRNA markedly enhanced the expression level of the key epithelial gene E-cadherin and decreased the expression level of the key mesenchymal gene vimentin in all four cell lines (Figures 4, 5). We further detected the EMT transcription factors Snail and Slug, and found that the expression levels of these two molecules were significantly decreased when silencing ROR1 (Figure 5). Our data demonstrated that ROR1 promoted the EMT process in lung adenocarcinoma cells.
Figure 4.
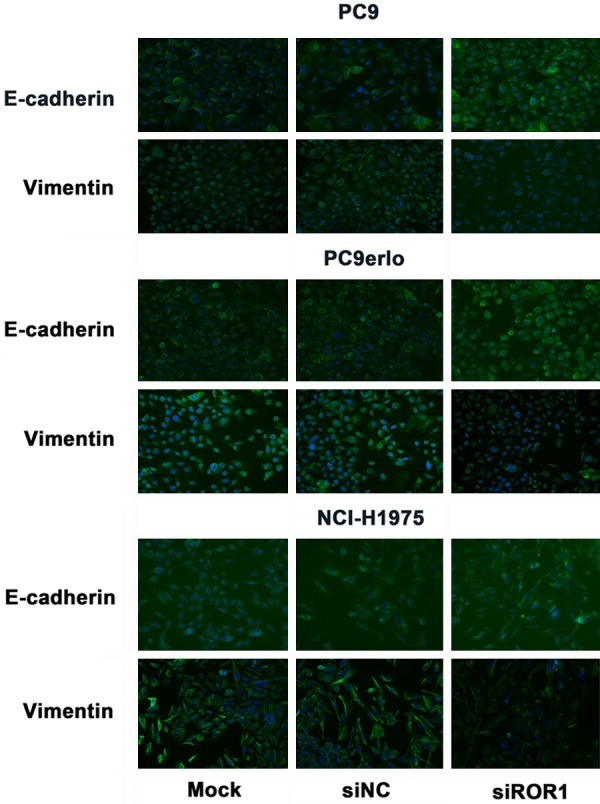
ROR1 knockdown inhibited the expression of vimentin and increased the expression of E-cadherin. PC9, PC9erlo, NCI-H358 and NCI-H1975 cells were treated with ROR1 siRNA (siROR1) or control siRNA (siNC) for 48 h and examined the expression of vimentin and E-cadherin by immunofluorescent assay using an inverted fluorescence microscope (magnification, 200×).
Figure 5.
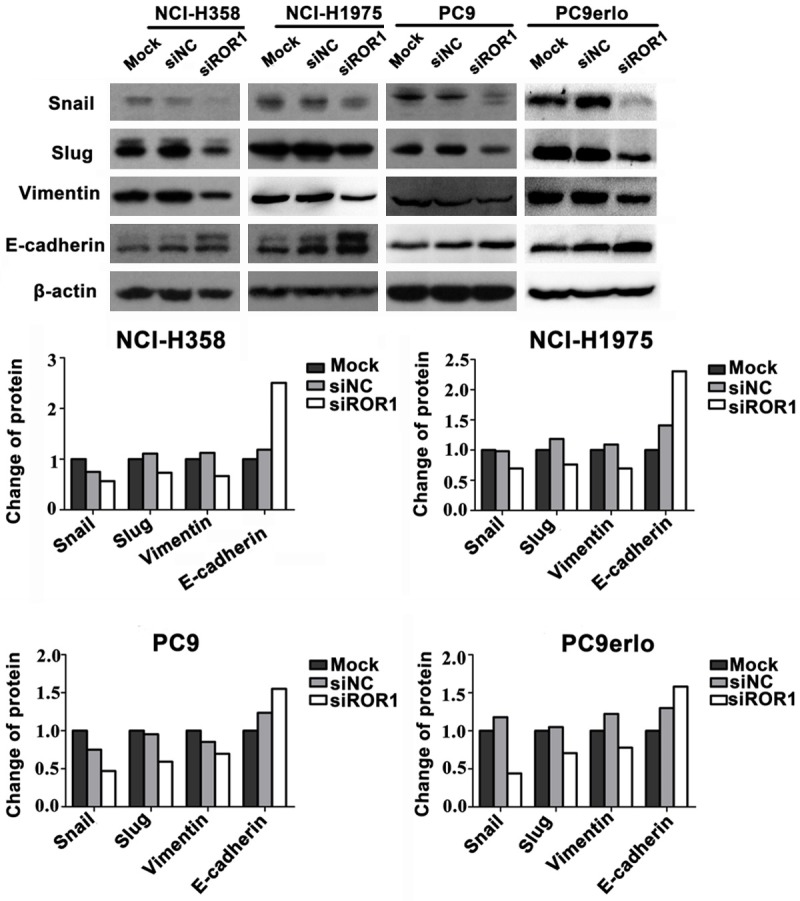
Effect of ROR1 knockdown on the expression of proteins involved in EMT process. PC9, PC9erlo, NCI-H358 and NCI-H1975 cells were treated with ROR1 siRNA (siROR1) or control siRNA (siNC) for 48 h and examined for Snail, Slug, vimentin and E-cadherin expression by immunoblot analysis.
ROR1 silencing inhibits PI3K/AKT/mTOR signaling pathway
Recent studies demonstrated that aberrations of the PI3K/Akt/mTOR pathway are very common in many human cancers including NSCLC, and mediate tumor cell migration, invasion, survival, and anti-apoptosis pathways. To investigate whether silencing ROR1 inhibited the PI3K/Akt/mTOR signaling pathway, we used western blotting to assess the expression of various proteins involved in this signaling cascade in the erlotinib-sensitive PC9, partial erlotinib-resistant NCI-H358, acquired erlotinib-resistant PC9erlo, and erlotinib-resistant NCI-H1975 cell lines. As shown in Figure 6, silencing ROR1 via siRNA significantly inhibited the phosphorylation of Akt (Ser473), mTOR (Ser2448), Raptor (Ser792) and p70S6K (Thr389) in all four cell lines, while the total expression of these proteins was not altered. Inhibiting the phosphorylation-dependent activation of these proteins likely plays a significant role in the observed silencing ROR1-induced migration and invasion in lung adenocarcinoma cells.
Figure 6.
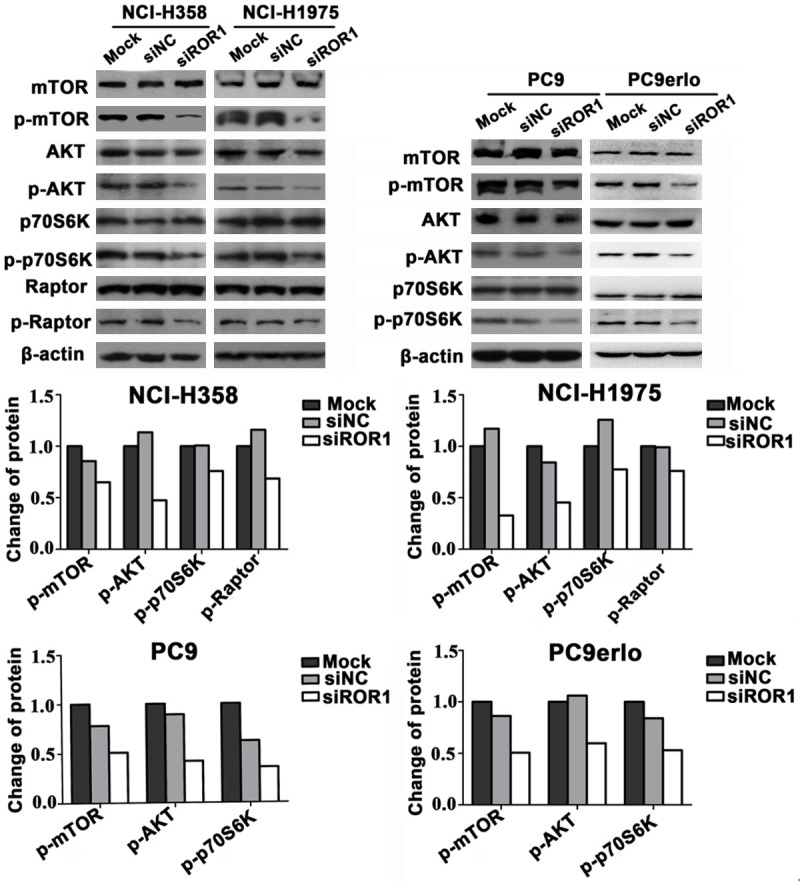
ROR1-mediated tumor cell invasion and migration via PI3K/AKT/mTOR signaling pathway. PC9, PC9erlo, NCI-H358 and NCI-H1975 cells were treated with ROR1 siRNA (siROR1) or control siRNA (siNC) for 72 h and examined for mTOR, p-mTOR, AKT, p-AKT, Raptor, p-Raptor, p70S6K and p-p70S6K by immunoblot analysis.
Discussion
Metastasis of epithelial cancer cells to distant sites is a particularly critical stage of cancer progression that typically marks the incurability of the disease. It is governed by a complex series of events including invasion and intravasation of tumor cells into the stroma and blood, respectively. EMT, a phenotypic change marked by the loss of epithelial characteristics and the acquisition of invasive mesenchymal properties, is implicated in the dissemination of tumor cells [41].
Kipps lab first reported the role of ROR1 in breast cancer metastasis in 2013 [34], then several additional studies also demonstrated that targeting ROR1 inhibits invasion and adhesion in ovarian, breast cancer, and chronic lymphocytic leukemia metastasis [35-39], but the roles and underlying mechanisms of ROR1-mediated metastasis in lung adenocarcinoma, especially those with EGFR-TKI resistance is unclear. For this reason, in the present study we choose adenocarcinoma cell lines, PC9 (erlotinib-sensitive), NCI-H358 (partial erlotinib-resistant), and NCI-H1975 (erlotinib-resistant) as cell models. In order to simulate the clinical situation, we also established an acquired erlotinib-resistant cell line PC9erlo from its parental cell line PC9 under gradient erlotinib exposure pressure. We found that silencing ROR1 with siRNA significantly reduced the migration and invasion in both erlotinib-sensitive and erlotinib-resistant cell lines. Blocking ROR1 upregulated the expression level of E-Cadherin which represents epithelial characteristics while down-regulated the expression level of vimentin which represents mesenchymal properties in all four cell lines. We also found that two master regulators of EMT, Snail and Slug, were significantly reduced after blocking ROR1 expression. Our data indicated that ROR1 signaling may promote EMT in lung adenocarcinoma cells.
Until now the potentially signaling pathway that might mediate ROR1-induced metastasis is still unknown. In a previous study, we found that ROR1 enhanced tumor cell proliferation and anti-apoptosis properties via over-activation of the PI3K/AKT/mTOR signaling pathway in two lung adenocarcinoma cell lines. Many studies have shown that over-activation of mTOR signaling significantly contributes to the initiation and development of tumors and mTOR activity was found to be deregulated in many types of cancer including non-small cell lung cancer [42-46], so here we extended our study and detected the level of critical molecules, such as Akt (Ser473), mTOR (Ser2448), Raptor (Ser792) and p70S6K (Thr389) involved in mTOR, especially mTOR Complex 1 (mTORC1) signaling in four clinically relevant cell line models. Our data reinforced our previous findings and further indicate that the components in mTORC1 most likely play a significant role in ROR1-induced migration and invasion in lung adenocarcinoma cells. Further work needs to be done to explore the key players in the mTOR signaling pathway. This could pave the way for ROR1-targeted therapy in lung adenocarcinoma patients, especially those with EGFR-TKI acquired resistance either as monotherapy or in combinations including chemotherapy and/or drugs that target mTOR signaling transduction pathways.
Recent evidence indicates that EMT of tumor cells not only causes increased metastasis but also contributes to drug resistance [47-51]. In lung cancer, treatments with EGFR tyrosine kinase inhibitors (EGFR-TKIs) bring significant benefits for patients harboring EGFR mutations, but the first-generation EGFR-TKIs such as gefitinib and erlotinib have only achieved limited clinical benefits because of acquired resistance to such drugs [52-54]. It will be of interest to know whether ROR1 could overcome EGFR-TKI resistance via the EMT process in lung cancer. In preliminary experiments we have shown that blocking ROR1 partially overcomes erlotinib-resistance in several erlotinib-resistant lung adenocarcinoma cell lines (unpublished data). Further work is needed to provide more direct evidence to interpret the direct or indirect interactions between EMT and EGFR-TKI-resistance in lung adenocarcinoma.
In conclusion, our study demonstrated that ROR1 acted as a key player in mediating tumor cell migration and invasion through EMT in lung adenocarcinoma cell lines. Moreover, we provided in vitro evidence that mTOR, especially the mTORC1, signaling pathway plays a central role in the ROR1-mediated MET process. These results indicate a potential clinical use of ROR1 as a target in the therapy of lung adenocarcinoma, regardless of its EGFR-TKI sensitivity status.
Acknowledgements
We thank Drs. Christoph Rader and Rose Mage for critical reading of this manuscript. We also thank Professor Yong Duan and Dr. Jun Zhang for providing NSCLC cell lines. This work was supported by the grant from the National Natural Science Foundation of China (No. 81401904, www.nsfc.gov.cn) and Science & Technology Department of Sichuan Province, P. R. China (No. 2018HH0005, http://www.scst.gov.cn/). Jia-Hui Yang received the two fundings. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 3.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thiery JP, Chouaib S. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11:824–846. doi: 10.1002/1878-0261.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaianigo N, Melisi D, Carbone C. EMT and treatment resistance in pancreatic cancer. Cancers (Basel) 2017;9 doi: 10.3390/cancers9090122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wade CA, Kyprianou N. Profiling prostate cancer therapeutic resistance. Int J Mol Sci. 2018;19:904. doi: 10.3390/ijms19030904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song W, Mazzieri R, Yang T, Gobe GC. Translational significance for tumor metastasis of tumor-associated macrophages and epithelial-mesenchymal transition. Front Immunol. 2017;8:1106. doi: 10.3389/fimmu.2017.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cyprian FS, Al-Farsi HF, Vranic S, Akhtar S, Al Moustafa AE. Epstein-barr virus and human papillomaviruses interactions and their roles in the initiation of epithelial-mesenchymal transition and cancer progression. Front Oncol. 2018;8:111. doi: 10.3389/fonc.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo Y, Yu T, Zhang Q, Fu Q, Hu Y, Xiang M, Peng H, Zheng T, Lu L, Shi H. Upregulated n-cadherin expression is associated with poor prognosis in epithelial-derived solid tumours: a meta-analysis. Eur J Clin Invest. 2018:48. doi: 10.1111/eci.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda S, Nakagawa H. Roles of e-cadherin in hepatocarcinogenesis. In: Nakao K, Minato N, Uemoto S, et al., editors. Innovative Medicine: Basic Research and Development. Tokyo: 2015. pp. 71–77. [Google Scholar]
- 11.Wang Y, Sun Y, Wu Y, Zhang J. Cucurbitacin e inhibits osteosarcoma cells proliferation and invasion through attenuation of PI3K/AKT/mTOR signaling. Biosci Rep. 2016;36:e00405. doi: 10.1042/BSR20160165. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Xiao W, Tang H, Wu M, Liao Y, Li K, Li L, Xu X. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci Rep. 2017;37 doi: 10.1042/BSR20170658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan W, Wu Q, Yao W, Li Y, Liu Y, Yuan J, Han R, Yang J, Ji X, Ni C. MiR-503 modulates epithelial-mesenchymal transition in silica-induced pulmonary fibrosis by targeting PI3K p85 and is sponged by lncRNA MALAT1. Sci Rep. 2017;7:11313. doi: 10.1038/s41598-017-11904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao M, Shang YY, Zhou ZW, Yang YX, Wu YS, Guan LF, Wang XY, Zhou SF, Wei X. The research on lapatinib in autophagy, cell cycle arrest and epithelial to mesenchymal transition via Wnt/ErK/PI3K-AKT signaling pathway in human cutaneous squamous cell carcinoma. J Cancer. 2017;8:220–226. doi: 10.7150/jca.16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D, Wang S, Chen J, Liu H, Lu J, Jiang H, Huang A, Chen Y. Fibulin-4 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition via the PI3K/Akt/mTOR pathway. Int J Oncol. 2017;50:1513–1530. doi: 10.3892/ijo.2017.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Yan Y, Cheng Z, Hu Y, Liu T. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-alpha/NF-kappaB and PI3K/AKT signaling pathway. Cell Death Discov. 2018;4:26. doi: 10.1038/s41420-018-0026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Gao MH, Lin ZH, Chen LY, Jin Y, Zhu G, Wang YW, Jin TF. DEK promoted EMT and angiogenesis through regulating PI3K/AKT/mTOR pathway in triple-negative breast cancer. Oncotarget. 2017;58:98702–98722. doi: 10.18632/oncotarget.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aamir A, Bernhard B, Li Y, Kong D, Bao B, Schobert R, Padhye SB, Sarkar FH. Targeted regulation of PI3K/Akt/mTOR/NF-κB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy. Anticancer Agents Med Chem. 2013;13:1002–1013. doi: 10.2174/18715206113139990078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siriwardena S, Tsunematsu T, Qi G, Ishimaru N, Kudo Y. Invasion-related factors as potential diagnostic and therapeutic targets in oral squamous cell carcinoma-a review. Int J Mol Sci. 2018;19:1462. doi: 10.3390/ijms19051462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baulida J. Epithelial-to-mesenchymal transition transcription factors in cancer-associated fibroblasts. Mol Oncol. 2017;11:847–859. doi: 10.1002/1878-0261.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng J, Wang L, Chen H, Hao J, Ni J, Chang L, Duan W, Graham P, Li Y. Targeting epithelial-mesenchymal transition and cancer stem cells for chemoresistant ovarian cancer. Oncotarget. 2016;37:55771–55788. doi: 10.18632/oncotarget.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Zhang HY, Du ZX, Li C, An MX, Zong ZH, Liu BQ, Wang HQ. Induction of epithelial-mesenchymal transition (EMT) by beclin 1 knockdown via posttranscriptional upregulation of ZEB1 in thyroid cancer cells. Oncotarget. 2016;43:70364–70377. doi: 10.18632/oncotarget.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ning X, Zhang K, Wu Q, Liu M, Sun S. Emerging role of twist1 in fibrotic diseases. J Cell Mol Med. 2018;22:1383–1391. doi: 10.1111/jcmm.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green J, Nusse R, van Amerongen R. The role of Ryk and Ror receptor tyrosine kinases in wnt signal transduction. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoda A, Oishi I, Minami Y. Expression and function of the Ror-family receptor tyrosine kinases during development: lessons from genetic analyses of nematodes, mice, and humans. J Recept Signal Transduct Res. 2003;23:1–25. doi: 10.1081/rrs-120018757. [DOI] [PubMed] [Google Scholar]
- 26.Zheng YZ, Ma R, Zhou JK, Guo CL, Wang YS, Li ZG, Liu LX, Peng Y. ROR1 is a novel prognostic biomarker in patients with lung adenocarcinoma. Sci Rep. 2016;6:36447. doi: 10.1038/srep36447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shabani M, Asgarian-Omran H, Jeddi-Tehrani M, Vossough P, Faranoush M, Sharifian RA, Toughe GR, Kordmahin M, Khoshnoodi J, Roohi A, Tavoosi N, Mellstedt H, Rabbani H, Shokri F. Overexpression of orphan receptor tyrosine kinase Ror1 as a putative tumor-associated antigen in Iranian patients with acute lymphoblastic leukemia. Tumour Biol. 2007;28:318–326. doi: 10.1159/000121405. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Chen L, Wang-Rodriguez J, Zhang L, Cui B, Frankel W, Wu R, Kipps TJ. The oncoembryonic antigen ROR1 is expressed by a variety of human cancers. Am J Pathol. 2012;181:1903–1910. doi: 10.1016/j.ajpath.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Chen L, Cui B, Chuang HY, Yu J, Wang-Rodriguez J, Tang L, Chen G, Basak GW, Kipps TJ. ROR1 is expressed in human breast cancer and associated with enhanced tumorcell growth. PLoS One. 2012;7:e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Qiu J, Ye C, Yang D, Gao L, Su Y, Tang X, Xu N, Zhang D, Xiong L, Mao Y, Li F, Zhu J. ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Sci Rep. 2014;4:5811. doi: 10.1038/srep05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaguchi T, Yanagisawa K, Sugiyama R, Hosono Y, Shimada Y, Arima C, Kato S, Tomida S, Suzuki M, Osada H, Takahashi T. NKX2-1/TITF1/TTF-1-Induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer cell. 2012;21:348–361. doi: 10.1016/j.ccr.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Hojjat-Farsangi M, Moshfegh A, Daneshmanesh AH, Khan AS, Mikaelsson E, Osterborg A, Mellstedt H. The receptor tyrosine kinase ROR1-an oncofetal antigen for targeted cancer therapy. Semin Cancer Biol. 2014;29:21–31. doi: 10.1016/j.semcancer.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Yang H, Chen T, Luo Y, Xu Z, Li Y, Yang J. Silencing of receptor tyrosine kinase ROR1 Inhibits tumor-cell proliferation via PI3K/AKT/mTOR signaling pathway in lung adenocarcinoma. PLoS One. 2015;10:e0127092. doi: 10.1371/journal.pone.0127092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui B, Zhang S, Chen L, Yu J, Widhopf GF 2nd, Fecteau JF, Rassenti LZ, Kipps TJ. Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis. Cancer Res. 2013;73:3649–3660. doi: 10.1158/0008-5472.CAN-12-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan H, He Q, Gong G, Wang Y, Li J, Wang J, Zhu D, Wu X. miR-382 inhibits migration and invasion by targeting ROR1 through regulating EMT in ovarian cancer. Int J Oncol. 2016;48:181–190. doi: 10.3892/ijo.2015.3241. [DOI] [PubMed] [Google Scholar]
- 36.Henry CE, Llamosas E, Djordjevic A, Hacker NF, Ford CE. Migration and invasion is inhibited by silencing ROR1 and ROR2 in chemoresistant ovarian cancer. Oncogenesis. 2016;5:e226. doi: 10.1038/oncsis.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry C, Hacker N, Ford C. Silencing ROR1 and ROR2 inhibits invasion and adhesion in an organotypic model of ovarian cancer metastasis. Oncotarget. 2017;68:112727–112738. doi: 10.18632/oncotarget.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janovska P, Poppova L, Plevova K, Plesingerova H, Behal M, Kaucka M, Ovesna P, Hlozkova M, Borsky M, Stehlikova O, Brychtova Y, Doubek M, Machalova M, Baskar S, Kozubik A, Pospisilova S, Pavlova S, Bryja V. Autocrine signaling by wnt-5a deregulates chemotaxis of leukemic cells and predicts clinical outcome in chronic lymphocytic leukemia. Clin Cancer Res. 2016;22:459–469. doi: 10.1158/1078-0432.CCR-15-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasan MK, Yu J, Chen L, Cui B, Widhopf GF, Rassenti L, Shen Z, Briggs SP, Kipps TJ. Wnt5a induces ROR1 to complex with HS1 to enhance migration of chronic lymphocytic leukemia cells. Leukemia. 2017;31:2615–2622. doi: 10.1038/leu.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, Baskar S, Kwong KY, Kennedy MG, Wiestner A, Rader C. Therapeutic potential and challenges of targeting receptor tyrosine kinase ROR1 with monoclonal antibodies in Bcell malignancies. PLoS One. 2011;6:e21018. doi: 10.1371/journal.pone.0021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasquier J, Abu-Kaoud N, Al Thani H, Rafii A. Epithelial to mesenchymal transition in a clinical perspective. J Oncol. 2015;2015:792182. doi: 10.1155/2015/792182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 43.Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7:209–219. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- 44.Liu AL, Liao HQ, Li Zh L, Liu J, Zhou CL, Guo ZF, Xie HY, Peng CY. New insights into mTOR signal pathways in ovarian-related diseases: polycystic ovary syndrome and ovarian cancer. Asian Pac J Cancer Prev. 2016;17:5087–5094. doi: 10.22034/APJCP.2016.17.12.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Z, Wang J, Liu M, Chen D, Qiu C, Sun K. CC-223 blocks mTORC1/C2 activation and inhibits human hepatocellular carcinoma cells in vitro and in vivo. PLoS One. 2017;12:e0173252. doi: 10.1371/journal.pone.0173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bajwa P, Nielsen S, Lombard JM, Rassam L, Nahar P, Rueda BR, Wilkinson JE, Miller RA, Tanwar PS. Overactive mTOR signaling leads to endometrial hyperplasia in aged women and mic. Oncotarget. 2017;31:7265–7275. doi: 10.18632/oncotarget.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding L, Wang C, Cui Y, Han X, Zhou Y, Bai J, Li R. S-phase kinase-associated protein 2 is involved in epithelial-mesenchymal transition in methotrexate-resistant osteosarcoma cells. Int J Oncol. 2018;52:1841–1852. doi: 10.3892/ijo.2018.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang KA, Ryu YS, Piao MJ, Shilnikova K, Kang HK, Yi JM, Boulanger M, Paolillo R, Bossis G, Yoon SY, Kim SB, Hyun JW. DUOX2-mediated production of reactive oxygen species induces epithelial mesenchymal transition in 5-fluorouracil resistant human colon cancer cells. Redox Biol. 2018;17:224–235. doi: 10.1016/j.redox.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qi XK, Han HQ, Zhang HJ, Xu M, Li L, Chen L, Xiang T, Feng QS, Kang T, Qian CN, Cai MY, Tao Q, Zeng YX, Feng L. OVOL2 links stemness and metastasis via fine-tuning epithelial-mesenchymal transition in nasopharyngeal carcinoma. Theranostics. 2018;8:2202–2216. doi: 10.7150/thno.24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei M, Mao S, Lu G, Li L, Lan X, Huang Z, Chen Y, Zhao M, Zhao Y, Xia Q. Valproic acid sensitizes metformin-resistant human renal cell carcinoma cells by upregulating H3 acetylation and EMT reversal. BMC Cancer. 2018;18:434. doi: 10.1186/s12885-018-4344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Lu Y, Zhang C, Huang D, Wu W, Zhang Y, Shen J, Cai Y, Chen W, Yao W. FSCN1 increases doxorubicin resistance in hepatocellular carcinoma through promotion of epithelial-mesenchymal transition. Int J Oncol. 2018;52:1455–1464. doi: 10.3892/ijo.2018.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu S, Cao H, Chen D, Yu S, Sha H, Wu J, Ma R, Wang Z, Jing C, Zhang J, Feng J. LXR ligands induce apoptosis of EGFR-TKI-resistant human lung cancer cells in vitro by inhibiting Akt-NF-kappaB activation. Oncol Lett. 2018;15:7168–7174. doi: 10.3892/ol.2018.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishikawa S, Kimura H, Koba H, Yoneda T, Watanabe S, Sakai T, Hara J, Sone T, Kasahara K, Nakao S. Selective gene amplification to detect the T790M mutation in plasma from patients with advanced non-small cell lung cancer (NSCLC) who have developed epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) resistance. J Thorac Dis. 2018;10:1431–1439. doi: 10.21037/jtd.2018.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang M, Chang A. Molecular mechanism of action and potential biomarkers of growth inhibition of synergistic combination of afatinib and dasatinib against gefitinib-resistant nonsmall cell lung cancer cells. Oncotarget. 2018;23:16533–16546. doi: 10.18632/oncotarget.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]


