Abstract
This study aimed to explore the correlation of circulating angiogenic microRNAs (miRNAs) with the occurrence of cardiotoxicity in triple-negative breast cancer (TNBC) patients who underwent epirubicin/cyclophosphamide-docetaxel (EC-D) neoadjuvant chemotherapy. One hundred seventy-nine TNBC patients were consecutively enrolled and received EC-D neoadjuvant chemotherapy. Plasma samples were collected before neoadjuvant treatment, and relative expression of angiogenic miRNAs was measured by real-time quantitative polymerase chain reaction. Cardiotoxicity was defined by any one of the following symptoms: heart failure, acute coronary artery syndrome, fatal arrhythmia and a left ventricular ejection fraction (LVEF) declined by 10% from baseline to an absolute value below 53%. The LVEF level was decreased, while cardiac troponin I (cTnI) and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were increased by EC-D neoadjuvant chemotherapy. In total, 9 cases (5.0%) of cardiotoxicity occurred. Let-7f, miR-126 and miR-210 were negatively associated with the cTnI level, while let-7f, miR-19a and miR-130a were negatively correlated with the NT-proBNP level. Compared to noncardiotoxicity patients, the expression levels of let-7f, miR-19a, miR-20a, miR-126, and miR-210 were decreased in cardiotoxicity patients. A multivariate logistic regression analysis revealed that let-7f and miR-126 independently predicted low cardiotoxicity risk, and a receiver operating characteristics curve illustrated that let-7f (area under curve [AUC]=0.815, 95% CI: 0.725-0.906) and miR-126 (AUC=0.731, 95% CI: 0.624-0.838) as well as the combination of these two miRNAs (AUC=0.885, 95% CI: 0.818-0.952) could effectively distinguish cardiotoxicity patients from noncardiotoxicity patients. The angiogenic miRNAs let-7f and miR-126 might serve as novel and convincing biomarkers for reduced cardiotoxicity risk in TNBC patients who receive EC-D neoadjuvant chemotherapy.
Keywords: Angiogenesis, miRNAs, cardiotoxicity, neoadjuvant chemotherapy, TNBC
Introduction
Triple-negative breast cancer (TNBC), a common subtype of breast cancer (BC) accounting for 13% of all BC cases worldwide, is defined by a lack of hormonal (estrogen and progesterone) receptors as well as a lack of human epidermal growth factor receptor 2 (HER2). TNBC is related to aggressive disease progression and worse prognosis among the major subtypes of BC [1,2]. Chemotherapies are the mainstay for treatment of TNBC, among which neoadjuvant chemotherapy is administered before radical treatments to reduce the size and stage of the tumor as well as the development of a drug-resistant cell strain [3,4]. Although neoadjuvant chemotherapy has achieved improved survival, it still leads to an increased risk of cardiotoxicity and has worsened the cardiologic prognosis in patients with BC including TNBC [5,6]. Therefore, there is a great need to identify novel biomarkers for monitoring cardiotoxicity and to improve the cardiac prognosis in TNBC patients who undergo neoadjuvant chemotherapy.
MicroRNAs (miRNAs) are single-stranded, small, noncoding RNAs containing approximately 20 nucleotides that participate in various biological processes and play critical roles in the development and progression of different diseases, including cardiovascular diseases, carcinomas and neurogenic diseases [7]. Based on previous studies, specific miRNAs (such as let-7, miR-210 and miR-378) can promote angiogenesis (a complex process of new blood formation in response to ischemia and hypoxia) to facilitate blood supply for ischemic tissues. The stimulation of angiogenesis is beneficial for reducing the risk of ischemic cardiovascular diseases by boosting the blood supply and retaining cardiac functions [8-11]. Considering that neoadjuvant chemotherapy is complicated by the risk of cardiotoxicity and that angiogenesis protects cardiac function, we were interested in discovering whether or not angiogenic miRNAs could predict the occurrence of cardiotoxicity in TNBC patients who underwent epirubicin/cyclophosphamide-docetaxel (EC-D) neoadjuvant chemotherapy. In this study, we selected a group of circulating angiogenic miRNAs to explore their association with the occurrence of cardiotoxicity in TNBC patients who underwent EC-D neoadjuvant chemotherapy.
Patients and methods
Patients
One hundred and seventy-nine TNBC patients who underwent EC-D neoadjuvant chemotherapy at Tongji Hospital from July 2013 to June 2016 were consecutively enrolled in this study. The inclusion criteria included the following: (1) Diagnosed as primary TNBC confirmed by clinical findings and immunohistochemical testing (IHC); (2) a TNM stage of II-III and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1; (3) age older than 18 years; (4) normal left ventricular ejection fraction (LVEF) ≥55%; (5) about to receive EC-D neoadjuvant chemotherapy followed by the surgery; (6) likely to be regularly followed up. Patients were excluded if they had: (1) evidence of metastatic breast cancer, concurrent bilateral invasive breast cancer or inflammatory breast cancer; (2) a history of congenital heart disease, myocardial infarction, heart failure, cerebral infarction or other severe cardiovascular or cerebrovascular diseases; (3) chemotherapy contraindications; (4) previous treatment with chemotherapy, immunotherapy, targeted agents, antitumor vaccines or radiation therapy for other diseases; (5) active infection requiring systemic therapy; (6) severe renal or liver dysfunction; (7) autoimmune disease, malignant solid tumors or hematologic malignancy. Patients who were pregnant or breast-feeding women were also excluded from this study.
Ethics statement
This study was performed according to the provisions of the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Tongji Hospital. All patients signed the informed consents before enrollment.
Samples collection
Whole blood samples were collected using ethylenediaminetetraacetic acid (EDTA) tubes from all patients prior to neoadjuvant chemotherapy and naturally coagulated for approximately 20 minutes at room temperature, then centrifuged at 2000 r/min for 10 minutes. After that, the plasma was collected and stored at -80°C for further detection.
Measurements of miRNAs
Total RNA was extracted from plasma with the use of the miRNeasy Plasma Kit (Canspec Scientific Instruments, Shanghai, China) according to the manufacturer’s instructions. The concentration and purity of the RNA were determined by spectrophotometer. After that, reverse transcription of RNA was conducted using the miRCURY LNA Universal RT cDNA Synthesis Kit (Aksomics, Shanghai, China). Real-time quantitative polymerase chain reaction (RT-qPCR) was carried out with the use of the SYBR Premix Ex Taq kit (Takara, Dalian, China) to measure the relative quantity of miRNAs. The relative expression levels of miRNAs were calculated using the 2-ΔΔCt method and normalized to U6 expression. The primer sequences used in the present study are shown in Table 1.
Table 1.
Primers used in the present study
| RNAs | Forward (5’->3’) | Reverse (5’->3’) |
|---|---|---|
| let-7b | ACACTCCAGCTGGGTGAGGTAGTAGGTTGTGT | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| let-7f | ACACTCCAGCTGGGTGAGGTAGTAGATTGTAT | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-17-5p | ACACTCCAGCTGGGTGAGGTAGTAGGTTGTGT | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-17-3p | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-18a | ACACTCCAGCTGGGTAAGGTGCATCTAGTGCA | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-19a | ACACTCCAGCTGGGAGTTTTGCATAGTTGCAC | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-19b-1 | ACACTCCAGCTGGGAGTTTTGCAGGTTTGCAT | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-20a | ACACTCCAGCTGGGTAAAGTGCTTATAGTGCA | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-92a | ACACTCCAGCTGGGTATTGCACTTGTCCCGGC | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-126 | ACACTCCAGCTGGGCATTATTACTTTTGGTAC | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-130a | ACACTCCAGCTGGGTTCACATTGTGCTACTGT | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-210 | ACACTCCAGCTGGGAGCCCCTGCCCACCGCAC | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-296 | ACACTCCAGCTGGGAGGGCCCCCCCTCAATCC | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| miR-378 | ACACTCCAGCTGGGCTCCTGACTCCAGGTCCT | ACACTCCAGCTGGGACTGCAGTGAAGGCACTT |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
Treatment
According to the disease condition and personal willingness, patients received the EC-D neoadjuvant chemotherapy regimen, which was administered as follows: epirubicin (E), at a dose of 100 mg/m2 on day 1 and cyclophosphamide (C), at a dose of 600 mg/m2 on day 1. E and C were repeated every 21 days for 4 cycles, then followed by docetaxel (D), at a dose of 75-100 mg/m2 on day 1 and repeated every 21 days for 4 cycles. After completing the neoadjuvant chemotherapy, all patients received surgical treatment followed by adjuvant therapy based on the disease condition and clinical requirements.
Assessments of cardiotoxicity
In the present study, cardiotoxicity was defined as the occurrence of the any of following symptoms [12,13]: (1) heart failure; (2) acute coronary artery syndrome; (3) fatal arrhythmia; (4) the LVEF (assessed by echocardiography) declined by 10% from baseline to an absolute value below 53% (the lower limit of normal). According to the American Society of Echocardiography recommendations [14], LVEF was assessed before neoadjuvant chemotherapy (Cycle 0, C0), at the end of 4 cycles of EC chemotherapy (C4), at the end of 4 cycles of docetaxel treatment (C8), and at 3th months (M3), 6th months (M6), 9th months (M9) and 12th months (M12) after surgery. Furthermore, the levels of cardiac troponin I (cTnI) and N-terminal pro-brain natriuretic peptide (NT-proBNP) were also used to monitor the myocardial injury and cardiac function. Both cTnI and NT-proBNP were measured at C0, C4 and C8.
Statistics
SPSS 21.0 statistical software (SPSS Inc, Chicago, USA) was used for statistical analysis and GraphPad Prism 6.01 software (GraphPad Software Inc, San Diego, USA) was used for chart making. Normally distributed continuous variables are presented as the mean value ± standard deviation, continuous variables with a skewed distribution are presented as the median (25th-75th quantiles), and the categorized variables are presented as count (percentage). Comparisons at paired time points were determined by a Wilcoxon signed-rank sum test, and comparisons between two groups were determined by a Wilcoxon rank sum test. A correlation of the miRNAs relative expressions with the levels of cTnI and NT-proBNP was determined by Spearman’s rank correlation coefficient. Univariate and multivariate logistic regression models were used to determine the value of miRNAs in predicting cardiotoxicity risk. The miRNAs with an independent value in predicting cardiotoxicity risk were further determined by a receiver operating characteristic (ROC) curve. A P value <0.05 was considered significant.
Results
Study flow
At the initiation of this study, a total of 331 TNBC patients were invited, and 67 patients were excluded (including 43 patients who missed the invitation and 24 patients who declined to attend a prescreening meeting). A total of 264 patients were then screened for eligibility, and 85 patients were excluded (including 47 patients who did not meet the inclusion criteria and 38 patients who disagreed with the informed consent). The remaining 179 TNBC patients were enrolled in the study, and 25 patients withdrew during the study (including 22 patients who were lost during follow-up and 3 patients who withdrew their informed consents). Finally, 154 TNBC patients completed the whole study (Figure 1). In the present study, all179 TNBC patients were included in the analysis, and the analysis was performed based on the intention-to-treat (ITT) principles with the last observation carried forward (LOCF) method from any of the post-baseline measures.
Figure 1.
Study flow. TNBC, triple-negative breast cancer.
Baseline characteristics
The mean age of TNBC patients (N=179) was 45.9±6.1 years. The numbers of patients with pathological grade 1, 2 and 3 were 44 (24.6%), 117 (65.3%) and 18 (10.1%) respectively. The numbers of patients with TNM stage II and III were 145 (81.0%) and 34 (19.0%) respectively. In addition, the median LVEF level, cTnI and NT-proBNP concentrations were 67.0 (64.0-71.0)%, 0.021 (0.010-0.049) ng/mL and 0.076 (0.060-0.096) ng/mL respectively. Other clinical characteristics of the TNBC patients are listed in Table 2.
Table 2.
Baseline characteristics
| Parameters | TNBC patients (N=179) |
|---|---|
| Age (years) | 45.9±6.1 |
| BMI (kg/m2) | 23.3±2.0 |
| Obesity (n/%) | 35 (19.6) |
| Smoke (n/%) | 32 (17.9) |
| Hypertension (n/%) | 41 (22.9) |
| Diabetes mellitus (n/%) | 8 (4.5) |
| Dyslipidemia (n/%) | 32 (17.9) |
| Hyperuricemia (n/%) | 31 (17.3) |
| Chromic kidney disease (n/%) | 4 (2.2) |
| Pathological grade (n/%) | |
| Grade 1 | 44 (24.6) |
| Grade 2 | 117 (65.3) |
| Grade 3 | 18 (10.1) |
| Tumor size (cm) | 3.0 (2.2-4.0) |
| T stage (n/%) | |
| T1 | 34 (19.0) |
| T2 | 135 (75.4) |
| T3 | 10 (5.6) |
| N stage (n/%) | |
| N0 | 79 (44.1) |
| N1 | 68 (38.0) |
| N2 | 32 (17.9) |
| TNM stage (n/%) | |
| II | 145 (81.0) |
| III | 34 (19.0) |
| ECOG performance (n/%) | |
| 0 | 138 (77.1) |
| 1 | 41 (22.9) |
| LVEF (%) | 67.0 (64.0-71.0) |
| cTnI (ng/mL) | 0.021 (0.010-0.049) |
| NT-proBNP (ng/mL) | 0.076 (0.060-0.096) |
Data were presented as the mean ± standard deviation, count (%) or median (25th-75th quantiles). Obesity was defined as BMI ≥25 kg/m2. TNBC, triple-negative breast cancer; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; LVEF, left ventricular ejection fraction; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro brain natriuretic peptide.
LVEF level, cTnI and NT-proBNP concentrations in TNBC patients
Compared to C0, the LVEF level was decreased at C4 (P<0.001), C8 (M0) (P<0.001), M3 (P<0.001), M6 (P<0.001), M9 (P<0.01) and M12 (P<0.001) (Figure 2A). For cardiac function indicators, both cTnI (Figure 2B) and NT-proBNP (Figure 2C) concentrations were increased at C4 and C8 compared to C0 (all P<0.001).
Figure 2.
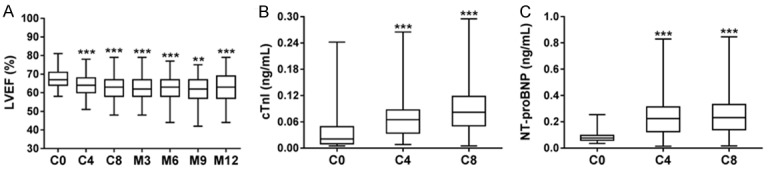
LVEF level, cTnI and NT-proBNP concentrations in TNBC patients during and post neoadjuvant chemotherapy. LVEF (A) level at C4, C8 (M0), M3, M6, M9 and M12 was decreased compared to C0. Concentrations of cTnI (B) and NT-proBNP (C) were higher at C4 and C8 compared to C0. The comparison between paired time points was carried out using Wilcoxon signed-rank sum test. **P<0.01, ***P<0.001. TNBC, triple-negative breast cancer; C, cycle; M, month; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro-brain natriuretic peptide.
The occurrence of cardiotoxicity in TNBC patients
The numbers of patients with heart failure, acute coronary syndrome, life-threatening arrhythmias and LVEF that declined by 10% from baseline to the absolute value below 53% were 1 (0.6%), 0 (0.0%), 0 (0.0%) and 9 (5.0%) respectively (one patient had both heart failure and LVEF declined by 10% from baseline to the absolute value below 53%). In total, 9 cases (5.0%) of cardiotoxicity occurred (Table 3).
Table 3.
Occurrence of cardiotoxicity
| Parameters | Count (n/%) |
|---|---|
| Heart failure | 1 (0.6) |
| Acute coronary syndrome | 0 (0.0) |
| Life threatening arrhythmias | 0 (0.0) |
| LVEF declined by 10% from baseline to the absolute value below 53% (the lower limit of normal) | 9 (5.0) |
| Cardiotoxicity | 9 (5.0) |
Data were presented as count (%). One patient had heart failure concurrently with the LVEF declined by 10% from baseline to the absolute value below 53%. LVEF, left ventricular ejection fraction.
Correlation of angiogenic miRNAs expressions levels with the levels of cTnI and NT-proBNP in TNBC patients
Let-7f (r=-0.281, P<0.001), miR-126 (r=-0.401, P<0.001) and miR-210 (r=-0.343, P<0.001) expressions levels were negatively correlated with the cTnI level. In addition, let-7f (r=-0.156, P=0.037), miR-19a (r=-0.153, P=0.041) and miR-130a (r=-0.152, P=0.043) expressions levels were negatively associated with the NT-proBNP level. There was no correlation of other candidate miRNAs with cTnI or NT-proBNP levels (Table 4).
Table 4.
Correlation of miRNAs relative expressions with the levels of cTnI and NT-proBNP
| miRNAs | cTnI | NT-proBNP | ||
|---|---|---|---|---|
|
| ||||
| R | P value | R | P value | |
| Let-7b | -0.091 | 0.226 | -0.078 | 0.298 |
| Let-7f | -0.281 | <0.001 | -0.156 | 0.037 |
| miR-17-5p | -0.084 | 0.265 | -0.026 | 0.730 |
| miR-17-3p | 0.010 | 0.889 | -0.031 | 0.680 |
| miR-18a | 0.041 | 0.587 | -0.127 | 0.090 |
| miR-19a | -0.061 | 0.827 | -0.153 | 0.041 |
| miR-19b-1 | -0.075 | 0.318 | -0.072 | 0.338 |
| miR-20a | -0.001 | 0.992 | -0.060 | 0.428 |
| miR-92a | -0.127 | 0.090 | 0.058 | 0.439 |
| miR-126 | -0.401 | <0.001 | -0.128 | 0.088 |
| miR-130a | -0.097 | 0196 | -0.152 | 0.043 |
| miR-210 | -0.343 | <0.001 | -0.146 | 0.051 |
| miR-296 | -0.030 | 0.695 | 0.050 | 0.506 |
| miR-378 | -0.069 | 0.357 | 0.035 | 0.639 |
Correlation of miRNAs relative expressions with the levels of cTnI and NT-proBNP was determined by Spearman’s test. P value <0.05 was considered significant. cTnI, cardiac troponin I; NT-proBNP, N-terminal pro brain natriuretic peptide.
Comparison of angiogenic miRNAs expressions levels between cardiotoxicity patients and noncardiotoxicity patients
There were 9 cardiotoxicity patients and 170 noncardiotoxicity patients. The expression of let-7f (P=0.001), miR-19a (P=0.023), miR-20a (P=0.040), miR-126 (P=0.020) and miR-210 (P=0.032) were decreased in cardiotoxicity patients compared to noncardiotoxicity patients (Table 5).
Table 5.
Comparison of miRNAs relative expression between cardiotoxicity patients and non-cardiotoxicity patients
| miRNAs | Cardiotoxicity patients (N=9) | Non-cardiotoxicity patients (N=170) | P value |
|---|---|---|---|
| Let-7b | 1.051 (0.396-1.788) | 1.129 (0.564-1.720) | 0.810 |
| Let-7f | 0.408 (0.176-0.689) | 1.124 (0.604-2.249) | 0.001 |
| miR-17-5p | 0.642 (0.465-1.450) | 1.060 (0.682-1.671) | 0.332 |
| miR-17-3p | 1.302 (0.696-2.692) | 1.355 (0.649-2.195) | 0.771 |
| miR-18a | 1.039 (0.380-1.914) | 1.181 (0.619-1.960) | 0.535 |
| miR-19a | 0.853 (0.464-1.800) | 2.100 (0.987-3.298) | 0.023 |
| miR-19b-1 | 0.485 (0.172-1.756) | 1.358 (0.644-2.038) | 0.083 |
| miR-20a | 0.727 (0.518-0.920) | 1.175 (0.524-2.025) | 0.040 |
| miR-92a | 0.883 (0.572-1.265) | 1.294 (0.655-1.786) | 0.160 |
| miR-126 | 0.559 (0.416-1.165) | 1.594 (0.748-2.744) | 0.020 |
| miR-130a | 3.183 (1.572-5.371) | 2.970 (1.714-4.921) | 0.815 |
| miR-210 | 0.591 (0.326-1.231) | 1.218 (0.642-2.056) | 0.032 |
| miR-296 | 0.668 (0.361-1.041) | 0.678 (0.403-1.411) | 0.853 |
| miR-378 | 0.645 (0.371-1.159) | 1.036 (0.535-1.790) | 0.104 |
Data were presented as median (25th-75th quantiles). Comparison was determined by a Wilcoxon rank sum test. P value <0.05 was considered significant.
Correlation of angiogenic miRNAs with cardiotoxicity risk in TNBC patients
Univariate logistic regression revealed that let-7f (P=0.015), miR-19a (P=0.036), miR-20a (P=0.050) and miR-126 (P=0.035) were negatively correlated with cardiotoxicity risk. All miRNAs were included in the following multivariate logistic regression, which illustrated that let-7f (P=0.033) and miR-126 (P=0.023) were independent predictive factors for decreased cardiotoxicity risk (Table 6).
Table 6.
Univariate and multivariate logistic regression analysis of miRNAs in predicting cardiotoxicity risk
| Univariate logistic regression | Multivariate logistic regression | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| P value | OR | 95% CI | P value | OR | 95% CI | |||
|
|
|
|||||||
| Lower | Higher | Lower | Higher | |||||
| Let-7b | 0.891 | 0.944 | 0.412 | 2.161 | 0.848 | 0.853 | 0.169 | 4.317 |
| Let-7f | 0.015 | 0.120 | 0.022 | 0.663 | 0.033 | 0.011 | 0.000 | 0.695 |
| miR-17-5p | 0.446 | 0.702 | 0.282 | 1.747 | 0.640 | 0.683 | 0.138 | 3.379 |
| miR-17-3p | 0.868 | 1.051 | 0.585 | 1.888 | 0.411 | 0.569 | 0.148 | 2.186 |
| miR-18a | 0.485 | 0.760 | 0.352 | 1.641 | 0.668 | 0.704 | 0.141 | 3.505 |
| miR-19a | 0.036 | 0.488 | 0.249 | 0.954 | 0.056 | 0.218 | 0.045 | 1.043 |
| miR-19b-1 | 0.115 | 0.479 | 0.192 | 1.195 | 0.187 | 0.365 | 0.082 | 1.628 |
| miR-20a | 0.050 | 0.321 | 0.103 | 0.998 | 0.293 | 0.289 | 0.029 | 2.923 |
| miR-92a | 0.172 | 0.499 | 0.184 | 1.354 | 0.163 | 0.257 | 0.038 | 1.736 |
| miR-126 | 0.035 | 0.358 | 0.138 | 0.931 | 0.023 | 0.146 | 0.028 | 0.768 |
| miR-130a | 0.951 | 1.009 | 0.749 | 1.361 | 0.248 | 1.399 | 0.792 | 2.471 |
| miR-210 | 0.055 | 0.331 | 0.107 | 1.023 | 0.335 | 0.405 | 0.064 | 2.545 |
| miR-296 | 0.856 | 0.912 | 0.340 | 2.450 | 0.758 | 0.778 | 0.157 | 3.858 |
| miR-378 | 0.125 | 0.416 | 0.136 | 1.276 | 0.244 | 0.361 | 0.065 | 2.004 |
Data were presented as P value, OR (odds ratio) and 95% CI (confidence interval). P value <0.05 was considered significant.
The value of angiogenic miRNAs let-7f and miR-126 for predicting cardiotoxicity risk in TNBC patients
Among the selected angiogenic miRNAs, let-7f and miR-126 independently predicted cardiotoxicity risk. Therefore, an ROC curve analysis was performed to further evaluate their predictive value on cardiotoxicity risk, which demonstrated that let-7f (AUC=0.815, 95% CI: 0.725-0.906) and miR-126 (AUC=0.731, 95% CI: 0.624-0.838) had good potential to distinguish cardiotoxicity patients from noncardiotoxicity patients. In addition, a combination of these two miRNAs presented with great value on predicting cardiotoxicity risk with an AUC of 0.885 (95% CI: 0.818-0.952). The sensitivity and specificity were as high as 88.9% and 84.7% at the best cut-off point where the sum of the two values was the largest (Figure 3).
Figure 3.
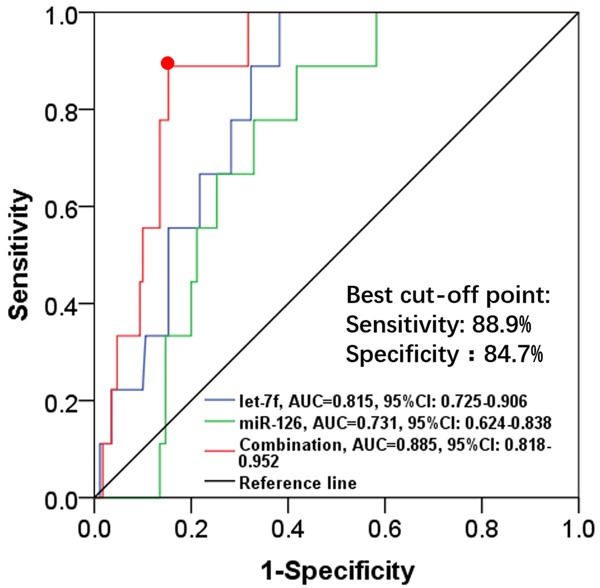
Predictive value of let-7f and miR-126 for cardiotoxicity risk in TNBC patients. Let-7f, miR-126 and combination of these two miRNAs were good predictive factors for distinguishing cardiotoxicity patients from non-cardiotoxicity patients. The predictive value of let-7f, miR-126 and the combination of the two miRNAs were assessed by ROC curve analysis. TNBC, triple-negative breast cancer; miRNAs, micro RNAs; ROC, receiver operating characteristics.
Discussion
In this study, we discovered that: (1) The LVEF level was reduced, while cTnI and NT-proBNP concentrations were increased during and post EC-D neoadjuvant chemotherapy, and the total occurrence of cardiotoxicity in TNBC patients was 5.0%. (2) Angiogenic miRNAs let-7f and miR-126 independently predicted a reduced risk of cardiotoxicity, and the ROC curve disclosed that both angiogenic miRNAs presented with good predictive values for predicting cardiotoxicity risk.
In clinical practices, neoadjuvant chemotherapy exerts great therapeutic effect on improving the survival of TNBC patients, while the application of cytotoxic antineoplastic drugs in neoadjuvant chemotherapy are frequently reported to be complicated with cardiotoxicity [15-17]. For instance, anthracyclines (epirubicin and doxorubicin) as well as alkylating agents (cyclophosphamide) are able to induce a relatively high incidence of left ventricular dysfunction in cancer patients [18]. In addition, anthracycline-based neoadjuvant chemotherapy has been shown to increase the risk of heart failure, as well as to elevate cTnI and NT-proBNP levels in BC patients [19-21]. These studies suggest that the occurrence of cardiotoxicity might be increased by neoadjuvant chemotherapy in cancer patients including BC patients. In line with this earlier evidence, we observed decreased LVEF but elevated cTnI and NT-proBNP levels in TNBC patients during and post EC-D neoadjuvant chemotherapy. The total occurrence rate of cardiotoxicity was 5.0%. This might be because the antineoplastic drugs in the EC-D neoadjuvant chemotherapy regimen could induce the production of cytotoxic agents such as free radicals to aggravate the oxidative stress and inflammation response in cardiac cells, thereby increasing the risk of cardiotoxicity in TNBC patients [22].
Angiogenic miRNAs participate in numerous cellular processes, and their dysregulation is involved in the alteration of normal cardiac function [23,24]. Let-7f, belonging to the let-7 family, is a common pro-angiogenic miRNA that facilitates the extending of the existing vascular network by directly targeting growth factors including transforming growth factor (TGF)-β and vascular epithelial growth factor (VEGF) [22,25]. For miR-126, it is highly expressed in endothelial cells and exerts a pro-angiogenic function by blocking the inhibitor of the vascular endothelial cells’ growth factor [26]. An elevated expression of miR-126 has been shown to relieve acute myocardial ischemic injury via protecting myocardial cells from apoptosis [24]. In addition, both let-7 and miR-126 expression levels are negatively associated with LVEF in dilated cardiomyopathy [23]. Therefore, these studies suggest that the angiogenic miRNAs let-7f and miR-126 could reduce the risk of cardiac dysfunction caused by ischemia and might protect TNBC patients from drug-induced cardiotoxicity. Moreover, a previous animal study reveals that downregulation of angiogenic let-7 increases the risk of myocardial injury in anthracycline-treated rats [27]. Few studies have investigated the role of miR-126 on cardiac function in cancer patients receiving antineoplastic drug therapy. In our study, let-7f and miR-126 were initially observed to be upregulated in noncardiotoxicity patients compared to cardiotoxicity patients and they independently predicted a low occurrence of cardiotoxicity in TNBC patients who underwent EC-D neoadjuvant chemotherapy. Additionally, an ROC curve analysis disclosed that let-7f and miR-126 has a good predictive value on cardiotoxicity risk. The possible explanations might be that: (1) let-7f and miR-126 were proangiogenic miRNAs and could induce the angiogenesis of vascular network by regulating angiogenic growth factors (such as VEGF and TGF-β) to increase blood and oxygen supply to the cardiac cells, reducing ischemia and hypoxia in the heart, thereby decreasing the risk of cardiotoxicity, or (2) the upregulation of let-7f or miR-126 might attenuate the activity of apoptotic agents (e.g., nuclear factor κB) induced by cytotoxic drugs, protecting cardiac cells from apoptosis, hence reducing the risk of cardiotoxicity. However, the mechanism of angiogenic miRNAs underlying cardiotoxicity is still unclear.
There were several limitations in our study. Firstly, only logistic regression and the ROC curve were performed to evaluate the predictive potential of angiogenic miRNAs for cardiotoxicity risk. This was because the follow-up period for cardiotoxicity was just 1 year in this study, and the occurrence of cardiotoxicity was more frequent at 6-12 months after neoadjuvant chemotherapy. The observed number of cardiotoxicity cases was too small to support the Kaplan-Meier curve or Cox’s proportional hazards regression analysis. Secondly, the sample size was small, which could mitigate the statistical power. Thirdly, the selection of patients was restricted to one hospital, leading to selection bias. Therefore, a larger number of patients from multiple centers with a longer follow-up time is necessary for further study.
In conclusion, the angiogenic miRNAs let-7f and miR-126 might serve as novel and convincing biomarkers for reduced cardiotoxicity risk in TNBC patients who undergo EC-D neoadjuvant chemotherapy.
Disclosure of conflict of interest
None.
References
- 1.Irvin WJ Jr, Carey LA. What is triple-negative breast cancer? Eur J Cancer. 2008;44:2799–2805. doi: 10.1016/j.ejca.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Chalakur-Ramireddy NK, Pakala SB. Combined drug therapeutic strategies for the effective treatment of triple negative breast cancer. Biosci Rep. 2018;38 doi: 10.1042/BSR20171357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAndrew N, DeMichele A. Neoadjuvant chemotherapy considerations in triple-negative breast cancer. J Target Ther Cancer. 2018;7:52–69. [PMC free article] [PubMed] [Google Scholar]
- 4.Omarini C, Guaitoli G, Pipitone S, Moscetti L, Cortesi L, Cascinu S, Piacentini F. Neoadjuvant treatments in triple-negative breast cancer patients: where we are now and where we are going. Cancer Manag Res. 2018;10:91–103. doi: 10.2147/CMAR.S146658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolazzi MA, Carnicelli A, Fuorlo M, Scaldaferri A, Masetti R, Landolfi R, Favuzzi AM. Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer. Eur Rev Med Pharmacol Sci. 2018;22:2175–2185. doi: 10.26355/eurrev_201804_14752. [DOI] [PubMed] [Google Scholar]
- 6.Sparano JA, Makhson AN, Semiglazov VF, Tjulandin SA, Balashova OI, Bondarenko IN, Bogdanova NV, Manikhas GM, Oliynychenko GP, Chatikhine VA, Zhuang SH, Xiu L, Yuan Z, Rackoff WR. Pegylated liposomal doxorubicin plus docetaxel significantly improves time to progression without additive cardiotoxicity compared with docetaxel monotherapy in patients with advanced breast cancer previously treated with neoadjuvant-adjuvant anthracycline therapy: results from a randomized phase III study. J Clin Oncol. 2009;27:4522–4529. doi: 10.1200/JCO.2008.20.5013. [DOI] [PubMed] [Google Scholar]
- 7.Dhahri W, Dussault S, Haddad P, Turgeon J, Tremblay S, Rolland K, Desjarlais M, Caceres-Gorriti KY, Mathieu R, Rivard A. Reduced expression of let-7f activates TGF-beta/ALK5 pathway and leads to impaired ischaemia-induced neovascularization after cigarette smoke exposure. J Cell Mol Med. 2017;21:2211–2222. doi: 10.1111/jcmm.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deveza L, Choi J, Yang F. Therapeutic angiogenesis for treating cardiovascular diseases. Theranostics. 2012;2:801–814. doi: 10.7150/thno.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei Y, Hu L, Yang G, Piao L, Jin M, Cheng X. Dipeptidyl peptidase-IV inhibition for the treatment of cardiovascular disease-recent insights focusing on angiogenesis and neovascularization. Circ J. 2017;81:770–776. doi: 10.1253/circj.CJ-16-1326. [DOI] [PubMed] [Google Scholar]
- 10.Wu F, Yang Z, Li G. Role of specific microRNAs for endothelial function and angiogenesis. Biochem Biophys Res Commun. 2009;386:549–553. doi: 10.1016/j.bbrc.2009.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo M, Shi JH, Wang PL, Shi DZ. Angiogenic growth factors for coronary artery disease: current status and prospects. J Cardiovasc Pharmacol Ther. 2018;23:130–141. doi: 10.1177/1074248417735399. [DOI] [PubMed] [Google Scholar]
- 12.Kitayama H, Kondo T, Sugiyama J, Kurimoto K, Nishino Y, Kawada M, Hirayama M, Tsuji Y. High-sensitive troponin T assay can predict anthracycline-and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer. 2017;24:774–782. doi: 10.1007/s12282-017-0778-8. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Biswas T, Efird JT, Prasad S, Jindal C, Walker PR. The survival benefit of neoadjuvant chemotherapy and pCR among patients with advanced stage triple negative breast cancer. Oncotarget. 2017;8:112712–112719. doi: 10.18632/oncotarget.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 17.Hatzis C, Symmans WF, Zhang Y, Gould RE, Moulder SL, Hunt KK, Abu-Khalaf M, Hofstatter EW, Lannin D, Chagpar AB, Pusztai L. Relationship between complete pathologic response to neoadjuvant chemotherapy and survival in triple-negative breast cancer. Clin Cancer Res. 2016;22:26–33. doi: 10.1158/1078-0432.CCR-14-3304. [DOI] [PubMed] [Google Scholar]
- 18.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 19.Gulati M, Mulvagh SL. The connection between the breast and heart in a woman: breast cancer and cardiovascular disease. Clin Cardiol. 2018;41:253–257. doi: 10.1002/clc.22886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu H, Zhang W, Huang D, Yang Q, Li J, Gao Y. Cardiotoxicity of anthracycline (ANT) treatment in children with malignant tumors. Pediatr Hematol Oncol. 2018 doi: 10.1080/08880018.2018.1459983. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Freres P, Bouznad N, Servais L, Josse C, Wenric S, Poncin A, Thiry J, Moonen M, Oury C, Lancellotti P, Bours V, Jerusalem G. Variations of circulating cardiac biomarkers during and after anthracycline-containing chemotherapy in breast cancer patients. BMC Cancer. 2018;18:102. doi: 10.1186/s12885-018-4015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schimmel KJ, Richel DJ, van den Brink RB, Guchelaar HJ. Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev. 2004;30:181–191. doi: 10.1016/j.ctrv.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Jiao M, You HZ, Yang XY, Yuan H, Li YL, Liu WX, Jin M, Du J. Circulating microRNA signature for the diagnosis of childhood dilated cardiomyopathy. Sci Rep. 2018;8:724. doi: 10.1038/s41598-017-19138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from MiR-126-overexpressing adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem. 2017;44:2105–2116. doi: 10.1159/000485949. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Popel AS. Computational model of MicroRNA control of HIF-VEGF pathway: insights into the pathophysiology of ischemic vascular disease and cancer. PLoS Comput Biol. 2015;11:e1004612. doi: 10.1371/journal.pcbi.1004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing BQ, Ou Y, Zhao L, Xie Q, Zhang YX. Experimental study on the prevention of liver cancer angiogenesis via miR-126. Eur Rev Med Pharmacol Sci. 2017;21:5096–5100. doi: 10.26355/eurrev_201711_13825. [DOI] [PubMed] [Google Scholar]
- 27.Fu J, Peng C, Wang W, Jin H, Tang Q, Wei X. Let-7 g is involved in doxorubicin induced myocardial injury. Environ Toxicol Pharmacol. 2012;33:312–317. doi: 10.1016/j.etap.2011.12.023. [DOI] [PubMed] [Google Scholar]


