Abstract
Background: The aim of this retrospective study is to review the clinical, radiologic and pathological features of GN and to bring awareness of GNs that may occur in unusual locations to clinicians. Methods: Data from 11 patients from the Lishui Center Hospital, Zhejiang University, (Lishui, China) were analyzed between January 1999 and May 2016, and the clinical, radiologic and pathological features in these patients are discussed here. Results: Our retrospective study involved 11 patients, 5 males and 6 females, with an average age of 34.1 (1-76) years, who underwent surgical intervention for GN. Tumors occurred in the following locations: one tumor in the cervical cord, one tumor in the subcutaneous layer, two tumors in the posterior mediastinum, two tumors in the nerve root, two tumors in the posterior peritoneum, and three tumors in the adrenal gland. Two patients presented with lumbocrural pain, one patient presented with neck and shoulder pain, and one patient presented with abdominal discomfort, with the remaining patients being asymptomatic. Homogenous density, oval mass and well-defined borders were characteristic radiologic features of GN. All patients underwent surgery, and their tumors were completely resected. Histopathological examinations showed that the tumors were characteristic of GN and consisted of nerve fibers and mature ganglion cells. The immunohistochemical reactions for S-100 were positive in all patients. The mean length of the hospital stays was 15.7 d (range: 8-28 d). The mean duration of follow-up was 96 mo (range: 5-180 mo). Two patients did not follow-up, and nine patients were asymptomatic. Conclusions: GNs are rare benign tumors, and their diagnosis is challenging. Complete surgical excision is an effective and successful treatment, and long-term follow-up is necessary.
Keywords: Ganglioneuroma, retroperitoneum, surgery, pathology
Introduction
Ganglioneuromas (GNs) are rare and benign neural tumors arising from the paravertebral sympathetic plexus and occasionally from other sites [1]. The most common site for GNs is the posterior mediastinum and the retroperitoneum [2]. Over the past several decades, ganglioneuromas have been identified in numerous locations, including the adrenal gland, kidney, conus medullaris, nerve root, and so on. They grow slowly and may occasionally secrete catecholamine or a steroid hormone. Unless ganglioneuromas become large in size, they are usually asymptomatic. As the tumor grows, patients present with different symptoms that are a result of tumor size and location and whether hormones are being secreted or not and include abdominal pain, distention and fatigue. They can occur in all ages but occur more frequently in individuals between the ages of 10 and 40 years [2].
To our knowledge, due to the rarity of GN, there are few detailed reports concerning the clinical, radiologic and pathological features of these tumors. In this study, 11 cases of GN are presented and the clinical, radiologic and pathological features are described.
Methods
Between January 1999 and May 2016, the data from 11 patients from Lishui Center Hospital, Zhejiang University, (Lishui, China) were analyzed. The clinicopathologic information from hospital records is as follows: patient gender, age, clinical presentation, tumor size and location, radiologic appearance, operative strategy, tumor cellularity, necrosis and prognosis.
Results
Clinical findings
In our study, there were 11 patients ranging in age from 7 to 76 years old (mean, 34.1 y). The male:female ratio was 1:1.8 (4 men and 7 women). Tumors occurred in the following areas: one tumor in the cervical cord, one tumor in the subcutaneous layer, two tumors in the posterior mediastinum, two tumors in the nerve root, two tumors in the posterior peritoneum and three tumors in the adrenal gland. The preoperative symptoms of these patients, based on the site of the tumor, included lumbocrural pain (No. 4 and No. 5), neck and shoulder pain (No. 3) and abdominal discomfort (No. 9). The remaining patients were asymptomatic (Table 1). Patients had no history of hypertensive disease, diabetes mellitus, coronary disease or tuberculosis, and only one patient had a history of hepatitis (No. 5).
Table 1.
Clinical findings of ganglioneuromas
| Case | Gender | Age | Tumor site | Symptom | Received examination |
|---|---|---|---|---|---|
| 1 | Male | 26 | Posterior peritoneum | Asymptomatic | CT/contrast-enhanced CT |
| 2 | Female | 76 | Adrenal gland | Asymptomatic | MRI/CT/contrast-enhanced CT |
| 3 | Male | 50 | Cervical cord | Neck and shoulder pain | MRI |
| 4 | Female | 52 | Nerve root | Lumbocrural pain | MRI |
| 5 | Female | 34 | Nerve root | Lumbocrural pain | CT |
| 6 | Female | 20 | Adrenal gland | Asymptomatic | Ultrasound/CT |
| 7 | Female | 1 | Subcutaneous | Asymptomatic | CT |
| 8 | Male | 38 | Adrenal gland | Asymptomatic | CT/contrast-enhanced CT |
| 9 | Male | 7 | Posterior peritoneum | Abdominal discomfort | Ultrasound/CT |
| 10 | Female | 27 | Posterior mediastinum | Asymptomatic | CT/contrast-enhanced CT |
| 11 | Male | 44 | Posterior mediastinum | Asymptomatic | CT/PET-CT |
Radiologic features
Two patients received ultrasonic examination (No. 6 and No. 9), which revealed a homogenous, oval, well-defined and low echo mass in contact with the right adrenal gland. The mass was 66×32 mm in size (No. 6), and the other mass was 89×67 mm in size (No 8). Nine patients received computed tomography (CT) scanning, including contrast-enhanced CT. The contrast-enhanced CT revealed a homogenous, oval and relatively well-defined mass occupying the posterior peritoneum in contact with the right renal artery (No. 1) (Figure 1). Three patients received magnetic resonance imaging (No. 2, No. 3 and No. 4). An MRI revealed an irregular, intraspinal mass, 5 cm in length and 1.3 cm in diameter, at the C2 and C4 level (No. 3). The tumor was isointense with the spinal cord on T1-weighted images and hyperintense on T2-weighted images. The tumor was not uniform on contrast-enhanced T1-weighted images. One patient (No. 11) received positron emission tomography-computed tomography (PET-CT). The results showed that the tumor had no fluorodeoxyglucose (FDG) uptake (Figure 2).
Figure 1.
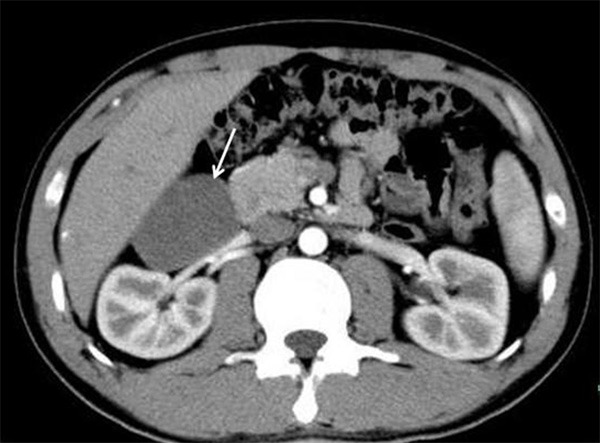
The contrast-enhanced CT reveals a homogenous, oval and relatively well-defined mass (arrows) occupying the posterior peritoneum in contact with the right renal artery (Case 1).
Figure 2.
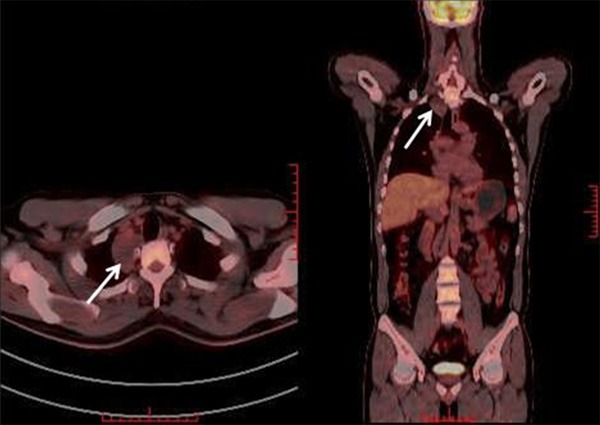
PET-CT shows an oval mass in the posterior mediastinum (arrows). The tumor did not have FDG uptake (Case 11).
Treatment and follow-up
All patients underwent surgery, and the tumors were completely resected (Table 2). Four patients (No. 2, No. 6, No. 8, and No. 11) underwent tumor resection by VATS. The mean operating time was 92 min (range, 60-150 min), and the mean blood loss was 236 mL (range, 50-1200 mL). The average duration of the hospital stays was 15.7 (8-28) days, with no mortality. The mean duration of follow-up time was 96 mo (range: 5-180 mo). Two patients did not follow-up, and nine patients were asymptomatic (Table 2).
Table 2.
Treatment history and follow-up
| Case | Surgical procedures | Operating time (min) | Blood loss (mL) | Hospital stay (days) | Follow-up (months) | Recurrence | Current status |
|---|---|---|---|---|---|---|---|
| 1 | Tumor excision | 90 | 50 | 22 | 14 | No | NED |
| 2 | VATS | 100 | 200 | 28 | 32 | No | NED |
| 3 | Tumor excision | 150 | 1200 | 20 | - | - | - |
| 4 | Tumor excision | 110 | 300 | 18 | 180 | No | NED |
| 5 | Tumor excision | 60 | 200 | 8 | 162 | No | NED |
| 6 | VATS | 90 | 50 | 22 | 156 | No | NED |
| 7 | Tumor excision | 70 | 50 | 10 | 165 | No | NED |
| 8 | VATS | 80 | 100 | 9 | 108 | No | NED |
| 9 | Tumor excision | 130 | 100 | 14 | - | - | - |
| 10 | Thoracotomy | 70 | 200 | 11 | 46 | No | NED |
| 11 | VATS | 60 | 150 | 11 | 5 | No | NED |
Histology
The tumors ranged from 0.5 cm to 7.5 cm in diameter and were round or ovoid (Figure 3). Histopathological examinations showed that the tumors were composed of nerve fibers and mature ganglion cells, the typical histological findings of GN (Figure 4). Immunohistochemical reactions for the S-100 protein were positive in all patients (Figure 5). Desmin, smooth-muscle actin (SMA) and CD34 were negative (Table 3).
Figure 3.
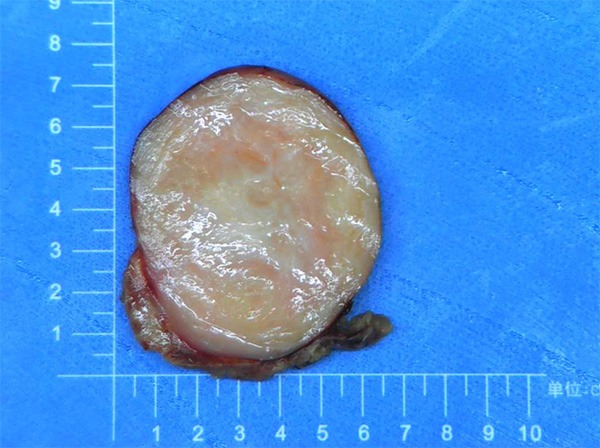
Cross-section of GN attached to the adrenal gland. The tumor was firm (Case 1).
Figure 4.
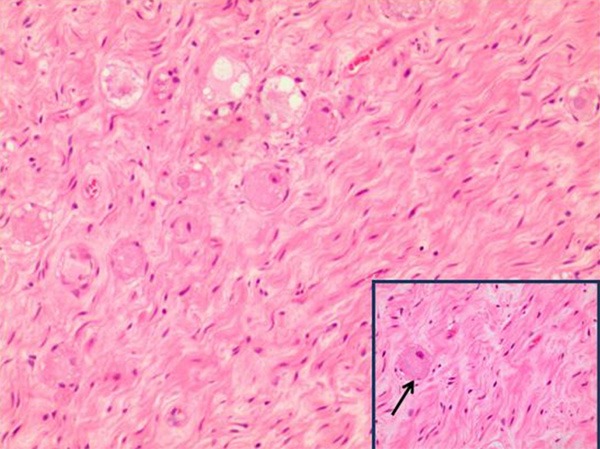
GN composed of nerve fibers and mature ganglion cells (100×); insert showing an enlarged morphology of gangliocytes, indicated by arrows (200×).
Figure 5.
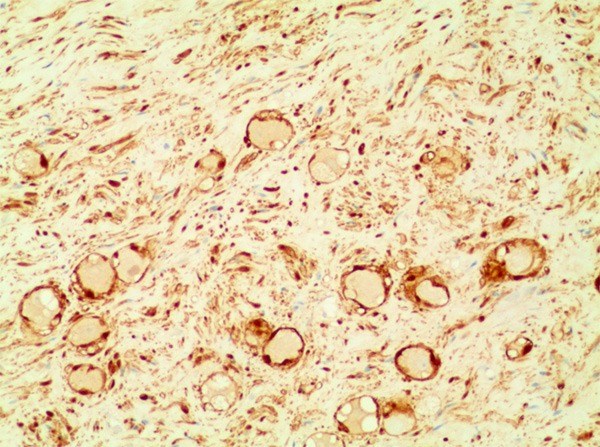
The immunohistochemical reactions for S-100 protein were positive (100×).
Table 3.
Pathologic features of ganglioneuromas
| Case | Size | Consistency | Shape | Border | Cellular pattern | S-100 | Desmin | SMA |
|---|---|---|---|---|---|---|---|---|
| 1 | 7.5×6×6 cm | Firm | Round | Well-defined | Spindle | + | - | - |
| 2 | 4.7×3×2 cm | Softening | Ovoid | Well-defined | Spindle | + | - | - |
| 3 | 5.0×2×1.3 cm | Firm | Ovoid | Unremarkable | Spindle | + | - | - |
| 4 | 1×1×0.5 cm | Solid | Ovoid | Unremarkable | Spindle | + | - | - |
| 5 | 1×1×1 cm | Firm | Round | Well-defined | Spindle | + | - | - |
| 6 | 4×3×3 cm | Firm | Ovoid | Well-defined | Spindle | + | - | - |
| 7 | 5×5×3 cm | Solid, cystic | Ovoid | Unremarkable | Spindle | + | - | - |
| 8 | 6×4×3 cm | Firm | Ovoid | Well-defined | Spindle | + | - | - |
| 9 | 9×7×6 cm | Softening | Round | Well-defined | Spindle | + | - | - |
| 10 | 7×5×2 cm | Firm | Ovoid | Well-defined | Spindle | + | - | - |
| 11 | 5.9×4.7×1.9 cm | Solid | Ovoid | Well-defined | Spindle | + | - | - |
Discussion
According to the degree of cellular and extracellular maturation, neuroblastic tumors originate from the neural crest tissue of the sympathetic nervous system and are divided into 3 subgroups: GNs, ganglioneuroblastomas and neuroblastomas [3]. GNs are slow-growing benign tumors usually found at any age but more frequently in children over the age of 10 [4]. The posterior mediastinum and retroperitoneum are the most common locations for GNs. Other rare sites of occurrence include the heart, spermatic cord, bone, pelvis, gastrointestinal tract, cervical region, parapharyngeal area and the supraclavivular area [5-7]. To date, most existing literature on GNs is limited to case reports.
In recent years, the characteristics of GNs have become increasingly recognized. In their early stage, most GNs are asymptomatic and usually found incidentally. As the tumor gradually grows, patients present with different symptoms that depend on tumor size and location and whether hormones are being secreted or not and include abdominal pain, distention, fatigue and so on. In our study, two patients presented with lumbocrural pain, one patient presented with neck and shoulder pain, and one patient presented with abdominal discomfort, with the remaining patients being asymptomatic. Therefore, masses in the nerve root, relative to the chest and abdominal cavity, are more likely to show symptoms due to their narrow space.
Because of the lack of specific distinguishing factors, GNs are relatively difficult to discern from other tumors [8]. The differential diagnosis of GNs includes numerous malignant and benign tumors, including neurofibroma, schwannoma, neuroblastoma, ganglioneuroblastoma, pheochromocytoma and so on [9]. Imaging examinations, including US, CT and MRI, are used for assessing GNs. According to the location of the tumor, different imaging examinations were used. For example, mediastinal, retroperitoneal or abdominal masses were identified by US or CT. The tumor origin from the nerve root was identified by MRI. US usually shows a homogenous, hypoechoic mass, and unenhanced CT reveals a homogenous, low attenuation mass [1]. MRI findings of GN showed low signal intensity on T1-weighted images and high signal intensity on T2-weighted images according to the Koktener [1] study. In our study, US showed a homogenous, oval, well-defined and low echo mass. The contrast-enhanced CT revealed a homogenous, oval and relatively well-defined mass. The tumor was isointense with the spinal cord on T1-weighted images and hyperintense on T2-weighted images. The tumor was not homogenous on contrast-enhanced T1-weighted images. It is not possible to determine whether a mass is benign or malignant by imaging examinations. However, their findings can reveal the extent of a tumor, its regional invasion, organ of origin, vascular encasement calcification, and adenopathy [10]. The PET-CT findings of GNs have been rarely reported. In our case, the tumor had no FDG uptake. This case indicates that the FDG uptake degree may be related to the tumor’s aggressive behavior.
The most successful management of GNs is by surgical resection, which can be either radical or staged [5]. Unless the GNs are associated with ganglioneuroblastoma changes, preoperative or postoperative radiotherapy or chemotherapy is ineffective [6]. In the past, the majority of retroperitoneal tumor excisions have been approached via laparotomy. With the improvement of laparoscopic surgical skills, surgery is increasingly being performed by laparoscope. Laparoscopic retroperitoneal surgeries are routinely performed for adrenalectomy, retroperitoneal lymphadenectomy and nephrectomy [5]. However, the number of retroperitoneal ganglioneuroma excisions approached via laparoscopic surgery is minimal due to their rarity. Ruiz-Tovar et al. [8] described the case of a 53-year-old female who was diagnosed with retroperitoneal ganglioneuroma based on histopathology. The patient received a laparoscopic anterior transperitoneal approach with two 10-mm trocars in both the iliac fossae and infraumbilical Hasson trocar. The patient was discharged following an unsuccessful recovery. This study illustrates that laparoscopic surgery is a safe and effective alternative to conventional open surgery for resection of retroperitoneal tumors [8].
Conclusions
In conclusion, we have described one cervical cord, one subcutaneous, two posterior mediastinal, two nerve roots, two posterior peritoneal, and three adrenal gland GNs, which have been treated by complete surgical resection. The goal of this study is to heighten the awareness of clinicians that GNs may arise within unusual locations.
Acknowledgements
We would like to acknowledge the patients for allowing their cases to be published. Medical and Health and Technology Project of Zhejiang Province (2016KYB336).
Disclosure of conflict of interest
None.
References
- 1.Koktener A, Kosehan D, Akin K, Bozer M. Incidentally found retroperitoneal ganglioneuroma in an adult. Indian J Surg. 2015;77(Suppl 1):3–5. doi: 10.1007/s12262-013-1030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acín-Gándara D, Carabias A, Bertomeu A, Giménez-Alvira L, Colao L, Limones M. Giant retroperitoneal ganglioneuroma. Rev Esp Enferm Dig. 2010;102:205–7. doi: 10.4321/s1130-01082010000300008. [DOI] [PubMed] [Google Scholar]
- 3.Esen HK, Esen O, Irsi C. Retroperitoneal ganglioneuroma: minicking an ovarian mass in a child. Pak J Med Sci. 2015;31:724–6. doi: 10.12669/pjms.313.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JY, Lee KS, Han J, Yoon HK, Kim TS, Han BK, Kim J, Shim YM. Spectrum of neurogenic tumors in the thorax: CT and pathologic findings. J Comput Assist Tomogr. 1999;23:399–406. doi: 10.1097/00004728-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Zugor V, Schott GE, Kühn R, Labanaris AP. Retroperitoneal ganglioneuroma in childhood-a presentation of two cases. Pediatr Neonatol. 2009;50:173–6. doi: 10.1016/S1875-9572(09)60058-9. [DOI] [PubMed] [Google Scholar]
- 6.Hayes FA, Green AA, Rao BN. Clinical manifestations of ganglioneuroma. Cancer. 1989;63:1211–4. doi: 10.1002/1097-0142(19890315)63:6<1211::aid-cncr2820630628>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Cobellis L, Messalli EM, Rossiello R, Montone L, Cobellis G. Bilateral pelvic ganglioneuroma: clinicopathologic findings: a case report. Eur J Obstet Gynecol Reprod Biol. 2004;117:242–244. doi: 10.1016/j.ejogrb.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Tovar J, Gamallo-Amat C. Laparoscopic excision of retroperitoneal ganglioneuroma through anterior transperitoneal approach. Cir Cir. 2012;80:274–7. [PubMed] [Google Scholar]
- 9.Strollo DC, Rosado-de-Christenson ML, Jett JR. Primary mediastinal tumors part II. Tumors of the middle and posterior mediastinum. Chest. 1997;112:1344–57. doi: 10.1378/chest.112.5.1344. [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa T, Ohtomo K, Araki T, Fujimoto H, Nemoto K, Nanbu A, Onoue M, Aoki K. Ganglioneuroma: computed tomography and magnetic resonance features. Br J Radiol. 1996;69:114–21. doi: 10.1259/0007-1285-69-818-114. [DOI] [PubMed] [Google Scholar]


