Abstract
Background/Aims: To explore the effect of octreotide on pancreatic fibrosis induced by high-fat diet (HFD) and its mechanism of action. Methods: Sprague-Dawley (SD) rats were assigned to control, HFD, or octreotide treatment groups. Glucose and insulin tolerance tests (GTT and ITT), fasting plasma glucose (FPG), and fasting insulin (FINS), serum and pancreatic lipid levels, were measured, and the Lee’s index and the homeostatic model assessment (HOMA) index were calculated. The expression levels of alpha-smooth muscle actin (α-SMA), desmin, connective tissue growth factor (CTGF), transforming growth factor beta1 (TGF-β1), Smad3, and Smad7 in the pancreas were quantified. The LTC-14 cell line, which has features of primary rat pancreatic stellate cells (PSCs), was used for in vitro studies. Results: The AUC of ipGTT and ipITT, and FPG, FINS, lipid levels, were elevated after HFD feeding; however, they decreased after octreotide administration. The expression of α-SMA, CTGF, TGF-β1, and Smad3 in the HFD group were increased relative to the control group, but Smad7 expression was decreased. After treatment with octreotide, α-SMA, CTGF, TGF-β1, and Smad3 expression decreased, whereas the expression of Smad7 increased. In vitro studies showed that the expression of CTGF, TGF-β1, and Smad3 increased with palmitate treatment (PA), which mimics HFD treatment; and octreotide treatment decreased the expression of these proteins. The α-SMA and Smad7 expression levels remained unchanged among the three groups. Conclusions: Octreotide can ameliorate pancreatic fibrosis and improve pancreatic beta-cell function induced in HFD treated rats, possibly by inhibiting PSC activation and by decreasing pancreatic extracellular matrix (ECM) through the TGF-β1/Smad signaling pathway.
Keywords: Octreotide, high-fat diet, pancreatic stellate cells, pancreatic fibrosis, TGF-β1/Smad signaling pathway
Introduction
Pancreatic fibrosis is a common histopathological characteristic of chronic pancreatitis caused by many varied etiologies [1]. High-fat diet (HFD) can be a cause of inflammatory reactions of the pancreas, ultimately leading to pancreatic fibrosis, which is connected to the activation of pancreatic stellate cells (PSCs) [2-5]. Pancreatic fibrosis damages normal anatomic structures and impairs the exocrine and endocrine functions of the pancreas [1,6]. Long-term HFD intake increases the concentration of total triglyceride (TG) in the pancreas. TG is subsequently broken down to produce free fatty acids (FFA), which can obstruct the pancreatic capillary network or cause vascular endothelial injury [7,8]. Currently, therapies for pancreatic fibrosis are limited and their efficacy is poor. There is therefore an urgent need to study drugs that can alleviate pancreatic tissue fibrosis [9].
Watari et al [10] observed a type of vitamin A-storing cell located in the rat pancreas through scanning electron microscopy and immunofluorescence analysis in 1982; however, it was not until 1998 that Apte et al [11] first isolated and cultured these vitamin A-storing cells. These cells resemble hepatic stellate cells (HSCs) and were therefore named pancreatic stellate cells, and they are the major cell type involved in pancreatic fibrosis. The overexpression of α-smooth muscle actin (SMA) is a marker of activated PSCs [11]. Quiescent pancreatic stellate cells, which express desmin, represent 4% of the total number of pancreatic cells [12]. In a normal pancreas, only a handful of PSCs exist in the activated state for the maintenance of balanced extracellular matrix (ECM) secretion and degradation. When many PSCs are activated, the generation of ECM is upset and excessive amounts of ECM are formed, providing the foundation for development of pancreatic tissue fibrosis [13].
The transforming growth factor beta (TGF-β) signaling pathway is involved in many cellular processes including cell growth, differentiation, apoptosis, cellular homeostasis and other functions [14]. Smads are intracellular proteins that transduce extracellular signals from TGF-β ligands to the nucleus, where they activate downstream gene transcription [15]. There are three classes of Smads: Receptor-regulated Smads (R-Smad), common-mediator Smads (co-Smad), and inhibitory Smads (I-Smad). R-Smads (Smad2, Smad3) are directly phosphorylated by TGF-β1 receptors, then a co-Smad (Smad4) and Smad3 form a complex that can bind to DNA and modify the expression of several genes related to various cellular activities. I-Smads (Smad6, Smad7) can inhibit the TGF-β1 signaling pathway by blocking the activation of R-Smads and co-Smads [15]. Connective tissue growth factor (CTGF) plays important roles in many biological processes, including cell migration, proliferation, angiogenesis and tissue wound repair, and is critically involved in fibrotic disease of theliver and pancreas through its mitogenesis and chemotaxis [16]. Somatostatin is a neuroendocrine hormone primarily produced by the hypothalamus, which can suppress the exocrine secretory activity of the pancreas and the release of pancreatic hormones, and suppress the release of gastrointestinal hormones. Octreotide is an octapeptide that mimics natural somatostatin pharmacologically, though it is a more potent inhibitor of growth hormone, glucagon, and insulin than the natural hormone and has a much longer half-life [17]. Some studies have shown that octreotide can alleviate hepatic fibrosis by inhibiting HSC activation and by inhibiting PSC activation [18,19].
The effects of octreotide on pancreatic fibrosis in HFD-induced obese rats remain uncertain. In the present study, the authors explored whether octreotide can alleviate pancreatic fibrosis induced by HFD.
Materials and methods
Animals and experimental designs
The Institutional Animal Care and Ethics Committee of Sichuan University (Chengdu, China; approval no. SYXK 2008-119) approved all animal studies. A total of 60 healthy, 21-day-old male Sprague-Dawley rats were purchased from the Animal Center of Sichuan University (Chengdu, China) and were fed at the Animal Center of West China Hospital (Chengdu, China). All rats were housed in individual cages with 12 h light-dark cycles and were given free access to water and chow. Following 7 days of adapted feeding to a standard diet, the rats were divided into a control group (standard diet 320 kcal/100 g, 4.65% calories derived from fat; n=15) and an HFD group (500 kcal/100 g, 60% calories derived from fat; n=45). The body weights and lengths were measured every week for 24 weeks. Lee’s index [body weight (g)1/3 × 1,000/body length (cm)] was calculated to evaluate the degree of obesity. At the 24th week, 31 eligible obese rats with a body weight at least 1.4 times heavier than the control group were selected from among the HFD rats and were randomly separated into an HFD group (n=15) and an octreotide-treated group (n=16). Both groups of rats continued with an HFD; the octreotide-treated group received a subcutaneous injection of octreotide at a dosage of 40 μg/kg body weight every 12 h for 8 days.
Rats were fasted for 12 h and anesthetized with 2% sodium pentobarbital administered intraperitoneally at the end of the experiment. The pancreas and abdominal adipose tissue were collected and weighed. Parts of the pancreatic tissues were put into liquid nitrogen and then stored at -80°C for later use. Remaining pancreatic tissues were fixed in 4% paraformaldehyde and glutaraldehyde for histopathological analysis and transmission electron microscopy (TEM), respectively. Blood samples were centrifuged (100×g, 4°C, 15 min) to separate the plasma/serum then stored at -80°C for later use.
Rat PSC culture and treatment
The immortalized LTC-14 cell line established by Sparmann et al that has been proven to maintain essential characteristics of primary PSCs was used for the in vitro studies. The LTC-14 cell line was provided by Professor Robert Jaster from University of Rostock (Rostock, Germany), and cultured in Iscove’s modified Dulbecco’s medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (Biological Industries, Beit Haemek, Israel), 100 U/ml penicillin and 100 μg/ml streptomycin (HyClone; GE Healthcare Life Sciences). When the cell confluence reached 60%, the cells were starved for 6 h and then divided into three groups with different treatments: (1) A standard control group (cultured with 0.5% BSA for 24 h); (2) a palmitate (PA) treatment group (cultured with 50 μM PA + 0.5% BSA for 24 h); (3) a PA + octreotide treatment group (cultured with 50 μM PA + 0.5% BSA for 24 h and then with 1×10-9 mmol/l octreotide for 4 h). Radioimmunoprecipitation (Nanjing KeyGen Biotech Co. Ltd, Nanjing, China) assay lysis buffer, mixed with a protease inhibitor and a phosphatase inhibitor, was used to isolate total protein. TRIzol reagent (Takara Bio, Inc, Otsu, Japan) was used to extract total mRNA.
Intraperitoneal glucose tolerance test (ipGTT) and insulin tolerance test (ipITT)
Rats were subjected to the ipGTT and ipITT after fasting for 12 h. For the ipGTT and ipITT assays, rats were given an intraperitoneal injection of glucose at 2.0 g/kg and, separately, of insulin at 7.5 U/kg of body weight. Blood samples were taken from the tail vein at 0, 15, 30, 60 and 120 min after injection. Blood levels were measured with an Accu-Chek active glucometer (Roche Diagnostics GmbH, ACCU-CHEK Active, Mannheim, Germany). GraphPad Prism 7 was used to calculate the area under the curve (AUC) of ipGTT and ipITT, and results were expressed as mean ± standard deviation.
FPG, lipids, insulin, FFA and homeostatic model assessment (HOMA) index
The FPG concentration and serum TG and TC levels were determined by enzymatic methods (Changchun Huili Biotech Co. Ltd, Changchun, China). The fasting serum insulin level was measured with an ELISA kit (EZHIASF-14K, EMD Millipore, Billerica, MA, USA). The HOMA index was used to estimate the degree of insulin resistance and was calculated as [Fasting plasma glucose (mmol/l) × fasting serum insulin (μU/ml)/22.5]. FFA levels were also measured (Randox, Crumlin, UK).
Pancreas TG and FFA levels
Pancreatic tissue (30 mg per rat) was put in 800 µl of a chloroform/methanol mixed solution (v/v, 2:1), shaken for 20 min, and then centrifuged (310×g, 10 min, 4°C). The supernatant was collected and mixed with a 0.9% NaCl solution at ~0.2× the volume of the supernatant. This mixture was centrifuged (550×g, 20 min, 4°C), and the resulting subnatant was collected. The subnatant was dried, and then 0.5 ml of a 2% Triton X-100 solution was used to redissolve the residue and tissue TG was extracted. The pancreatic TG content was measured by an enzymatic method (Changchun Huili Biotech Co, Ltd). Pancreatic tissue (~50 mg) was put in pH 7.4 PBS to obtain tissue homogenate and then centrifuged (860×g, 20 min, 4°C). The supernatant was collected, and the BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc, Waltham, MA, USA) was used to measure protein concentrations. A FFA Assay kit (Randox, Crumlin, UK) was then used to determine pancreatic FFA content.
Histopathological and immunohistochemical studies of pancreas
Pancreatic tissues were fixed in formalin, dehydrated and embedded in paraffin, and then sectioned (thickness of 5 μm) for hematoxylin-eosin and Masson trichrome staining. Glutaraldehyde-fixed pancreatic specimens were used for TEM analysis (Hitachi Corporation, Tokyo, Japan).
For deparaffinization, sections of pancreatic tissue were subjected to high-pressure antigen retrieval for 10 min in citrate buffer at pH 6.0 and treated with 3% H2O2 for 10 min. After blocking with goat serum, the sections were incubated with anti-desmin (1:100, bs-1026R, BIOSS, Beijing, China) and anti-α-SMA (1:5,000, ab124964, Abcam, Cambridge, UK) antibodies overnight at 4°C. The desmin and α-SMA proteins were detected by incubating the sections with biotinylated secondary antibodies (SP-9002, Beijing Zhongshan Golden Bridge Biotechnology, Co, Ltd, Beijing, China) at 37°C for 30 min to form streptavidin-biotin-complexes. Finally, the immunocomplex was visualized using a solution of diaminobenzidine tetrahydrochloride. All photomicrographs were captured under a microscope (Olympus Corporation, Tokyo, Japan).
RNA isolation and quantitative reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
An innuPREP RNA Mini kit (Analytik Jena AG, Jena, Germany) was used to extract total RNA from pancreatic tissue. A total of 3 μg RNA was reverse transcribed to cDNA with a Revert Aid First Strand cDNA Synthesis kit (Thermo Fisher Scientific Inc.) following the instructions of the manufacturer. RT-qPCR was performed using 2X SYBR Green Master Mix (Biotool, LLC, Houston, TX, USA). The amplification reactions were run in duplicate and performed at 95°C for 5 min followed by 39 cycles of 95°C for 15 sec and 60°C for 30 sec on a CFX96 PCR Thermocycle Instrument (Bio-Rad Laboratories, Inc, Hercules, CA, USA). The mRNA expression was normalized to β-actin. And the following primer pairs were used (Table 1). The relative quantitative method was applied to calculate the threshold, and the results were expressed as 2-ΔΔCq [20].
Table 1.
Primers used for RT-qPCR
| Gene | Database | Forward sequence (5’-3’) | Reverse sequence (5’-3’) | Product Size |
|---|---|---|---|---|
| β-actin (rat) | NM_031144 | CGAGTACAACCTTCTTGCAGC | CCTTCTGACCCATACCCACC | 209 bp |
| TGF-β1 (rat) | NM_021578 | ATTCCTGGCGTTACCTTGG | AGCCCTGTATTCCGTCTCCT | 120 bp |
| α-SMA (rat) | NM_031004 | ACCATCGGGAATGAACGCTT | CTGTCAGCAATGCCTGGGTA | 199 bp |
| CTGF (rat) | NM_022266 | CGGGAAATGCTGTGAGGAGT | GGCTCGCATCATAGTTGGGT | 121 bp |
| Smad3 (rat) | NM_013095 | AACGGGCAGGAGGAGAAGTG | TGGGGATGGTAATGCACTTGG | 136 bp |
| Smad7 (rat) | NM_030858 | TGCAACCCCCATCACCTTAG | ATTCGTTCCCCCGGTTTCAT | 141 bp |
The primers were synthesized by TSINGKE Biological Technology, Ltd. (Chengdu, China).
Total protein extraction and western blotting
Total protein was extracted from pancreatic tissues or rat PSCs with a KeyGen Whole Cell Assay kit (Nanjing KeyGen Biotech Co. Ltd, Nanjing, China). An enhanced BCA Protein Assay kit was used to measure protein concentrations. The extracted protein (~40 μg) was electrophoresed on 10% SDS-PAGE and then transferred to polyvinylidene difluoride membranes (EMD Millipore). Nonspecific binding sites were blocked with 5% nonfat milk at room temperature for 2 h, and membranes were then incubated with anti-α-SMA rabbit monoclonal antibody (1:20,000, ab124964, Abcam), anti-Smad3 rabbit monoclonal antibody (1:5,000, ab40854, Abcam), anti-Smad7 rabbit polyclonal antibody (1:1,000, D160746-0025, BBI Life Sciences, Shanghai, China), anti-TGF-β1 rabbit polyclonal antibody (1:400, D121324-0025, BBI Life Sciences), anti-CTGF rabbit polyclonal antibody (1:800, D160212-0025, BBI Life Sciences) or anti-β-actin mouse monoclonal antibody (1:3,000, mAbcam8226, Epitomics, Hangzhou, China) at 4°C overnight. The membranes were washed with 0.1% TBST, then incubated with the appropriate HRP-conjugated secondary antibodies (Beijing Zhongshan Golden Bridge Biotechnology, Co, Ltd, Beijing, China) for 2 h at room temperature. Specific bands were visualized using enhanced chemiluminescence (Engreen Biosystem, Auckland, New Zealand) detection. The housekeeping protein β-actin was analyzed for normalization. The intensity of the bands was analyzed by Quantity One software (version, 4.6.2; Bio-Rad Laboratories, Inc.).
Statistical analysis
All of the data were analyzed using SPSS software (version, 20.0; IBM SPSS, Armonk, NY, USA), and the results were expressed as mean ± standard deviation. Comparisons among the three groups were performed by one-way ANOVA, and the Student-Newman-Keuls test was applied to detect significant differences. A test for the homogeneity of variances was also used. P<0.05 was considered a significant difference.
Results
Body weight, Lee’s Index, pancreas weight, abdominal fat and index
The body weight, Lee’s Index, pancreas weight, abdominal fat, and abdominal fat index were calculated at the end of the experiment. The body weight of the HFD group was increased by 27.8% compared with the control group (P<0.01). Lee’s Index, an estimate of obesity in adult rats, was also increased by 5.0% in the HFD group when compared with that of the control group (P< 0.01). Abdominal fat in the HFD group was heavier than in the control rats (P<0.01). The abdominal fat index in obese rats was also higher than in the control group (P<0.01). Octreotide treatment significantly reduced these parameters (P<0.01 or P<0.05). Pancreas weight in the HFD group was lighter than in the control group (P<0.01; Table 2).
Table 2.
Parameters in each group
| Control group (n=15) | HFD group (n=15) | Octreotide-treated group (n=16) | |
|---|---|---|---|
| Final body weight (g) | 470.07±28.94 | 600.27±58.38** | 512.56±47.90## |
| Lee’s Index | 306.82±4.96 | 322.30±8.82** | 315.32±12.28 |
| Pancreas weight (g) | 1.39±0.38 | 0.91±0.23** | 0.87±0.25 |
| Abdominal fat (g) | 10.36±4.72 | 28.87±8.76** | 20.02±4.83## |
| Abdominal fat Index | 2.16±0.88 | 4.72±1.23** | 3.75±0.81# |
Values are expressed as mean ± SD;
P<0.01 vs. control group;
P<0.01 vs. HFD group;
P<0.05 vs. HFD group.
Plasma glucose, serum lipids, insulin levels, HOMA-index and pancreas lipids levels
Compared with the control group, the plasma glucose concentration in obese rats was significantly elevated (P<0.01). Following octreotide administration, plasma glucose levels decreased (P<0.01). Obese rats’ serum insulin, FFA, TG, and TC levels were higher than in the control group (P<0.05 or P<0.01). Octreotide intervention distinctly decreased these parameters, with the exception of serum TC levels (P>0.05). The HOMA-index, derived from plasma glucose and serum insulin levels, was significantly increased in the HFD group compared with the control group, but was decreased in the octreotide-treated group (P<0.01). In HFD-fed rats, the pancreatic TG and FFA levels significantly increased compared with the control group (P<0.05 or P<0.01), and octreotide treatment significantly decreased their levels (P< 0.05 or P<0.01; Table 3).
Table 3.
Data of blood biochemistry, pancreatic TG levels, and HOMA-index in the three groups
| Control group (n=15) | HFD group (n=15) | Octreotide-treated group (n=16) | |
|---|---|---|---|
| Plasma glucose (mmol/) | 4.60±1.17 | 6.26±1.55** | 4.75±1.60## |
| Serum insulin (mmol/l) | 118.33±37.08 | 157.68±43.55* | 108.85±66.36# |
| Serum FFA (mmol/l) | 225.36±88.20 | 391.99±105.77** | 278.99 ±63.65## |
| Serum TG (mmol/l) | 0.35±0.14 | 0.70±0.30** | 0.49±0.14# |
| Serum TC (mmol/l) | 1.68±0.44 | 2.54±0.62** | 2.31±0.44 |
| HOMA-index | 23.47±8.86 | 39.57±13.48** | 23.40±16.71## |
| Pancreas TG (mg/g tissue) | 15.40±5.78 | 31.76±10.53** | 23.82±11.99# |
| Pancreas FFA (mmol/g protein) | 104.01±43.20 | 138.09±62.20* | 107.66±37.03# |
Values are expressed as mean ± SD;
P<0.01 vs. control group;
P<0.05 vs. control group;
P<0.01 vs. HFD group;
P<0.05 vs. HFD group.
Intraperitoneal insulin tolerance test (ipITT) and Intraperitoneal glucose tolerance test (ipGTT)
The blood insulin and glucose concentrations of the HFD group at the five time points were higher than in the control group (Figure 1A and 1B). The relative AUC increased by 32.5% or increased by 28.4%, respectively (P<0.05, Figure 1C and 1D). Although the AUC of ipITT and ipGTT in the octreotide-treated group decreased by 15.4% and 5%, respectively, compared with the HFD group, these decreases did not meet statistical significance (P>0.05).
Figure 1.
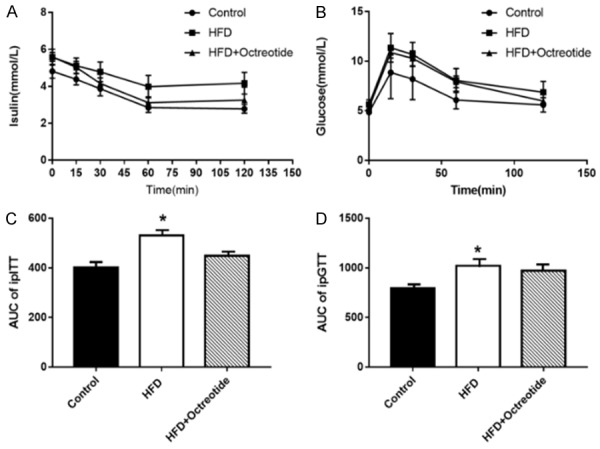
(A and B) Results of ipITT and ipGTT. The glycemic curve in ipITT and ipGTT (C) The AUC of ipITT increased by 32.5% in the HFD group compared to the control group, and decreased by 15.4% after octreotide treatment (C). The AUC of ipGTT increased by 28.4% in the HFD group compared to the control group, and decreased by 5% following octreotide treatment (D). Values are expressed as the mean ± standard deviation. *P<0.05 vs. control group. ipITT, insulin tolerance test; ipGTT, intraperitoneal glucose tolerance test; AUC, area under the curve; HFD, high-fat diet.
Morphology of the pancreata
It can be seen in Figure 2A that the pancreatic tissues of the HFD group became harder and smaller, pancreatic weights were lighter than in the control group (P<0.01, Table 2), and pancreatic calcification was conspicuous. Hematoxylin and eosin (Figure 2B) and Masson trichrome staining (Figure 2C) show histopathological features of the pancreata. In contrast with the control group, the structure of pancreatic acinar cells was atrophic and accompanied by inflammatory cell infiltration in the HFD group. The pancreatic interlobular fibers in the HFD group were also increased. Octreotide treatment reduced the inflammatory cell infiltration and alleviated pancreatic tissue fibrosis (Figure 2). Study of pancreatic ultramicrostructure by TEM further showed reduced zymogen granules, dilated rough endoplasmic reticula in the acinar cells of rats in the HFD group, indicating that the secretion function was impaired. In contrast, octreotide treatment increased zymogen granules and the dilation degree of rough endoplasmic reticula was alleviated (Figure 2D).
Figure 2.
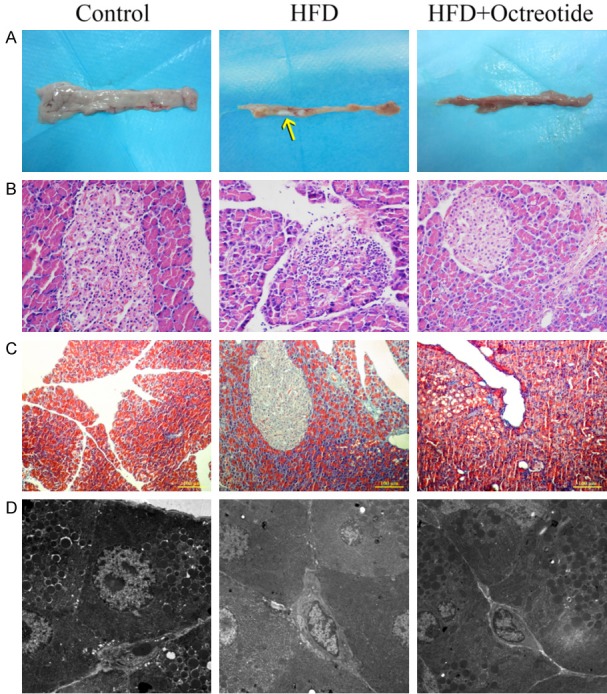
Pancreatic morphology of each group. Pancreatic tissues of the HFD group became harder and smaller, and pancreatic calcification was observed (A, yellow arrow). Hematoxylin and eosin and Masson-Trichrome staining presented pancreatic acinar inflammatory infiltration and atrophy in the HFD group, and pancreatic interlobular fibers also increased in obese rats (B and C, magnification, ×200). Pancreatic ultramicrostructure was visualized by TEM: red arrow, zymogen granules; white arrow, rough endoplasmic reticula. TEM showed reduced zymogen granules, dilated rough endoplasmic reticula in the acinar cells in the HFD group, and octreotide treatment increased zymogen granules and the dilation degree of rough endoplasmic reticula was alleviated (D, magnification, ×0.7). HFD, high-fat diet; TEM, transmission electron microscopy.
Immunohistochemical staining for α-SMA and desmin
The increased expression of α-SMA (Figure 3A) and decreased expression of desmin (Figure 3B) were thought to be markers of activated PSCs. Immunohistochemical staining indicated that the expression of α-SMA increased and desmin decreased in the HFD group. The α-SMA protein significantly decreased and the desmin protein increased in response to octreotide treatment.
Figure 3.
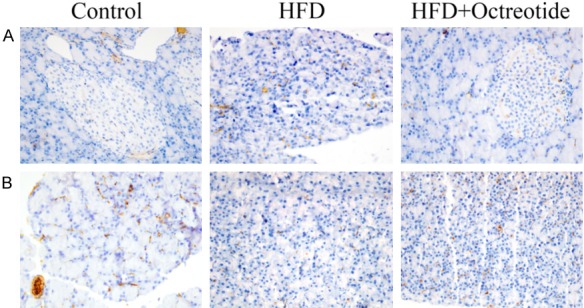
Immunohistochemical staining for α-SMA and desmin. (A) α-SMA and (B) desmin were primarily localized in the periacinar regions of the pancreas. The expression of α-SMA was increased and desmin was decreased in the HFD group. Following octreotide treatment, α-SMA protein significantly decreased and desmin protein increased (magnification, ×400). α-SMA, α-smooth muscle actin; HFD, high-fat diet.
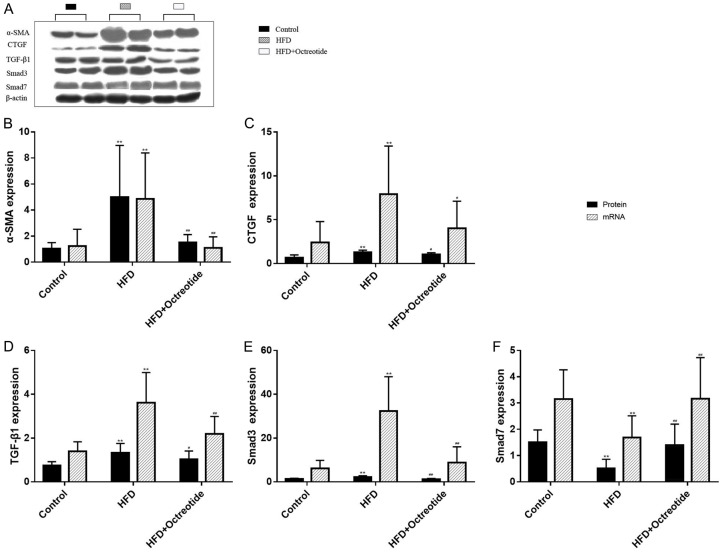
Expression of pancreatic protein and mRNA for α-SMA, CTGF, TGF-β1, Smad3 and Smad7
The protein and mRNA expression of α-SMA, CTGF, TGF-β1 and Smad3 in the HFD group were higher than in the control group (Figure 4, P<0.01), but the expression level of Smad7 was lower than in the control group (Figure 4F, P<0.01). Octreotide treatment significantly reduced α-SMA, Smad3, CTGF and TGF-β1 protein and mRNA expression in the obese rats (Figure 4, P<0.01 or P< 0.05). The Smad7 protein and mRNA expression increased in the octreotide treatment group (Figure 4, P<0.01).
Figure 4.
α-SMA, CTGF, TGF-β1, Smad3 and Smad7 protein and mRNA levels of each group. (A) The western blot bands of α-SMA, CTGF, TGF-β1, Smad3 and Smad7. (B-E) The protein and mRNA expression levels of α-SMA, CTGF, TGF-β1 and Smad3 in the obese group were higher than those in the control group, and the expression level of Smad7 was lower than in (F) the control group. Octreotide treatment significantly decreased α-SMA, Smad3, CTGF, and TGF-β1 protein and mRNA expression levels in obese rats. The Smad7 protein and mRNA expression level increased in the octreotide treatment group. Values are expressed as mean ± standard deviation. *P<0.05, **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the HFD group. α-SMA, α-smooth muscle actin; CTGF, connective tissue growth factor; TGF-β1, transforming growth factor-β1.
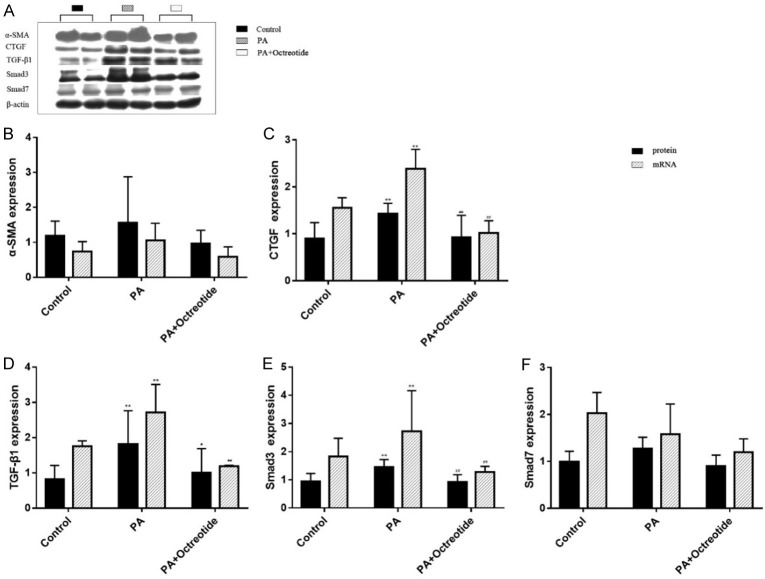
Expression of protein and mRNA for α-SMA, CTGF, TGF-β1, Smad3 and Smad7 of the PSCs
In in vitro studies, the authors found the protein and mRNA expression of α-SMA and Smad7 displayed no significant differences between the three groups (Figure 5). The protein and mRNA expression levels of CTGF, TGF-β1 and Smad3 in the PA group were higher than in the control group (Figure 5, P<0.01 or P<0.05), and octreotide treatment significantly decreased their expression (Figure 5, P<0.01 or P<0.05).
Figure 5.
α-SMA, CTGF, TGF-β1, Smad3 and Smad7 protein and mRNA levels of rat PSCs. The western blot images of α-SMA, CTG, TGF-β1, Smad3, and Smad7. The protein and mRNA expression levels of α-SMA and Smad7 displayed no significant difference between the three groups. The protein and mRNA expression levels of CTGF, TGF-β1, and Smad3 in the palmitate (PA) group were higher than in the control group, and octreotide treatment significantly decreased their expression levels. Values are expressed as mean ± standard deviation. *P<0.05, **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the HFD group. α-SMA, α-smooth muscle actin; CTGF, connective tissue growth factor; TGF-β1, transforming growth factor-β1.
Discussion
Long-term HFD-intake leads to energy metabolism disequilibrium and increased blood lipid (especially TG) content, which produces an increased burden on pancreatic insulin secretion [3]. Excessive TG accumulation in pancreatic tissue results from the increased serum TG content, and subsequent TG breakdown to FFA are main causes of chronic pancreatic injuries [7,21]. In the present study, the TG and FFA content increased in HFD rats, subsequently, pancreatic acinar cells were destroyed by inflammatory cell infiltration and pancreatic tissue fibrosis formed, indicating the establishment of an animal model of chronic pancreatitis induced by HFD. Interestingly, octreotide can inhibit the activation of PSCs and improve pancreatic endocrine function.
The pancreatic ECM is important for maintaining normal pancreatic structure and secretory functions [12]. The generation of ECM primarily occurs by PSCs, and ECM degradation is primarily regulated by matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases [22]. PSCs are mostly located in the periacinar areas and function similarly to fibroblasts, which can switch between quiescent and activated phenotypes. When stimulated by pathological factors such as acute or chronic pancreatitis, quiescent PSCs are activated and migrate to injured areas and subsequently generate ECM to repair the tissues [23,24]. HFD is closely correlated with insulin resistance [25]. Yang et al [26] reported that high levels of insulin cannot activate quiescent PSCs, but can prompt the proliferation of activated PSCs and increased generation of ECM. Previous studies have found similar biologic characteristics between PSCs and HSCs, such as that both have quiescent and activated phenotypes and both contain vitamin A lipid droplets when quiescent [27,28]. They also play an important role in fibrosis [27]. Research focused on proteomic analysis of HSCs and PSCs found that there were 177 proteins highly expressed in HSCs, and 157 of these proteins were also highly expressed in PSCs [28]. Other research has found that octreotide can alleviate liver fibrosis through inhibiting the activation of HSCs [18]. Long et al [19] found that octreotide can effectively inhibit PSC activation and proliferation in vitro. The current study shows that the insulin level and HOMA index of the HFD group was higher than the control group, and octreotide treatment can reduce these levels. In addition, α-SMA, which is an important marker of activated PSCs, was significantly increased in the HFD group, but decreased following octreotide treatment, showing the inhibitory effect of octreotide. Desmin is highly expressed in quiescent PSCs, and HFD treatment decreased while octreotide treatment increased its expression, indicating an inhibitory effect of octreotide on PSCs. However, the authors reported that that α-SMA expression was not significantly changed between the three groups in the in vitro studies. The LTC-14 cell line tends to display a more activated phenotype when compared to primary PSCs [30], which is speculated to explain these results.
CTGF is an ECM-associated protein and is involved in its intercellular signaling [31]. TGF-β1 can promote the generation of ECM, collagen and fibronectin, while inhibiting the degradation of ECM [14]. CTGF can cooperate with TGF-β1 to induce sustained fibrosis and to exacerbate extracellular matrix production. TGF-β1 can regulate the TGF-β1 reaction components of CTGF, form the complex with Smad components, and finally induce the generation of CTGF [14,19]. Shek et al [32] used exogenous TGF-β1 (10 ng/ml) to stimulate PSCs and found that it can increase procollagen-1 mRNA and collagen protein expression levels, showing that TGF-β1 can promote collagen generation. The present study showed a decrease in CTGF and TGF-β1 protein expression levels in obese rats after using octreotide, which strongly supports the hypothesis that octreotide may inhibit the generation of ECM and alleviate pancreatic fibrosis.
The TGF-β1/Smad signaling pathway functions first through phosphorylating the Smad3 protein to transduce signals into the cytoplasm and later binds to Smad4 and forms a heterodimeric complex. These signals are transduced into the nucleus to combine with the target gene to regulate the protein product. However, Smad7 may block the activation of Smad3 and the complex formation with Smad4 [14,15]. The in vivo studies showed that the HFD group’s expression of Smad3 increased and Smad7 decreased compared with the control group. In contrast, following treatment with octreotide, Smad3 decreased and Smad7 reached a normal level. Consistent with these results, Qian et al [33] used exogenous TGF-β1 to stimulate rat primary PSCs and found Smad3 expression levels increased along with the concentration of TGF-β1, but Smad7 expression levels were reduced. However, the expression levels of Smad7 remained unchanged among the three groups in the in vitro studies, possibly because the concentration of PA, which mimics HFD to interfere with PSCs, could not reach an effective level to activate the Smad7 protein. Unfortunately, the lipotoxicity of PA increased with the increasing concentration required for effective interference, resulting in the death of PSCs.
As a somatostatin analogue, octreotide is commonly used to prevent complications following pancreatic surgeries and esophageal variceal hemorrhage in clinical settings [34,35]. Long et al [19] found that octreotide can also alleviate inflammatory infiltration, and slowed the occurrence of insulitis and vasculitis after rat pancreas transplantation. The study found that an HFD can destroy pancreatic acinar cells, causing inflammatory cell infiltration and resulting in small and tough pancreata. Octreotide reduced pancreatic inflammatory cell infiltration.
In summary, the current study indicated that octreotide can ameliorate pancreatic fibrosis and improve pancreatic beta-cell function in HFD-induced obese rats, possibly by inhibiting PSC activation and decreasing pancreatic extracellular matrix through TGF-β1/Smad signaling.
Acknowledgements
The project was supported by the National Natural Science Foundation of China (grant no. 30870919).
Disclosure of conflict of interest
None.
References
- 1.Majumder S, Chari ST. Chronic pancreatitis. Lancet. 2016;387:1957–66. doi: 10.1016/S0140-6736(16)00097-0. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda A, Makino N, Tozawa T, Shirahata N, Honda T, Ikeda Y, Sato H, Ito M, Kakizaki Y, Akamatsu M, Ueno Y, Kawata S. Pancreatic fat accumulation, fibrosis, and acinar cell injury in the zucker diabetic fatty rat fed a chronic highfat diet. Pancreas. 2014;43:735–43. doi: 10.1097/MPA.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castineira-Alvarino M, Lindkvist B, Luaces-Regueira M, Iglesias-García J, Lariño-Noia J, Nieto-García L, Domínguez-Muñoz JE. The role of high fat diet in the development of complications of chronic pancreatitis. Clin Nutr. 2013;32:830–6. doi: 10.1016/j.clnu.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Lingvay I, Szczepaniak LS, Ravazzola M, Orci L, Unger RH. Pancreatic steatosis: harbinger of type 2 diabetes in obese rodents. Int J Obes (Lond) 2010;34:396–400. doi: 10.1038/ijo.2009.245. [DOI] [PubMed] [Google Scholar]
- 5.Yan MX, Li YQ, Meng M, Kou Y. Long-term highfat diet induces pancreatic injuries via pancreatic microcirculatory disturbances and oxidative stress in rats with hyperlipidemia. Biochem Biophys Res Commun. 2006;347:192–9. doi: 10.1016/j.bbrc.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 6.Enrique Dominguez-Munoz J. Latest advances in chronic pancreatitis. Gastroenterol Hepatol. 2016;39(Suppl 1):87–92. doi: 10.1016/S0210-5705(16)30179-0. [DOI] [PubMed] [Google Scholar]
- 7.Mathur A, Marine M, Lu D, Swartz-Basile DA, Saxena R, Zyromski NJ, Pitt HA. Nonalcoholic fatty pancreas disease. HPB (Oxford) 2007;9:312–8. doi: 10.1080/13651820701504157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JP. Serum triglycerides, the liver and the pancreas. Curr Opin Lipidol. 2000;11:377–82. doi: 10.1097/00041433-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu K. Mechanisms of pancreatic fibrosis and applications to the treatment of chronic pancreatitis. J Gastroenterol. 2008;43:823–32. doi: 10.1007/s00535-008-2249-7. [DOI] [PubMed] [Google Scholar]
- 10.Watari N, Hotta Y, Mabuchi Y. Morphological studies on a vitamin A-storing cell and its complex with macrophage observed in mouse pancreatic tissues following excess vitamin A administration. Okajimas Folia Anat Jpn. 1982;58:837–58. doi: 10.2535/ofaj1936.58.4-6_837. [DOI] [PubMed] [Google Scholar]
- 11.Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–33. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–9. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch HJ, Hwang RF, Jaster R, Kleeff J, Klöppel G, Kordes C, Logsdon CD, Masamune A, Michalski CW, Oh J, Phillips PA, Pinzani M, Reiser-Erkan C, Tsukamoto H, Wilson J. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172–8. doi: 10.1136/gutjnl-2011-301220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wrana JL, Cárcamo J, Attisano L, Zentella A, Doody J, Laiho M, Wang XF, Massagué J. TGFβ signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–14. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 15.Moustakas A. Smad signalling network. J Cell Sci. 2002;115:3355–6. doi: 10.1242/jcs.115.17.3355. [DOI] [PubMed] [Google Scholar]
- 16.Charrier A, Brigstock DR. Regulation of pancreatic function by connective tissue growth factor (CTGF, CCN2) Cytokine Growth Factor Rev. 2013;24:59–68. doi: 10.1016/j.cytogfr.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L, Coy DH. Somatostatin and its analogs. Curr Drug Targets. 2016;17:529–37. doi: 10.2174/1389450116666141205163548. [DOI] [PubMed] [Google Scholar]
- 18.Klironomos S, Notas G, Sfakianaki O, Kiagiadaki F, Xidakis C, Kouroumalis E. Octreotide modulates the effects on fibrosis of TNF-alpha, TGF-beta and PDGF in activated rat hepatic stellate cells. Regul Pept. 2014;188:5–12. doi: 10.1016/j.regpep.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Long D, Lu J, Luo L, Guo Y, Li C, Wu W, Shan J, Li L, Li S, Li Y, Lin T, Feng L. Effects of octreotide on activated pancreatic stellate cell-induced pancreas graft fibrosis in rats. J Surg Res. 2012;176:248–259. doi: 10.1016/j.jss.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535–41. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel G, Tonon T, Scornet D, Cock JM, Kloareg B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol. 2010;188:82–97. doi: 10.1111/j.1469-8137.2010.03374.x. [DOI] [PubMed] [Google Scholar]
- 23.Jaster R. Molecular regulation of pancreatic stellate cell function. Mol Cancer. 2004;3:26. doi: 10.1186/1476-4598-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu K. Pancreatic stellate cells: molecular mechanism of pancreatic fibrosis. J Gastroenterol Hepatol. 2008;23(Suppl 1):S119–121. doi: 10.1111/j.1440-1746.2007.05296.x. [DOI] [PubMed] [Google Scholar]
- 25.Paniagua JA. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J Diabetes. 2016;7:483–514. doi: 10.4239/wjd.v7.i19.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Waldron RT, Su HY, Moro A, Chang HH, Eibl G, Ferreri K, Kandeel FR, Lugea A, Li L, Pandol SJ. Insulin promotes proliferation and fibrosing responses in activated pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2016;311:G675–G687. doi: 10.1152/ajpgi.00251.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kordes C, Sawitza I, Haussinger D. Hepatic and pancreatic stellate cells in focus. Biol Chem. 2009;390:1003–12. doi: 10.1515/BC.2009.121. [DOI] [PubMed] [Google Scholar]
- 28.Wehr AY, Furth EE, Sangar V, Blair IA, Yu KH. Analysis of the human pancreatic stellate cell secreted proteome. Pancreas. 2011;40:557–66. doi: 10.1097/MPA.0b013e318214efaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulo JA, Kadiyala V, Banks PA, Conwell DL, Steen H. Mass spectrometry-based quantitative proteomic profiling of human pancreatic and hepatic stellate cell lines. Genomics Proteomics Bioinformatics. 2013;11:105–13. doi: 10.1016/j.gpb.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparmann G, Hohenadl C, Tornoe J, Jaster R, Fitzner B, Koczan D, Thiesen HJ, Glass A, Winder D, Liebe S, Emmrich J. Generation and characterization of immortalized rat pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G211–9. doi: 10.1152/ajpgi.00347.2003. [DOI] [PubMed] [Google Scholar]
- 31.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–63. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shek FW, Benyon RC, Walker FM, McCrudden PR, Pender SL, Williams EJ, Johnson PA, Johnson CD, Bateman AC, Fine DR, Iredale JP. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787–98. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian ZY, Peng Q, Zhang ZW, Zhou LA, Miao Y. Roles of Smad3 and Smad7 in rat pancreatic stellate cells activated by transforming growth factor-beta 1. Hepatobiliary Pancreat Dis Int. 2010;9:531–36. [PubMed] [Google Scholar]
- 34.Gotzsche PC, Hrobjartsson A. Somatostatin analogues for acute bleeding oesophageal varices. Cochrane Database Syst Rev. 2008:Cd000193. doi: 10.1002/14651858.CD000193.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Sliwinska-Mosson M, Vesely M, Milnerowicz H. The clinical significance of somatostatin in pancreatic diseases. Ann Endocrinol (Paris) 2014;75:232–40. doi: 10.1016/j.ando.2014.06.004. [DOI] [PubMed] [Google Scholar]