Abstract
Background and purpose: CD133, which is considered a useful biomarker for the prediction of metastasis and prognosis for various cancers, is a biomarker of cancer stem cells (CSCs). Metastasis-associated in colon cancer 1 (MACC1) has been considered an oncogene that promotes invasion and metastasis in many solid tumors. KAI1 is a suppressor gene of metastasis and is also considered a valuable biomarker for the prediction of tumor invasion and metastasis. The purpose of this study is to investigate the expression of CD133, MACC1, and KAI1 in sebaceous gland carcinoma of the eyelid (SGCE) and to analyze their respective associations with clinicopathologic characteristics and survival in SGCE. Methods: Positive expression of CD133, MACC1, and KAI1 in 77 whole SGCE tissues and the corresponding normal tissues were detected by immunohistochemistry. Patient demographics, clinical data, and follow-up data were collected. Results: The positive expression of CD133 and MACC1 was significantly higher and KAI1 significantly lower in SGCE tissues compared to the control tissues. The Positive expression of CD133 and MACC1 was positively associated with local invasion, lymph node metastasis (LNM), and TNM stages. KAI1 expression was inversely associated with tumor grade, local invasion, LNM, and TNM stages. A Kaplan-Meier survival analysis demonstrated that CD133+ or MACC1+ patients had a significantly lower overall survival (OS) time when compared with CD133- or MACC1- patients. And KAI1+ patients had a significantly longer OS time compared with KAI1- patients. Multivariate analysis showed that positive expression of CD133, MACC1, and KAI1, as well as the TNM stages were independent prognostic factors in SGCE patients. Conclusion: The expression of CD133, MACC1, and KAI1 should be considered promising biomarkers for invasion, metastasis, and prognosis, as well as potential therapeutic targets for SGCE.
Keywords: Sebaceous gland carcinoma, CD133, MACC1, KAI1, metastasis, prognosis, eyelid
Introduction
Sebaceous gland carcinoma of the eyelid (SGCE), which is as common or even more common than eyelid basal cell carcinoma in China [1], is a highly malignant tumor. SGCE is capable of aggressive local invasion and metastasis to the regional lymph nodes [2]. Relapse and metastasis are the main reasons for initiating anti-cancer therapy.
The cancer may be associated with a subpopulation of tumor cells called cancer stem cells (CSCs) or tumor initiating cells (TICs). CSCs are characterized by their ability to self-renew and differentiate, and they are responsible for a natural resistance to cancer therapy (such as chemotherapy and radiotherapy) [3-6]. CSCs have been certified to play a pivotal role in SGCE progression and prognosis [7,8]. CD133, which is the most common biomarker for CSCs, is a 120 kDa, five pentaspan membrane glycoprotein. CD133 is identified as a biomarker of CSCs in various cancers including NSCLC, ovarian cancer, cervical cancer, liver cancer, cholangiocarcinoma, and sebaceous gland cancer [3,4,6,7,9,10]. Accumulating evidence has demonstrated that CD133 should be considered a valuable biomarker for the prediction of invasion, metastasis, and prognosis of many cancers.
Metastasis-associated in colon cancer 1 (MACC1) was originally found in the colon cancer cell line in 2009 [11]. MACC1 is a key transcriptional factor of the mesenchymal-epithelial transition (MET) gene and is bound to the promoter of the MET gene to regulate the hepatocyte growth factor/MET signaling pathway [11,12]. MACC1 has been reported to be involved in various fundamental biological behaviors, such as proliferation, migration, invasion, metastasis, and chemotherapy resistance [13-15]. Accumulating evidence has demonstrated that MACC1 plays an important role in promoting tumor cells metastases.
KAI1, which is also called CD82, is an important member of the tetraspanin family of proteins located in human chromosome 11p11.2. KAI1 was first defined as a suppressor of metastasis in prostate cancer cells. This gene encodes 10 exons and 9 introns. It has been demonstrated that the KAI1 gene should be involved in a series of biological behaviors including mobility, migration, adhesion, fusion, and invasion [16-18]. Previous studies have also demonstrated that the aberrant expression of KAI1 is closely correlated with the progression of various human cancers. Accumulating studies have indicated that KAI1 should also be considered a useful biomarker for the prediction of invasion, metastasis, and prognosis in many cancers, including colorectal, laryngeal, nasopharyngeal, and prostate cancer, and gastric carcinoma [18-22].
The purpose of the current study is to evaluate the expression of CD133, MACC1, and KAI1 in the SGCE tissues of patients and their associations between clinicopathologic characteristics and the prognosis of patients with SGCE. Immunohistochemistry was used to detect the expressions of CD133, MACC1, and KAI1 in SGCE tissues and the corresponding adjacent normal tissues of patients with SGCE.
Methods
Patients and tissue specimens
We recruited 77 patients (median age: 68.4 years, range: 56-77 years) with SGCE diagnosed at the Department of Pathology at our hospital from January 2011 to December 2012. Patients who had had any anti-cancer therapy were excluded. The study was performed in accordance with the Declaration of Helsinki guidelines and authorized by the Bengbu Medical College ethics committee. Patient data (such as demographics, clinicopathologic characteristics, and follow-up) were also collected. Overall survival (OS) time was calculated from the surgery date to the death date or to December 2017 (mean OS: 45.6 months; range: 11-71 months). TNM stages were assessed according to the 8th edition of the guidelines issued by the American Joint Committee on Cancer (AJCC). Tumor grades were assessed according to the standards issued by the World Health Organization (WHO). The patient data are shown in Table 1.
Table 1.
Patients characteristics
| Patients characteristics | Frequency (n) | Percentage (%) |
|---|---|---|
| Gender | ||
| Male | 22 | 28.6 |
| Female | 55 | 71.4 |
| Ages | ||
| < 60 | 11 | 14.3 |
| ≥ 60 | 66 | 85.7 |
| Size | ||
| < 2.0 cm | 63 | 81.8 |
| ≥ 2.0 cm | 14 | 18.2 |
| Location | ||
| Up | 45 | 58.4 |
| Down | 32 | 41.6 |
| Grade | ||
| Low | 50 | 64.9 |
| High | 27 | 35.1 |
| Invasion | ||
| No | 60 | 77.9 |
| Yes | 17 | 22.1 |
| Lymph node metastasis | ||
| No | 62 | 80.5 |
| Yes | 15 | 19.5 |
| TNM stage | ||
| I and II | 57 | 74.0 |
| III and IV | 20 | 26.0 |
Immunohistochemistry
The SGCE and control tissues were fixed in 10% buffered formation solution and embedded in paraffin. Then they were cut into 4-μm-thick slices. Immunohistochemical staining was performed using the ElivisionTM Plus method. All the slices were deparaffinized using xylene and dehydrated using alcohol. Then a routine H2O2 solution was used for quenching the endogenous peroxidase activity. The antigen was repaired using a citrate buffer. Then all the tissues were blocked with goat serum. CD133 (mouse monoclonal antibody, Abcam, US), MACC1 (rabbit polyclonal antibody, Santa Cruz Biotechnology, US), and KAI1 (mouse monoclonal antibody, Abcam, US) primary antibodies were added, then all slices were incubated overnight at 4°C. The enhancers (reagent A and reagent B) were added and then all the slices were developed in a diaminobenzidine (DAB) solution. Lastly, all the slices were re-dyed with hematoxylin, dehydrated, and mounted with gum.
Evaluation of the immunohistochemical staining
We randomly selected ten high-power-field (HPF) fields of each SGCE slice to avoid the intra-tumoral heterogeneity of any marker expression. According to the percentage of positive cells and the positive staining intensity, the staining results were multiplied using percentage and intensity scores (The percentage scores were recorded as follows: > 11% as 1, 11%-50% as 2, 51%-75% as 3, and > 75% as 4. The intensity scores were recorded as follows: no staining as 0, weak staining as 1, moderate staining as 2, and strong staining as 3) which ranged from 0-12. The expression of CD133 and KAI1 was considered positive when the final scores were > 2.
Statistical analysis
SPSS 19.0 software was used for analyzing all the data. For the countable data, we used a Chi-square test for the comparisons between the two groups. Univariate OS time analysis was conducted using the Kaplan-Meier method with a log-rank test. A multivariate OS time analysis was conducted using the COX regression model test. P < 0.05 was defined as indicative of statistically significant differences.
Results
Associations between the expressions of CD133, MACC1, and KAI1 in the SGCE tissues of patients and their clinicopathologic characteristics
As shown in Figure 1A and 1B, the CD133-positive expression was mainly located in the cytoplasms and membranes. The positive rate of CD133 expression in the SGCE samples (41.6%, 32/77) was significantly higher than it was in the control samples (9.1%, 7/77; P < 0.001). The positive rate of CD133 expression in SGCE was positively associated with gender, tumor size, tumor site, invasion, LNM, and TNM stages (Table 2).
Figure 1.
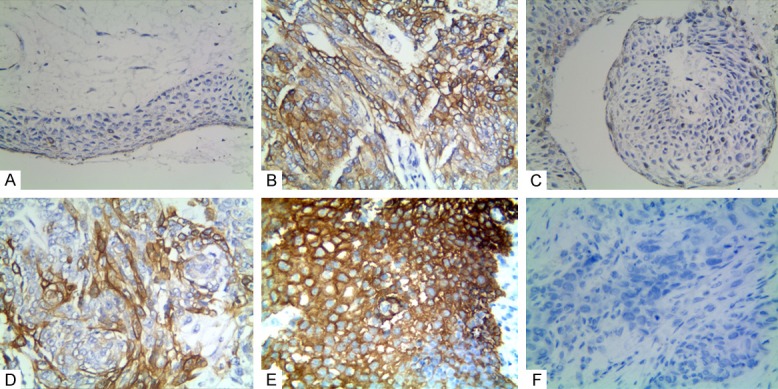
Immunostaining for CD133, MACC1, and KAI1 in sebaceous gland carcinoma of the eyelid and the control tissue. A. Negative CD133 in the control tissue (100 × magnification); B. Positive CD133 in the cytoplasm and membrane of SGCE tissue (400 × magnification); C. Negative MACC1 in the control tissues (100 × magnification); D. Positive MACC1 in the cytoplasms of SGCE tissue (400 × magnification); E. Positive KAI1 in the cytoplasms and membranes of the control tissue (400 × magnification); F. Negative KAI1 in the SGCE tissue (400 × magnification).
Table 2.
The associations between expression of CD133, or MACC1, or KAI1 and clinicopathological characteristics of sebaceous gland carcinoma of eyelid (SGCE)
| Variables | CD133 | P | MACC1 | P | KAI1 | P | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| - | + | - | + | - | + | ||||
| Gender | 0.007 | 0.999 | 0.428 | ||||||
| Male | 7 | 15 | 8 | 14 | 13 | 9 | |||
| Female | 33 | 22 | 20 | 35 | 27 | 28 | |||
| Ages | 0.196 | 0.014 | 0.642 | ||||||
| < 60 | 8 | 3 | 8 | 3 | 5 | 6 | |||
| ≥ 60 | 32 | 34 | 20 | 46 | 35 | 31 | |||
| Size | 0.017 | 0.013 | 0.038 | ||||||
| < 2.0 cm | 37 | 26 | 27 | 36 | 29 | 34 | |||
| ≥ 2.0 cm | 3 | 11 | 1 | 13 | 11 | 3 | |||
| Location | 0.013 | 0.861 | 0.452 | ||||||
| Up | 18 | 27 | 16 | 29 | 25 | 20 | |||
| Down | 22 | 10 | 12 | 20 | 15 | 17 | |||
| Grade | 0.148 | 0.367 | 0.004 | ||||||
| Low | 29 | 21 | 20 | 30 | 20 | 30 | |||
| High | 11 | 16 | 8 | 19 | 20 | 7 | |||
| Invasion | 0.002 | 0.003 | 0.022 | ||||||
| No | 37 | 24 | 27 | 33 | 27 | 33 | |||
| Yes | 3 | 13 | 1 | 16 | 13 | 4 | |||
| LNM | 0.043 | 0.007 | 0.021 | ||||||
| No | 36 | 26 | 27 | 35 | 28 | 34 | |||
| Yes | 4 | 11 | 1 | 14 | 12 | 3 | |||
| TNM stage | 0.001 | 0.030 | 0.004 | ||||||
| I and II | 36 | 21 | 25 | 32 | 24 | 33 | |||
| III and IV | 4 | 16 | 3 | 17 | 16 | 4 | |||
As shown in Figure 1C and 1D, the MACC1-positive expression was mainly located in the cytoplasm. The positive rate of MACC1 expression in the SGCE tissues (63.6%, 49/77) was significantly higher than it was in the control samples (7.8%, 6/77). The positive rate of MACC1 expression in SGCE was positively associated with the patients’ age, tumor size, invasion, LNM, and TNM stages (Table 2).
As shown in Figure 1E and 1F, the KAI1-positive expression was mainly located in the membranes and cytoplasms. Unlike CD133 and MACC1, the positive rate of KAI1 expression in the SGCE tissues (48.1%, 37/77) was significantly lower than it was in the control tissues (92.2%, 71/77; P < 0.001). Furthermore, the positive rate of KAI1 expression was negatively associated with tumor size, tumor grades, invasions, LNM, and TNM stages (Table 2).
Associations among CD133, MACC1, and KAI1 in SGCE
There was a negative association between KAI1 expression and CD133, or MACC1 expression (r = -0.249, P = 0.029; r = -0.246, P = 0.031, respectively). There was a positive association between CD133 expression and MACC1 expression (r = 0.241, P = 0.035) (Table 3).
Table 3.
Correlation among expression of CD133, MACC1, and KAI1 in SGCE
| Variable | CD133 | r | P | KAI1 | r | P | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| - | + | - | + | |||||
| CD133 | -0.301 | 0.008* | ||||||
| - | 15 | 25 | ||||||
| + | 25 | 12 | ||||||
| MACC1 | 0.295 | 0.009@ | -0.246 | 0.031* | ||||
| - | 20 | 8 | 10 | 18 | ||||
| + | 20 | 29 | 30 | 19 | ||||
negative association;
positive association.
Survival analysis
As shown in Figure 2A, a Kaplan-Meier survival analysis showed that the overall survival (OS) time of the CD133+ patients (38.1 ± 13.1 months) with SGCE was significantly shorter than it was for the CD133- patients (52.6 ± 11.3 months; log-rank = 28.530, P < 0.001). Similar to CD133, the OS time of the MACC1+ patients (41.5 ± 13.3 months) with SGCE was significantly shorter than it was for the MACC1- patients (52.9 ± 12.6 months; log-rank = 13.828, P < 0.001; Figure 2B). As shown in Figure 2C, the OS time of the KAI1+ patients (51.5 ± 12.7 months) with SGCE was significantly longer than it was for the KAI1- patients (40.2 ± 13.3 months; log-rank = 14.387, P < 0.001).
Figure 2.
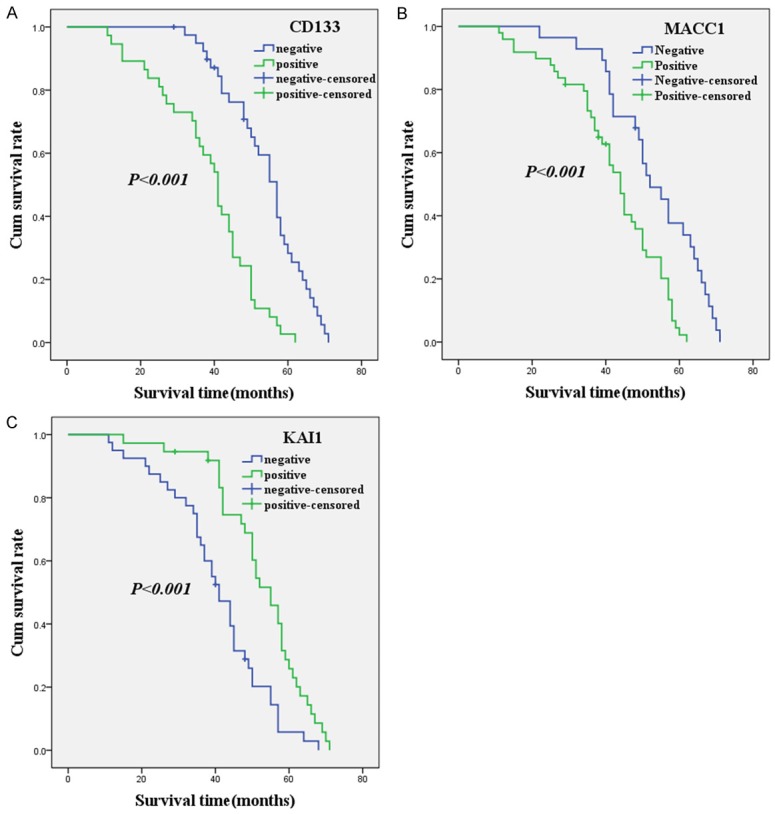
A Kaplan-Meier analysis curve of the survival rate of patients with SGCE. The y-axis means the percentage of patients; the x-axis means their survival in months. (A) OS analysis of all patients in relation to CD133 (log-rank = 28.530, P < 0.001); (B) OS analysis of all patients in relation to MACC1 expression (log-rank = 13.828, P < 0.001); (C) OS analysis of all patients in relation to KAI1 expression (log-rank = 14.387, P < 0.001). In the (A-C) analyses, the green line represents patients with positive CD133, or MACC1, or KAI1; the blue line represents the negative CD133, or MACC1, or KAI1 group.
A multivariate Cox regression analysis indicated that a positive expression of CD133, MACC1, and KAI1, as well as TNM stages, are independent prognostic factors for patients with SGCE (Table 4).
Table 4.
Results of multivariate analyses of overall survival (OS) time
| Covariate | B | SE | P | HR | 95% CI |
|---|---|---|---|---|---|
| CD133 | 1.050 | 0.342 | 0.002 | 2.859 | 1.461-5.592 |
| MACC1 | 0.819 | 0.341 | 0.016 | 2.269 | 1.163-4.424 |
| KAI1 | -0.611 | 0.285 | 0.032 | 0.543 | 0.310-0.950 |
| TNM stages | 1.794 | 0.657 | 0.006 | 6.015 | 1.660-21.796 |
Discussion
SGCE, which is able to infiltrate local tissues as well as metastasize to regional lymph nodes, is a highly malignant tumor. Because of the high heterogeneity of SGCE, it is difficult to completely assess the biomarker value and effectiveness. Previous studies have shown that CSCs can promote tumor cell proliferation, invasiveness, metastasis, and are responsible for resistance to anti-cancer therapy. In our study, we found that CD133+ expression in SGCE was positively associated with the grade of differentiation, tumor invasion, LNM, and TNM stages. A Kaplan-Meier survival analysis indicated that THE OS time of CD133+ patients with SGCE was significantly shorter when compared with CD133- patients. These results demonstrated that CD133 should be considered a valuable biomarker for the prediction of invasion and metastasis, which is consistent with previous studies [3,4,6-10,23].
It has been demonstrated that MACC1 not only promotes tumor cell proliferation, migration, invasion, and metastasis in vitro, but also in vivo [11,13,14]. The findings of this study showed that a positive expression of MACC1 in SGCE was positively associated with tumor invasion, LNM, and TNM stages. Survival analysis indicated that the OS time of MACC1+ patients was significantly shorter when compared with MACC1- patients. Several previous studies have investigated the invasive and metastatic significance of MACC1 and reached similar results [11,13,14,24]. These results demonstrated that MACC1 should be a valuable biomarker for the prediction of invasiveness and the metastasis of SGCE as well as for predicting prognosis.
The inactivation of tumor suppressor genes is an important event in tumor invasiveness and metastasis. KAI1 is widely considered a suppressor gene and also a metastatic suppressor gene in various human cancers [16-18,20]. Previous studies have demonstrated that KAI1 should inhibit tumor cell proliferation, motility, migration, and metastasis. In this study, we found that KAI1+ expression was negatively associated with the grade of differentiation, tumor invasion, LNM, and TNM stages. A survival analysis demonstrated that the OS time of KAI1+ patients was significantly longer when compared with KAI1- patients. Our results indicated that KAI1 should be considered a useful predictor for the invasiveness and metastasis of SGCE, even for prognosis [17-22].
Metastasis and relapse are the most common causes of treatment failure in SGCE. Therefore, it is urgent to find valuable and effective biomarkers to predict the invasiveness and metastasis of the disease. In our study, a multivariate regression model analysis demonstrated that a positive expression of CD133, MACC1, and KAI1, as well as TNM stages, were independent prognostic factors for patients with SGCE. Furthermore, our results also demonstrated that CD133, MACC1, and KAI1 were associated with the invasion, metastasis, and prognosis of SGCE. CSCs may induce the initiation of SGCE, and also promote the proliferation, invasion, and metastasis of SGCE cells. CSCs also induces the epithelial-mesenchymal transition (EMT) to promote tumor cell invasion and metastasis. The aberrant expression of CD133 is helpful for SGCE progression and metastasis. At the same time, the overexpression of MACC1 can promote tumor cells EMT through the HGF/MET signaling pathway [18,25]. The inactivation or loss of expression of KAI1 should further promote cancer cell invasion and metastasis [17-22].
Conclusions
This study found that a positive expression of CD133, MACC1, and KAI1 are associated with the duration of OS time among patients with SGCE. Thus, CD133, MACC1, and KAI1 should be considered valuable and effective biomarkers in SGCE and may be beneficial for the prognosis of SGCE.
Acknowledgements
This work was supported by the Nature Science Key Program of College and University of Anhui Province (no. KJ2016A488).
Disclosure of conflict of interest
None.
References
- 1.Shields JA, Demirci H, Marr BP, Eagle RC Jr, Shields CL. Sebaceous carcinoma of the ocular region: a review. Surv Ophthalmol. 2005;50:103–22. doi: 10.1016/j.survophthal.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Pe’er J. Pathology of eyelid tumors. Indian J Ophthalmol. 2016;64:177–90. doi: 10.4103/0301-4738.181752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu S, Yu L, Wang D, Zhou L, Cheng Z, Chai D, Ma L, Tao Y. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer. 2012;12:535. doi: 10.1186/1471-2407-12-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tao Y, Li H, Huang R, Mo D, Zeng T, Fang M, Li M. Clinicopathological and prognostic significance of cancer stem cell markers in ovarian cancer patients: evidence from 52 studies. Cell Physiol Biochem. 2018;46:1716–26. doi: 10.1159/000489586. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–43. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 6.Javed S, Sharma BK, Sood S, Sharma S, Bagga R, Bhattacharyya S, Rayat CS, Dhaliwal L, Srinivasan R. Significance of CD133 positive cells in four novel HPV-16 positive cervical cancer derived cell lines and biopsies of invasive cervical cancer. BMC Cancer. 2018;18:357. doi: 10.1186/s12885-018-4237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim N, Choung HK, Lee MJ, Khwarg SI, Kim JE. Cancer stem cell markers in eyelid sebaceous gland carcinoma: high expression of ALDH1, CD133, and ABCG2 correlates with poor prognosis. Invest Ophthalmol Vis Sci. 2015;56:1813–9. doi: 10.1167/iovs.14-15547. [DOI] [PubMed] [Google Scholar]
- 8.De Craene B, Denecker G, Vermassen P, Taminau J, Mauch C, Derore A, Jonkers J, Fuchs E, Berx G. Epidermal snail expression drives skin cancer initiation and progression through enhanced cytoprotection, epidermal stem/progenitor cell expansion and enhanced metastatic potential. Cell Death Differ. 2014;21:310–20. doi: 10.1038/cdd.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zerki AN, EI-Sisi ER, Youssef ASE, Kamel MM, Nassar A, Ahmed OS, EI Kassas M, Barakat AB, Abd EI-Molaleb AI, Bahnassy AA. MicroRNA Signatures for circulating CD133- positive cells in hepatocellular carcinoma with HCV infection. PLoS One. 2018;13:e0193709. doi: 10.1371/journal.pone.0193709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai X, Li J, Yuan X, Xiao J, Dooley S, Wang X, Weng H, Lu L. CD133 expression in cancer cells predicts poor prognosis of non-mucin producing intrahepatic cholangiocarcinoma. J Transl Med. 2018;16:50. doi: 10.1186/s12967-018-1423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, Birchmeier W, Schlag PM. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15:59–67. doi: 10.1038/nm.1889. [DOI] [PubMed] [Google Scholar]
- 12.Stein U, Smith J, Walther W, Arlt F. MACC1 controls Met: what a difference a Sp1 site makes. Cell Cycle. 2009;8:2467–9. doi: 10.4161/cc.8.15.9018. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Wu Y, Lin L, Liu P, Huang H, Liao W, Zheng D, Zuo Q, Sun L, Huang N, Shi M, Liao Y, Liao W. Metastasis-associated in colon cancer-1 upregulation predicts a poor prognosis of gastric cancer, and promotes tumor cell proliferation and invasion. Int J Cancer. 2013;133:1419–30. doi: 10.1002/ijc.28140. [DOI] [PubMed] [Google Scholar]
- 14.Chundong G, Uramoto H, Onitsuka T, Shimokawa H, Iwanami T, Nakagawa M, Oyama T, Tanaka F. Molecular diagnosis of MACC1 status in lung adenocarcinoma by immunohistochemical analysis. Anticancer Res. 2011;31:1141–5. [PubMed] [Google Scholar]
- 15.Wu ZZ, Chen LS, Zhou R, Bin JP, Liao YL, Liao WJ. Metastasis-associated in colon cancer-1 in gastric cancer: beyond metastasis. World J Gastroenterol. 2016;22:6629–37. doi: 10.3748/wjg.v22.i29.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miranti CK. Controlling cell surface dynamics and signaling: how CD82/KAI1 suppresses metastasis. Cell Signal. 2009;21:196–211. doi: 10.1016/j.cellsig.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Zhu B, Zhou L, Yu L, Wu S, Song W, Gong X, Wang D. Evaluation of the correlation of vasculogenic mimicry, ALDH1, KAI1 and microvessel density in the prediction of metastasis and prognosis in colorectal carcinoma. BMC Surg. 2017;17:47. doi: 10.1186/s12893-017-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu G, Zhou L, Zhang X, Zhu B, Wu S, Song W, Gong X, Wang D, Tao Y. The expression of metastasis-associated in colon cancer-1 and KAI1 in gastric adenocarcinoma and their clinical significance. World J Surg Oncol. 2016;14:276. doi: 10.1186/s12957-016-1033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae WK, Hong CS, Park MR, Sun EG, Lee JH, Kang K, Ryu KH, Shim HJ, Hwang JE, Cho SH, Chung IJ. Tap73 inhibits cell invasion and migration by directly activating KAI1 expression in colorectal carcinoma. Cancer Lett. 2018;415:106–16. doi: 10.1016/j.canlet.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Z, Tian R, Wang P. Roles of KAI1 and nm23 in lymphangiogenesis and lymph metastasis of laryngeal squamous cell carcinoma. World J Surg Oncol. 2017;15:211. doi: 10.1186/s12957-017-1279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Z, Wang Y, Yang J, Zhong J, Liu X, Xu M. KAI1 overexpression promotes apoptosis and inhibits proliferation, cell cycle, migration, and invasion in nasopharyngeal carcinoma cells. Am J Otolaryngol. 2017;38:511–7. doi: 10.1016/j.amjoto.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Lee MS, Jeoug DI, Kim YM, Lee H. Promoter CpG-site methylation of the KAI1 metastasis suppressor gene contributes to its epigenetic repression in prostate cancer. Prostate. 2017;77:350–60. doi: 10.1002/pros.23274. [DOI] [PubMed] [Google Scholar]
- 23.Lu G, Zhou L, Song W, Wu S, Zhu B, Wang D. Expression of ORAOV1, CD133 and WWOX correlate with metastasis and prognosis in gastric adenocarcinoma. Int J Clin Exp Pathol. 2017;10:8916–24. [PMC free article] [PubMed] [Google Scholar]
- 24.Yu L, Zhu B, Wu S, Zhou L, Song W, Gong X, Wang D. Evaluation of the correlation of vasculogenic mimicry, ALDH1, KiSS-1, and MACC1 in the prediction of metastasis and prognosis in ovarian carcinoma. Diag Pathol. 2017;12:23. doi: 10.1186/s13000-017-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burock S, Herrmann P, Wendler I, Niederstrasser M, Wernecke KD, Stein U. Circulating metastasis associated in colon cancer 1 transcripts in gastric cancer patient plasma as diagnostic and prognostic biomarker. World J Gastroenterol. 2015;21:333–41. doi: 10.3748/wjg.v21.i1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]


