Abstract
Colorectal cancer is the third most frequently diagnosed malignancy, and the prognosis at advanced tumor stages remains poor. FBXO2, a member of the F-box protein family, is a cytoplasmic protein and an ubiquitin ligase. The aim of this study was to investigate the role of FBXO2 in colorectal cancer. The expression levels of Ki67, N-cadherin and FBXO2 were detected in 195 pairs of primary CRC tissues using immunohistochemistry (IHC). The associations among Ki67, N-cadherin, and FBXO2 expression, as well as the clinicopathological parameters, were analyzed. Survival curves were calculated with the Kaplan-Meier method. Univariate and multivariate analyses were performed to explore the prognostic significance of Ki67, N-cadherin, and FBXO2 expression. We found that the positive rates of Ki67, N-cadherin and FBXO2 expression in CRC tissue samples were 55.9%, 65.1%, 62.6%, respectively. The high expression levels of Ki67 and N-cadherin were significantly correlated with CRC size (P = 0.01) and metastasis (P = 0.01), respectively. The high expression level of FBXO2 was significantly correlated with CRC metastasis (P = 0.04) and AJCC stage (P = 0.029). A Cox regression analysis revealed that FBXO2 is an independent prognostic factor for CRC patients (HR 1.817, 95% CI 1.106-2.983, P = 0.018). FBXO2 may serve as a biomarker for metastasis and a reliable predictor for poor prognosis in CRC patients.
Keywords: Colorectal cancer, Ki67, N-cadherin, FBXO2, immunohistochemistry
Introduction
Colorectal cancer (CRC), including colon cancer and rectal cancer, is the second most common cancer type, and the third leading cause of cancer-related deaths worldwide [1]. Approximately 15% to 25% of patients have distant metastases at the time of colorectal cancer diagnosis, and some have distant metastases within five years of partial colectomy. Most cancer patient deaths are caused by severe cachexia due to metastasis [2]. While multidisciplinary care is employed in CRC treatment, overall survival (OS) rates remain unsatisfactory. Clinical/pathological staging, also known as TNM staging, is widely used in the clinic and is highly associated with 5-year overall survival (OS). Initial patient management is defined by clinical/pathological staging at the time of diagnosis, based on the depth of tumor invasion, lymph node involvement, and distant metastasis [3]. However, the prognosis can vary for CRC patients with identical TNM staging who receive the same therapeutic schedule [3,4]. Hence, it is essential to find new biomarkers to predict the outcome of CRC by incorporating additional anatomic and nonanatomic prognostic factors, as stated by the AJCC [5]. Though substantial efforts and investments are devoted to discovering predictive biomarkers for aggressiveness of tumors and distant metastasis, there are no efficient biomarkers for the prediction of overall survival. Therefore, there is a need for the identification of clinically applicable molecular markers predictive of OS in CRC.
The ubiquitin proteasome system (UPS) is an evolutionarily conserved protein degradation mechanism that comprises ubiquitin activating (E1), ubiquitin-conjugating (E2) and ubiquitin ligase (E3) enzymes [6]. The F-box proteins constitute one of the four subunits of the ubiquitin protein ligase complex known as SCF (SKP1-cullin-F-box), which functions in phosphorylation-dependent ubiquitination. The F-box family of proteins plays pivotal and indispensable roles in cell cycle regulation by the ubiquitin proteasome system. These proteins regulate a variety of cellular processes, including cell proliferation, cell cycle, RNA transcription, and apoptosis, primarily through ubiquitination and subsequent degradation of the target protein [7,8]. F-box proteins can be categorized into the following three subclasses: FBXW, FBXL, and FBXO [9]. FBXO2, also known as FBG1 or Fbs1, is a member of the FBXO protein family that recognizes high-mannose-type asparagine-linked carbohydrate chains (N-glycans) [10]. FBXO2 is expressed in brain tissue and is tightly linked to brain diseases, such as Parkinson’s disease [11]. FBXO2 is a neuron-enriched ubiquitin ligase substrate adaptor protein, which binds glycoproteins containing high mannose N-linked glycans and facilitates their degradation through ER-associated-degradation (ERAD) [12]. Emerging experimental and clinical data indicate that F-box proteins function as tumor suppressors and oncoproteins. Abundant literature documents the fact that the expression of SKP2 and FBXW7, members of the F-box protein family, are associated with the invasion and metastasis of various cancer types [7,13,14]. There are published reports demonstrating that FBXO2 is highly expressed in gastric cancer (GC) and is tightly associated with cancer progression and poor outcome in GC patients [15]. However, the expression status of FBXO2 in CRC and its relationship to clinicopathological parameters have not been elucidated.
Ki-67 and its corresponding antibody were identified by Gerdes et al. in 1991. Ki-67 is a large nuclear nonhistone protein with a short half-life [16]. Ki-67 is expressed in the active phases of the cell cycle (G1, S, G2, and mitosis) of proliferating cells but is absent in resting cells (G0) [17,18]. The lack of expression in quiescent cells and the universal expression in proliferating tissues have generated profound interest in the potential use of Ki67 as a marker of cell proliferation. A large number of studies have confirmed that the percentage of Ki67-positive tumor cells, or the Ki67 index, has been widely used as a marker of cancer cell proliferation and a strong prognostic indicator for poor outcome in human neoplasms [19].
Cadherins are cell-surface molecules that mediate cell-cell adhesion, mainly through homophilic interactions [20]. Cadherins have important roles in normal development, morphogenesis, organogenesis, and carcinogenesis. N-cadherin, also known as cadherin-2, was first identified in 1982 as a 130 KD molecule in the chick neural tube, which was protected by calcium from proteolysis [21]. N-cadherin, encoded by the CDH1 gene, is a calcium-dependent adhesion protein located on chromosome 18q11.2 [22]. N-cadherin is composed of an extracellular part, a transmembrane part and a cytoplasmic part. The cytoplasmic part forms complexes with multiple molecules, such as β-catenin and α-catenin [21]. By regulating cell adhesion, N-cadherin plays multiple different important functions in various aspects of cell biology, including the control of cell polarization, differentiation, stemness, and cell motility [22].
In the current study, we investigate the role of FBXO2 in colorectal cancer. We hypothesized that FBXO2 is upregulated in CRC tumor tissues and can be used as a predictor of poor prognosis for CRC patients. To investigate the validity of this hypothesis, we detected the expression levels of FBXO2, Ki67 and N-cadherin to determine whether FBXO2 expression was correlated with the prognosis of CRC.
Materials and methods
Patient selection
Between January 2009 and December 2012, patients with CRC receiving treatment at the Department of General Surgery, Fifth Affiliated Hospital of Sun Yat-sen University, were enrolled. The patients included in this study met the following criteria: (i) having no other malignancy or inflammatory bowel disease diagnosed; (ii) having undergone curative resection without any treatment prior to surgery; (iii) having a diagnosis of CRC confirmed by postoperative histopathology; and (iv) having available follow-up information. TNM staging was assigned according to the American Joint Committee on Cancer (AJCC) TNM classification guidelines [23]. The study was approved by the Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University.
Immunohistochemical examinations
The tissue sections were dewaxed in xylene and then rehydrated through a graded alcohol series. Antigen retrieval was performed in a 95°C citrate-buffered solution, and a 30% hydrogen peroxide solution (H2O2) was used to block the endogenous peroxidase activity. After incubation with 10% BSA and a wash in PBS, the sections were incubated with the primary antibody at 4°C overnight. HRP-conjugated secondary antibody (Dako Cytomation, Glostrup, Denmark) was applied to the sections. The sections were subsequently washed in PBS, counterstained with hematoxylin, dehydrated, and mounted.
Immunohistochemical evaluation
The Ki67 staining intensities were quantified in the cell nucleus, N-cadherin and FBXO2 staining intensities were quantified in the cell cytoplasm. Two pathologists with expertise in gynecological pathology, who were blinded to the clinical and pathological data, independently performed the IHC assessment by using a light microscope (BX43 microscope; Olympus, Tokyo, Japan). The results were evaluated using a scoring system based on staining intensity and the percentage of positive cells, and the mean values for four representative fields were determined at a magnification of × 200. The immunostaining scores were calculated as immunostaining intensity multiplied by the proportion of positively stained tumor cells [15,22,24]. The immunostaining intensity was scored as 0 (lack of staining), 1 (weak staining), 2 (moderate staining), or 3 (strong staining), and the proportion of positively stained tumor cells was scored as 0 (<5%), 1 (6-25%), 2 (26-50%), 3 (51-75%), or 4 (>75%). The cases with IHC scores higher than 3 were defined as positive.
Follow-up visits
The clinical parameters and the overall survival (OS) were collected from the Health information department, and the patients or their families were contacted by telephone following a standardized protocol. Survival time was defined as the period from the date of primary surgery to the date of death or the date of the last follow-up phone call. The median follow-up time was 51 months (range, 21-87 months).
Statistical analysis
Statistical analysis for this study was executed with SPSS version 20.0 software for Windows (IBM, Armonk, NY, USA). The correlations between the clinicopathological factors and the expression levels of Ki67, N-cadherin, and FBXO2 were analyzed by the Chi-squared test. Pearson’s correlation analysis was used to analyze the correlations among Ki67, N-cadherin and FBXO2 expression. Survival curves were plotted using the Kaplan-Meier method, and group differences in survival times were assessed by the Log-rank test. Cox proportional hazards models were used to assess the correlations of the clinical variables with survival. The confidence intervals (CIs) were set to 95%, and a two-sided P-value of <0.05 was considered statistically significant.
Results
Clinicopathologic characteristics
According to the cutoff criteria, a total of 195 patients were included in this study. The median age of 195 patients was 59 (range 24-94) years. Among the patients, 94 (48.2%) were females and 101 (51.2%) were males; 132 (67.8%) patients had tumors located in the colon, and 63 (32.2%) had tumors located in the rectum. According to the imaging examination and the postoperative pathological results, 78 (40%) patients had positive lymph nodes, and 40 (20.5%) patients had distant metastasis. Clinical stage assignment of the CRC patients was performed according to the criteria established by the American Joint Committee on Cancer (AJCC) in 2010 [23]. A total of 97 (49.7%) cases were stage I or II tumors, and 98 (50.3%) cases were stage III or IV tumors at the time of diagnosis. Based on differentiation, 46 (23.6%) cases were poorly differentiated, and 149 (76.4%) cases were well or moderately differentiated. According to the currently established cut-off values, the positive rates of Ki67, N-cadherin and FBXO2 in the CRC tissue samples were 55.9%, 65.1% and 62.6%, respectively. The high expression levels of Ki67 and N-cadherin were significantly correlated with CRC size (P = 0.01) and metastasis (P = 0.01), respectively. The high expression of FBXO2 was significantly correlated with CRC metastasis (P = 0.04) and AJCC stage (P = 0.029) (Table 1). Representative IHC images for FBXO2 staining can be seen in Figure 1.
Table 1.
Clinicopathological parameters of CRC patients
| Variable | n | Ki-67 (-) | Ki-67 (+) | P-value | N-cadherin (-) | N-cadherin (+) | P-value | FBXO2 (-) | FBXO2 (+) | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| (n = 86) | (n = 109) | (n = 68) | (n = 127) | (n = 73) | (n = 122) | ||||||
| Gender | |||||||||||
| Female | 94 | 46 | 48 | 32 | 62 | 38 | 56 | ||||
| Male | 101 | 40 | 61 | 0.190 | 36 | 65 | 0.815 | 35 | 66 | 0.405 | |
| Age (years) | |||||||||||
| <65 | 104 | 49 | 55 | 32 | 72 | 39 | 65 | ||||
| ≥65 | 91 | 37 | 54 | 0.365 | 36 | 55 | 0.199 | 34 | 57 | 0.984 | |
| Location | |||||||||||
| Colon | 132 | 55 | 77 | 48 | 84 | 50 | 82 | ||||
| Rectum | 63 | 31 | 32 | 0.321 | 20 | 43 | 0.527 | 23 | 40 | 0.853 | |
| Size (cm) | |||||||||||
| <5 | 131 | 69 | 62 | 49 | 82 | 55 | 76 | ||||
| ≥5 | 64 | 17 | 47 | 0.01* | 19 | 45 | 0.288 | 18 | 46 | 0.06 | |
| T stage | |||||||||||
| T1+T2 | 52 | 29 | 23 | 20 | 32 | 21 | 31 | ||||
| T3+T4 | 143 | 57 | 86 | 0.048 | 48 | 95 | 0.526 | 52 | 91 | 0.608 | |
| N stage | |||||||||||
| N0 | 117 | 51 | 66 | 40 | 77 | 48 | 69 | ||||
| N1+N2 | 78 | 35 | 43 | 0.860 | 28 | 50 | 0.806 | 25 | 53 | 0.205 | |
| M stage | |||||||||||
| M0 | 155 | 71 | 84 | 61 | 95 | 64 | 91 | ||||
| M1 | 40 | 15 | 25 | 0.346 | 7 | 33 | 0.01* | 9 | 31 | 0.029* | |
| AJCC stage | |||||||||||
| I+II | 97 | 44 | 53 | 39 | 58 | 46 | 51 | ||||
| III+IV | 98 | 42 | 56 | 0.725 | 29 | 69 | 0.12 | 27 | 71 | 0.04* | |
| Differentiation | |||||||||||
| Well + Moderate | 149 | 63 | 86 | 49 | 100 | 59 | 90 | ||||
| Poor | 46 | 23 | 23 | 0.357 | 19 | 27 | 0.488 | 14 | 32 | 0.262 | |
P<0.05.
Figure 1.
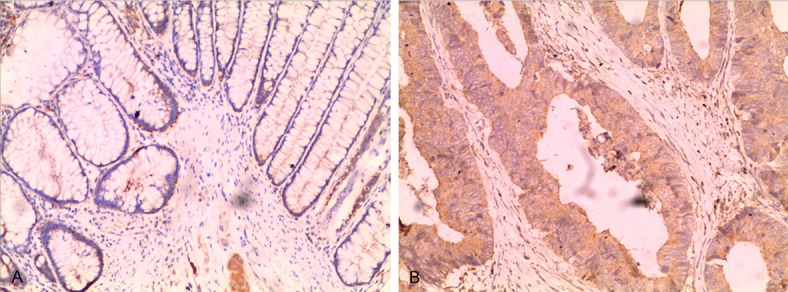
Immunohistochemical staining of FBXO2 in normal mucosa and CRC tissue (magnification, × 200). A. Immunohistochemical staining of FBXO2 in normal mucosa; B. Immunohistochemical staining of FBXO2 in CRC tissues.
Correlation between ki67, N-cadherin and FBXO2
As shown in Table 2, Pearson’s correlation analysis revealed a significant correlation between FBXO2, Ki67 and N-cadherin expression (r = 0.145, P = 0.043; r = 0.212, P = 0.003, respectively), while Ki67 displayed no correlation with N-cadherin (r = 0.022, P = 0.761) (Table 2).
Table 2.
Correlation between Ki67, N-cadherin and FBXO2
| Ki67 | N-cadherin | FBXO2 | ||
|---|---|---|---|---|
| Ki67 | Pearson Correlation | 1 | 0.022 | 0.145* |
| Sig. (2-tailed) | 0.761 | 0.043 | ||
| N | 195 | 195 | 195 | |
| N-cadherin | Pearson Correlation | 0.022 | 1 | 0.212** |
| Sig. (2-tailed) | 0.761 | 0.003 | ||
| N | 195 | 195 | 195 | |
| FBXO2 | Pearson Correlation | 0.145* | 0.212** | 1 |
| Sig. (2-tailed) | 0.043 | 0.003 | ||
| N | 195 | 195 | 195 | |
Correlation is significant at the 0.05 level (2-tailed);
Correlation is significant at the 0.01 level (2-tailed).
Correlation between tumor markers and overall survival
Survival curves were plotted using the Kaplan-Meier method, and group differences in survival times were assessed by the Log-rank test. For the combined group, those cases, where all 3 markers were positive, were defined as combined positive; the others were defined as combined negative. The statistical analysis showed that high expression of Ki67, N-cadherin, FBXO2 as well as the combined expression of all 3 markers were linked to shorter overall survival in CRC patients (P = 0.001, P = 0.022, P<0.001, and P<0.001, respectively) (Figure 2).
Figure 2.
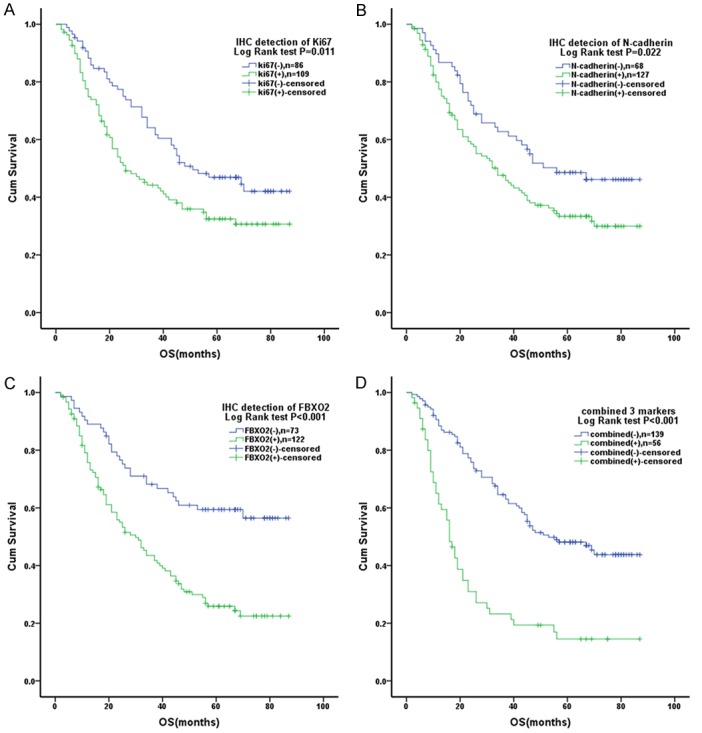
Kaplan-Meier survival analysis of CRC patients. A. Expression levels of Ki67 and OS. B. Expression levels of N-cadherin and OS. C. Expression levels of FBXO2 and OS. D. Expression levels of the combined 3 markers and OS.
Univariate and multivariate analysis of OS
As shown in Table 3, the univariate analysis revealed that M stage, AJCC stage, Ki67, N-cadherin, and FBXO2, as well as the combined expression of all 3 markers, were significantly associated with OS (P<0.001, P = 0.029, P = 0.012, P = 0.025, P<0.001, P<0.001, respectively). The multivariate analysis revealed that FBXO2 is an independent prognostic factor for CRC patients (HR 1.817, 95% CI 1.106-2.983, P = 0.018) (Table 3).
Table 3.
Univariate and multivariate analysis of OS
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender | ||||
| Female | 1 | |||
| Male | 1.225 (0.850-1.766) | 0.277 | ||
| Age (years) | ||||
| <65 | 1 | |||
| ≥65 | 1.148 (0.798-1.649) | 0.457 | ||
| Location | ||||
| Colon | 1 | |||
| Rectum | 1.077 (0.727-1.959) | 0.710 | ||
| Size (cm) | ||||
| <5 | 1 | |||
| ≥5 | 1.345 (0.918-1.972) | 0.129 | ||
| T stage | ||||
| T1+T2 | 1 | |||
| T3+T4 | 1.347 (0.880-2.060) | 0.17 | ||
| N stage | ||||
| N0 | 1 | |||
| N1+N2 | 1.156 (0.801-1.669) | 0.438 | ||
| M stage | ||||
| M0 | 1 | 1 | ||
| M1 | 2.154 (1.420-3.267) | <0.001* | 1.629 (0.988-2.686) | 0.056 |
| AJCC stage | ||||
| I+II | 1 | 1 | ||
| III+IV | 1.504 (1.044-2.169) | 0.029* | 1.004 (0.650-1.552) | 0.984 |
| Differentiation | ||||
| Well + Moderate | 1 | |||
| Poor | 1.159 (0.740-1.815) | 0.520 | ||
| Ki67 (negative vs positive) | 1.610 (1.109-2.335) | 0.012* | 1.032 (0.614-1.736) | 0.905 |
| N-cadherin (negative vs positive) | 1.576 (1.060-2.342) | 0.025* | 1.103 (0.660-1.842) | 0.708 |
| FBXO2 (negative vs positive) | 2.496 (1.644-3.790) | <0.001* | 1.817 (1.106-2.983) | 0.018* |
| Combined (negative vs positive) | 3.025 (2.070-4.421) | <0.001* | 1.913 (0.964-3.796) | 0.063 |
P<0.05.
Discussion
In this study, we investigated the role of FBXO2 in colorectal cancer by analyzing the correlations between clinicopathological characteristics and the expression level of FBXO2. At the beginning of the present study, we performed immunohistochemistry (IHC) to examine the expression level of FBXO2 in several paired CRC and normal tissue samples; the results of the preliminary experiments suggested that FBXO2 was over-expressed in 10 out of 15 CRC specimens. The expression of FBXO2 was then evaluated in an expanded population with 195 pairs of CRC samples. We observed that FBXO2 was mainly detected in the cytoplasm of CRC cells, and over-expression of FBXO2 was significantly correlated with CRC metastasis and AJCC stage; the Kaplan-Meier survival curves indicated that high expression of FBXO2 was related to the prognosis of CRC. The multivariate analysis revealed that over-expression of FBXO2 was a prognostic factor for overall survival.
FBXO2 is a member of the F-box protein family (FBP), which plays an important role in cell cycle regulation. By guiding a precise and timely turnover of myriad important cellular proteins, the F-box protein family plays a pivotal role in a number of key molecular events, such as cell division, signal transduction, and DNA replication, as well as guiding developmental pathways [25,26]. Emerging experimental and clinical data indicate that the F-box protein family members, such as SKP2, FBXW7, FBXO11, and FBXO5, are tightly linked to invasion and metastasis in various cancer types [7,13,14]. In CRC, some FBPs function as oncogenes, whereas other proteins display tumor suppressive functions. For example, SKP2 acts as an oncogene by recognizing cyclin-dependent kinase inhibitor 1B (CDKN1B) in S-phase, with the cooperation of SKP1, which leads to its degradation along with other tumor suppressor genes [9]; FBXW7 acts as a tumor suppressor by facilitating the destruction of oncogenic proteins via the ubiquitin proteasome pathway [27]. Unlike other FBXO family members, FBXO2 is less studied, and its mechanism of action is not very clear. Several studies showed that FBXO2 plays an important role in ER-associated-degradation (ERAD), which is a normal cellular process, whereby non-complex-forming or improperly modified proteins are removed from the endoplasmic reticulum (ER) and are degraded by the ubiquitin proteasome system [11,12]. FBXO2 is implicated in the ubiquitination of glycoproteins discarded from the endoplasmic reticulum; it binds the co-chaperone/ubiquitin ligase CHIP (C terminus of Hsc-70-interacting protein) through a unique N-terminal PEST domain [12]. Our results indicate that FBXO2 is highly expressed in CRC and is related to poor prognosis. Thus, we hypothesize that FBXO2 displays oncogenic functions in CRC, and it may degrade tumor suppressor proteins or other specific proteins via the ubiquitin proteasome pathway.
Our data indicate that FBXO2 might be involved in cell proliferation. Ki-67 is involved in the cell cycle, and within somatic tissues, it is only expressed in proliferating cells [28]. Ki-67 is frequently used as a static marker of proliferative activity. Although it is not considered an obligatory marker in the clinic, many studies have demonstrated that Ki67 is an early predictor of treatment efficacy and a prognostic factor for long-term outcomes [16,29]. Our data showed that high expression of FBXO2 correlates with the expression of Ki67. Furthermore, several studies have demonstrated that members of the F-box protein family, such as FBXO11 and FBXO5, regulate the cell cycle, cell proliferation and cell mobility by promoting the degradation of their substrates in several neoplastic diseases [30-32]. Hence, we have ample reason to conclude that FBXO2 might be involved in cell proliferation through ubiquitination and the subsequent degradation of key molecules.
Our data showed that the over-expression of FBXO2 was significantly correlated with CRC metastasis and the expression of N-cadherin. N-cadherin over-expression can affect the expression and localization of β-catenin, which plays an important role in the proliferative Wnt signaling pathway [33]. It has been demonstrated that aberrant N-cadherin expression is associated with epithelial-mesenchymal transition (EMT), a process whereby an epithelial cancer cell acquires invasiveness by losing epithelial features and gaining a mesenchymal phenotype [33,34]. Dong Hua et al. have demonstrated that the knockdown of FBXO2 inhibits gastric cancer cell migration, and low FBXO2 expression increased the expression of E-cadherin but reduced the expression of N-cadherin [15]. Thus, we inferred that FBXO2 was involved in tumor metastasis by participating in the epithelial-mesenchymal transition.
In summary, our study demonstrated that FBXO2 is highly expressed in colorectal cancer and that the expression level of FBXO2 could serve as a potential biomarker for metastasis as well as a reliable predictor for poor prognosis in CRC patients after curative enterectomy. We hypothesize that FBXO2 is an oncogenic protein in CRC, where it may play important roles in EMT and cancer cachexia by degrading tumor suppressor proteins or other specific proteins. The suppression of FBXO2 expression may present a therapeutic strategy to improve the prognosis for CRC patients.
Acknowledgements
This work was funded by the Zhuhai City, Guangdong Province Science and Technology Department (no. 20171009E030027). The funding was used to purchase experimental reagents.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 3.Dienstmann R, Mason MJ, Sinicrope FA, Phipps AI, Tejpar S, Nesbakken A, Danielsen SA, Sveen A, Buchanan DD, Clendenning M, Rosty C, Bot B, Alberts SR, Milburn Jessup J, Lothe RA, Delorenzi M, Newcomb PA, Sargent D, Guinney J. Prediction of overall survival in stage II and III colon cancer beyond TNM system: a retrospective, pooled biomarker study. Ann Oncol. 2017;28:1023–31. doi: 10.1093/annonc/mdx052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth AD, Delorenzi M, Tejpar S, Yan P, Klingbiel D, Fiocca R, d’Ario G, Cisar L, Labianca R, Cunningham D, Nordlinger B, Bosman F, Van Cutsem E. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst. 2012;104:1635–46. doi: 10.1093/jnci/djs427. [DOI] [PubMed] [Google Scholar]
- 5.Kattan MW, Hess KR, Amin MB, Lu Y, Moons KG, Gershenwald JE, Gimotty PA, Guinney JH, Halabi S, Lazar AJ, Mahar AL, Patel T, Sargent DJ, Weiser MR, Compton C members of the AJCC Precision Medicine Core. American Joint Committee on Cancer acceptance criteria for inclusion of risk models for individualized prognosis in the practice of precision medicine. CA Cancer J Clin. 2016;66:370–4. doi: 10.3322/caac.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sukari A, Muqbil I, Mohammad RM, Philip PA, Azmi AS. F-BOX proteins in cancer cachexia and muscle wasting: emerging regulators and therapeutic opportunities. Semin Cancer Biol. 2016;36:95–104. doi: 10.1016/j.semcancer.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz VM, de Herreros AG. F-box proteins: keeping the epithelial-to-mesenchymal transition (EMT) in check. Semin Cancer Biol. 2016;36:71–9. doi: 10.1016/j.semcancer.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nat Rev Cancer. 2014;14:233–47. doi: 10.1038/nrc3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uddin S, Bhat AA, Krishnankutty R, Mir F, Kulinski M, Mohammad RM. Involvement of F-BOX proteins in progression and development of human malignancies. Semin Cancer Biol. 2016;36:18–32. doi: 10.1016/j.semcancer.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Nishio K, Yoshida Y, Tanaka K, Mizushima T. Structural analysis of a function-associated loop mutant of the substrate-recognition domain of Fbs1 ubiquitin ligase. Acta Crystallogr F Struct Biol Commun. 2016;72:619–26. doi: 10.1107/S2053230X16011018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkin G, Hunt J, Minakawa E, Sharkey L, Tipper N, Tennant W, Paulson HL. F-box only protein 2 (Fbxo2) regulates amyloid precursor protein levels and processing. J Biol Chem. 2014;289:7038–48. doi: 10.1074/jbc.M113.515056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson RF, Glenn KA, Miller VM, Wen H, Paulson HL. A novel route for F-box protein-mediated ubiquitination links CHIP to glycoprotein quality control. J Biol Chem. 2006;281:20242–51. doi: 10.1074/jbc.M602423200. [DOI] [PubMed] [Google Scholar]
- 13.Hao Z, Huang S. E3 ubiquitin ligase Skp2 as an attractive target in cancer therapy. Front Biosci (Landmark Ed) 2015;20:474–90. doi: 10.2741/4320. [DOI] [PubMed] [Google Scholar]
- 14.Ishii N, Araki K, Yokobori T, Gantumur D, Yamanaka T, Altan B, Tsukagoshi M, Igarashi T, Watanabe A, Kubo N, Hosouchi Y, Kuwano H, Shirabe K. Reduced FBXW7 expression in pancreatic cancer correlates with poor prognosis and chemotherapeutic resistance via accumulation of MCL1. Oncotarget. 2017;8:112636–46. doi: 10.18632/oncotarget.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X, Wang T, Guan ZR, Zhang C, Chen Y, Jin J, Hua D. FBXO2, a novel marker for metastasis in human gastric cancer. Biochem Biophys Res Commun. 2018;495:2158–64. doi: 10.1016/j.bbrc.2017.12.097. [DOI] [PubMed] [Google Scholar]
- 16.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J. Clin. Oncol. 2005;23:7212–20. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 17.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Traut W, Endl E, Scholzen T, Gerdes J, Winking H. The temporal and spatial distribution of the proliferation associated Ki-67 protein during female and male meiosis. Chromosoma. 2002;111:156–64. doi: 10.1007/s00412-002-0202-8. [DOI] [PubMed] [Google Scholar]
- 19.Thakur SS, Li H, Chan AMY, Tudor R, Bigras G, Morris D, Enwere EK, Yang H. The use of automated Ki67 analysis to predict Oncotype DX risk-of-recurrence categories in early-stage breast cancer. PLoS One. 2018;13:e0188983. doi: 10.1371/journal.pone.0188983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q, Kerstetter AE, Azodi E, Marrs JA. Cadherin-1, -2, and -11 expression and cadherin-2 function in the pectoral limb bud and fin of the developing zebrafish. Dev Dyn. 2003;228:734–9. doi: 10.1002/dvdy.10401. [DOI] [PubMed] [Google Scholar]
- 21.Grunwald GB, Pratt RS, Lilien J. Enzymic dissection of embryonic cell adhesive mechanisms. III. Immunological identification of a component of the calcium-dependent adhesive system of embryonic chick neural retina cells. J Cell Sci. 1982;55:69–83. doi: 10.1242/jcs.55.1.69. [DOI] [PubMed] [Google Scholar]
- 22.DI Domenico M, Pierantoni GM, Feola A, Esposito F, Laino L, DE Rosa A, Rullo R, Mazzotta M, Martano M, Sanguedolce F, Perillo L, D’Angelo L, Papagerakis S, Tortorella S, Bufo P, Lo Muzio L, Pannone G, Santoro A. Prognostic significance of N-Cadherin expression in oral squamous cell carcinoma. Anticancer Res. 2011;31:4211–8. [PubMed] [Google Scholar]
- 23.Chen VW, Hsieh MC, Charlton ME, Ruiz BA, Karlitz J, Altekruse SF, Ries LA, Jessup JM. Analysis of stage and clinical/prognostic factors for colon and rectal cancer from SEER registries: AJCC and collaborative stage data collection system. Cancer. 2014;120(Suppl 23):3793–806. doi: 10.1002/cncr.29056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma YL, Peng JY, Zhang P, Liu WJ, Huang L, Qin HL. Immunohistochemical analysis revealed CD34 and Ki67 protein expression as significant prognostic factors in colorectal cancer. Med Oncol. 2010;27:304–9. doi: 10.1007/s12032-009-9210-3. [DOI] [PubMed] [Google Scholar]
- 25.Deshaies RJ. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu Rev Cell Dev Biol. 1999;15:435–67. doi: 10.1146/annurev.cellbio.15.1.435. [DOI] [PubMed] [Google Scholar]
- 26.Deshaies RJ. Structural biology: corralling a protein-degradation regulator. Nature. 2014;512:145–6. doi: 10.1038/nature13644. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Inuzuka H, Zhong J, Wan L, Fukushima H, Sarkar FH, Wei W. Tumor suppressor functions of FBW7 in cancer development and progression. FEBS Lett. 2012;586:1409–18. doi: 10.1016/j.febslet.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winking H, Gerdes J, Traut W. Expression of the proliferation marker Ki-67 during early mouse development. Cytogenet Genome Res. 2004;105:251–6. doi: 10.1159/000078196. [DOI] [PubMed] [Google Scholar]
- 29.Inwald EC, Klinkhammer-Schalke M, Hofstadter F, Zeman F, Koller M, Gerstenhauer M, Ortmann O. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139:539–52. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Zhang G, Gao Z, Li S, Li Z, Bi J, Liu X, Li Z, Kong C. Comprehensive analysis of differentially expressed genes associated with PLK1 in bladder cancer. BMC Cancer. 2017;17:861. doi: 10.1186/s12885-017-3884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun C, Tao Y, Gao Y, Xia Y, Liu Y, Wang G, Gu Y. F-box protein 11 promotes the growth and metastasis of gastric cancer via PI3K/AKT pathway-mediated EMT. Biomed Pharmacother. 2018;98:416–23. doi: 10.1016/j.biopha.2017.12.088. [DOI] [PubMed] [Google Scholar]
- 32.Schneider C, Kon N, Amadori L, Shen Q, Schwartz FH, Tischler B, Bossennec M, Dominguez-Sola D, Bhagat G, Gu W, Basso K, Dalla-Favera R. FBXO11 inactivation leads to abnormal germinal-center formation and lymphoproliferative disease. Blood. 2016;128:660–6. doi: 10.1182/blood-2015-11-684357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–11. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 34.Rosano L, Spinella F, Di Castro V, Nicotra MR, Dedhar S, de Herreros AG, Natali PG, Bagnato A. Endothelin-1 promotes epithelial-to-mesenchymal transition in human ovarian cancer cells. Cancer Res. 2005;65:11649–57. doi: 10.1158/0008-5472.CAN-05-2123. [DOI] [PubMed] [Google Scholar]


